化工学报 ›› 2022, Vol. 73 ›› Issue (2): 865-875.DOI: 10.11949/0438-1157.20211530
杨英杰1( ),杨赫1,朱家龙1,郭双淇1,尚妍2,李扬1,靳立军1,胡浩权1(
),杨赫1,朱家龙1,郭双淇1,尚妍2,李扬1,靳立军1,胡浩权1( )
)
收稿日期:2021-10-26
修回日期:2021-12-01
出版日期:2022-02-05
发布日期:2022-02-18
通讯作者:
胡浩权
作者简介:杨英杰(1996—),女,硕士研究生,基金资助:
Yingjie YANG1( ),He YANG1,Jialong ZHU1,Shuangqi GUO1,Yan SHANG2,Yang LI1,Lijun JIN1,Haoquan HU1(
),He YANG1,Jialong ZHU1,Shuangqi GUO1,Yan SHANG2,Yang LI1,Lijun JIN1,Haoquan HU1( )
)
Received:2021-10-26
Revised:2021-12-01
Online:2022-02-05
Published:2022-02-18
Contact:
Haoquan HU
摘要:
采用滴管炉,在短停留时间下,制备具有一定低温反应活性而消除主要低温交联位点的淖毛湖煤(NMHcoal)快速热解半焦(NRPchar),再将NMHcoal和NRPchar混合进行慢速热解,研究官能团间的相互作用。热重分析结果表明,NMHcoal/NRPchar混合比为5∶5,温度为500℃热解时具有较强的负协同作用。固定床热解结果表明,NMHcoal热解生成的挥发物部分扩散至NRPchar中,?CH3与芳碳自由基以及?O有更多的结合概率与时间,使焦油中含甲基的萘、酚类增多,半焦中烷基化邻氧芳碳结构与醚类结构增加。析出的酚类增多,使半焦中连氧芳碳结构减少。NRPchar中生成较多的多环芳烃前体,它们与酚类物质发生反应生成多环芳烃和CO,使共热解焦油中5、6环化合物含量增加,而另一部分滞留在半焦中使其比表面积降低。
中图分类号:
杨英杰, 杨赫, 朱家龙, 郭双淇, 尚妍, 李扬, 靳立军, 胡浩权. 淖毛湖煤慢速热解过程官能团相互作用[J]. 化工学报, 2022, 73(2): 865-875.
Yingjie YANG, He YANG, Jialong ZHU, Shuangqi GUO, Yan SHANG, Yang LI, Lijun JIN, Haoquan HU. Interaction between functional groups during slow pyrolysis of Naomaohu coal[J]. CIESC Journal, 2022, 73(2): 865-875.
| 样品 | 工业分析/%(质量) | 元素分析/%(质量,daf) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | C | H | N | O | S | |
| NMHcoal | 3.37 | 5.29 | 54.83 | 71.64 | 6.01 | 0.85 | 21.08 | 0.42 |
| NRPchar | 2.43 | 10.36 | 14.37 | 90.19 | 1.46 | 2.25 | 5.33 | 0.77 |
表1 NMHcoal和NRPchar的工业与元素分析
Table 1 Proximate and ultimate analyses of NMHcoal and NRPchar
| 样品 | 工业分析/%(质量) | 元素分析/%(质量,daf) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | C | H | N | O | S | |
| NMHcoal | 3.37 | 5.29 | 54.83 | 71.64 | 6.01 | 0.85 | 21.08 | 0.42 |
| NRPchar | 2.43 | 10.36 | 14.37 | 90.19 | 1.46 | 2.25 | 5.33 | 0.77 |
| 峰位置/cm-1 | 峰归属 | 峰面积的比例 | |
|---|---|---|---|
| NMHcoal | NRPchar | ||
| 2825 | O—CH3 | 3.20 | 3.39 |
| 2851、2920 | sym.R2CH2、asym.R2CH2 | 61.60 | 54.97 |
| 2874、2953 | sym.RCH3、asym.RCH3 | 24.69 | 22.94 |
| 2893 | R3CH | 10.51 | 18.69 |
表2 NMHcoal和NRPchar 2800~3000 cm-1拟合结果
Table 2 Fitting results of NMHcoal and NRPchar in 2800—3000 cm-1
| 峰位置/cm-1 | 峰归属 | 峰面积的比例 | |
|---|---|---|---|
| NMHcoal | NRPchar | ||
| 2825 | O—CH3 | 3.20 | 3.39 |
| 2851、2920 | sym.R2CH2、asym.R2CH2 | 61.60 | 54.97 |
| 2874、2953 | sym.RCH3、asym.RCH3 | 24.69 | 22.94 |
| 2893 | R3CH | 10.51 | 18.69 |
| 样品 | 初温/ ℃ | 峰温/ ℃ | 终温/ ℃ | 失重量/% | DTGmax/(%/℃) | ||
|---|---|---|---|---|---|---|---|
| 实验值 | 加权平均值 | 实验值 | 加权平均值 | ||||
| NMHcoal | 201 | 443 | 636 | 47.3 | — | -0.215 | — |
| NRPchar | 368 | — | — | 22.3 | — | -0.058 | — |
| 20% char | 197 | 443 | 637 | 41.8 | 42.3 | -0.166 | -0.175 |
| 40% char | 203 | 443 | 625 | 34.8 | 37.3 | -0.134 | -0.134 |
| 50% char | 209 | 442 | 625 | 32.7 | 34.8 | -0.101 | -0.114 |
| 60% char | 203 | 443 | 608 | 29.5 | 32.3 | -0.097 | -0.094 |
| 80% char | 207 | 443 | 583 | 24.6 | 27.3 | -0.055 | -0.054 |
表3 NMHcoal、NRPchar及NMHcoal/NRPchar共热解的特征参数
Table 3 Characteristic parameters of NMHcoal, NRPchar and NMHcoal/NRPchar pyrolysis
| 样品 | 初温/ ℃ | 峰温/ ℃ | 终温/ ℃ | 失重量/% | DTGmax/(%/℃) | ||
|---|---|---|---|---|---|---|---|
| 实验值 | 加权平均值 | 实验值 | 加权平均值 | ||||
| NMHcoal | 201 | 443 | 636 | 47.3 | — | -0.215 | — |
| NRPchar | 368 | — | — | 22.3 | — | -0.058 | — |
| 20% char | 197 | 443 | 637 | 41.8 | 42.3 | -0.166 | -0.175 |
| 40% char | 203 | 443 | 625 | 34.8 | 37.3 | -0.134 | -0.134 |
| 50% char | 209 | 442 | 625 | 32.7 | 34.8 | -0.101 | -0.114 |
| 60% char | 203 | 443 | 608 | 29.5 | 32.3 | -0.097 | -0.094 |
| 80% char | 207 | 443 | 583 | 24.6 | 27.3 | -0.055 | -0.054 |
| 序号 | 化合物 | NMHcoal-fb | NMHcoal/NRPchar-fb | NRPchar-fb |
|---|---|---|---|---|
| 1环芳香化合物 | ||||
| 1 | 甲苯 | 0.806 | 0.243 | 0.732 |
| 2 | 乙苯 | 0.366 | 0.206 | 0.058 |
| 3 | 对二甲苯 | 1.400 | 0.594 | 0.409 |
| 4 | 1,2,3-三甲苯 | 1.362 | 1.194 | — |
| 5 | 苯酚 | 6.274 | 5.582 | 1.548 |
| 6 | 2-甲基苯酚 | 9.222 | 8.639 | 0.916 |
| 7 | 2,6-二甲基苯酚 | 10.04 | 6.08 | — |
| 8 | 2-乙基苯酚 | 0.475 | 1.709 | — |
| 9 | 3-甲基-1,2-苯二酚 | 0.715 | 2.765 | — |
| 10 | 2,3,6-三甲基苯酚 | 0.371 | 0.840 | — |
| 11 | 4,5-二甲基-1,3-苯二酚 | 0.445 | 0.594 | — |
| 12 | 4-乙基-1,3-苯二酚 | 0.431 | 0.814 | — |
| 2环芳香化合物 | ||||
| 13 | 茚 | 0.437 | 0.280 | 0.061 |
| 14 | 萘 | 1.238 | 1.647 | 66.852 |
| 15 | 4,7-二甲基苯并呋喃 | 0.317 | 0.230 | — |
| 16 | 1,3-二甲基-1-氢茚 | 0.85 | 1.006 | — |
| 17 | 2,3-二氢化-5-羟基茚 | 0.744 | 1.236 | — |
| 18 | 2-甲基萘 | 3.377 | 2.912 | — |
| 19 | 2,6-二甲基萘 | 3.117 | 2.844 | — |
| 20 | 1,4,6-三甲基萘 | 3.266 | 4.052 | — |
| 21 | 2-甲基-1-萘酚 | 3.388 | 3.994 | — |
| 3环以上芳香化合物 | ||||
| 22 | 1-甲基-9H-笏 | 0.268 | 0.874 | — |
| 23 | 菲 | — | 1.132 | 5.773 |
| 24 | 3,6-二甲基菲 | 0.187 | — | — |
| 25 | 2,3,5-三甲基菲 | — | 0.131 | — |
| 26 | 芘 | 0.974 | 4.428 | |
| 27 | 荧蒽 | — | — | 5.437 |
| 28 | 苯并芘 | — | 0.340 | 1.080 |
| 29 | 苯并荧蒽 | — | 1.692 | — |
| 30 | 苝 | 0.166 | 0.405 | — |
| 31 | 苯并苝 | — | 0.548 | 0.117 |
| 长链脂肪烃化合物 | ||||
| 32 | 十一烷 | 0.741 | 0.552 | — |
| 33 | 十二烷 | 0.958 | 1.567 | — |
| 34 | 十四烷 | 1.058 | 1.383 | — |
| 35 | 十五烷 | 1.009 | 1.165 | — |
| 36 | 十七烷 | 1.004 | 1.100 | — |
| 37 | 十八烷 | 0.945 | 0.947 | — |
| 38 | 十九烷 | 0.873 | 0.962 | — |
| 39 | 二十烷 | 0.909 | 1.025 | — |
| 40 | 二十一烷 | 1.005 | 1.154 | — |
| 41 | 二十二烷 | 1.110 | 1.170 | — |
| 42 | 二十三烷 | 1.357 | 1.445 | — |
| 43 | 二十四烷 | 1.170 | 1.035 | — |
| 44 | 二十五烷 | 1.019 | 2.473 | — |
表4 NMHcoal、NRPchar及其混合物在500℃热解得到的焦油样品中检测到的主要单环、多环芳烃和长链脂肪烃
Table 4 Main single-ring, polycyclic aromatic compounds and long-chain aliphatic hydrocarbons detected in tar samples obtained from pyrolysis of NMHcoal, NRPchar and their mixtures at 500℃
| 序号 | 化合物 | NMHcoal-fb | NMHcoal/NRPchar-fb | NRPchar-fb |
|---|---|---|---|---|
| 1环芳香化合物 | ||||
| 1 | 甲苯 | 0.806 | 0.243 | 0.732 |
| 2 | 乙苯 | 0.366 | 0.206 | 0.058 |
| 3 | 对二甲苯 | 1.400 | 0.594 | 0.409 |
| 4 | 1,2,3-三甲苯 | 1.362 | 1.194 | — |
| 5 | 苯酚 | 6.274 | 5.582 | 1.548 |
| 6 | 2-甲基苯酚 | 9.222 | 8.639 | 0.916 |
| 7 | 2,6-二甲基苯酚 | 10.04 | 6.08 | — |
| 8 | 2-乙基苯酚 | 0.475 | 1.709 | — |
| 9 | 3-甲基-1,2-苯二酚 | 0.715 | 2.765 | — |
| 10 | 2,3,6-三甲基苯酚 | 0.371 | 0.840 | — |
| 11 | 4,5-二甲基-1,3-苯二酚 | 0.445 | 0.594 | — |
| 12 | 4-乙基-1,3-苯二酚 | 0.431 | 0.814 | — |
| 2环芳香化合物 | ||||
| 13 | 茚 | 0.437 | 0.280 | 0.061 |
| 14 | 萘 | 1.238 | 1.647 | 66.852 |
| 15 | 4,7-二甲基苯并呋喃 | 0.317 | 0.230 | — |
| 16 | 1,3-二甲基-1-氢茚 | 0.85 | 1.006 | — |
| 17 | 2,3-二氢化-5-羟基茚 | 0.744 | 1.236 | — |
| 18 | 2-甲基萘 | 3.377 | 2.912 | — |
| 19 | 2,6-二甲基萘 | 3.117 | 2.844 | — |
| 20 | 1,4,6-三甲基萘 | 3.266 | 4.052 | — |
| 21 | 2-甲基-1-萘酚 | 3.388 | 3.994 | — |
| 3环以上芳香化合物 | ||||
| 22 | 1-甲基-9H-笏 | 0.268 | 0.874 | — |
| 23 | 菲 | — | 1.132 | 5.773 |
| 24 | 3,6-二甲基菲 | 0.187 | — | — |
| 25 | 2,3,5-三甲基菲 | — | 0.131 | — |
| 26 | 芘 | 0.974 | 4.428 | |
| 27 | 荧蒽 | — | — | 5.437 |
| 28 | 苯并芘 | — | 0.340 | 1.080 |
| 29 | 苯并荧蒽 | — | 1.692 | — |
| 30 | 苝 | 0.166 | 0.405 | — |
| 31 | 苯并苝 | — | 0.548 | 0.117 |
| 长链脂肪烃化合物 | ||||
| 32 | 十一烷 | 0.741 | 0.552 | — |
| 33 | 十二烷 | 0.958 | 1.567 | — |
| 34 | 十四烷 | 1.058 | 1.383 | — |
| 35 | 十五烷 | 1.009 | 1.165 | — |
| 36 | 十七烷 | 1.004 | 1.100 | — |
| 37 | 十八烷 | 0.945 | 0.947 | — |
| 38 | 十九烷 | 0.873 | 0.962 | — |
| 39 | 二十烷 | 0.909 | 1.025 | — |
| 40 | 二十一烷 | 1.005 | 1.154 | — |
| 41 | 二十二烷 | 1.110 | 1.170 | — |
| 42 | 二十三烷 | 1.357 | 1.445 | — |
| 43 | 二十四烷 | 1.170 | 1.035 | — |
| 44 | 二十五烷 | 1.019 | 2.473 | — |
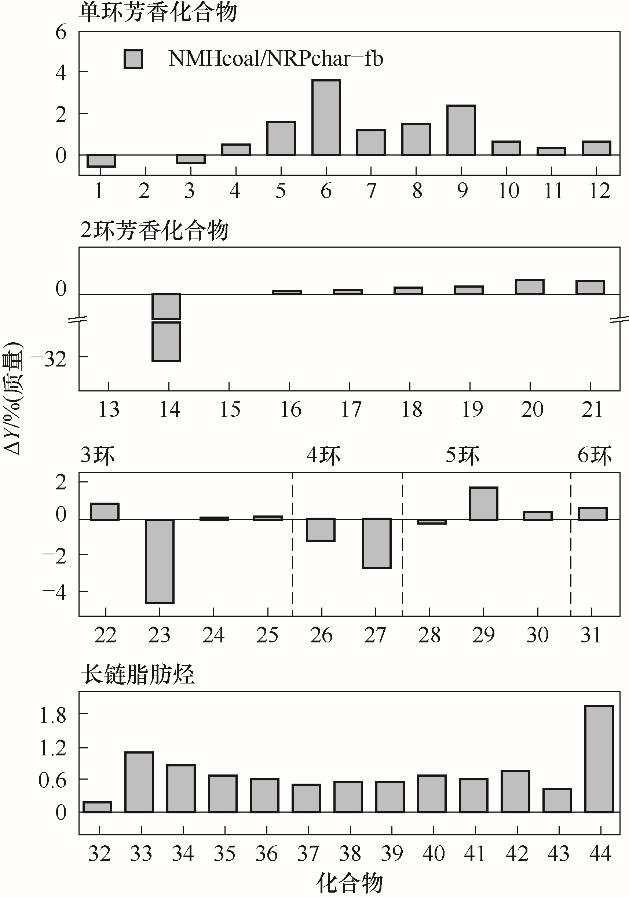
图8 单环、多环芳香族化合物和长链脂肪烃在500℃下的协同效应(x轴上的数字1~44对应于表4中的化合物)
Fig.8 Synergetic effects from single-ring, polycyclic aromatic compounds and long-chain aliphatic hydrocarbons obtained from NMHcoal/NRPchar (5∶5)at 500℃ (The numbers 1—44 on x-axis correspond to the compounds in Table 4)
| 样品 | 比表面积/ (m2/g) | 孔容/ (cm3/g) | 孔径/nm |
|---|---|---|---|
| NMHcoal-fb | 10.7 | 0.016 | 5.44 |
| NRPchar-fb | 285.9 | 0.171 | 4.27 |
| NMHcoal/NRPchar-fb(实验值) | 105.0 | 0.088 | 4.23 |
| NMHcoal/NRPchar -fb(加权平均值) | 148.3 | 0.093 | 4.86 |
表5 半焦的比表面积及孔隙结构参数
Table 5 Specific surface area and pore structure parameters of char
| 样品 | 比表面积/ (m2/g) | 孔容/ (cm3/g) | 孔径/nm |
|---|---|---|---|
| NMHcoal-fb | 10.7 | 0.016 | 5.44 |
| NRPchar-fb | 285.9 | 0.171 | 4.27 |
| NMHcoal/NRPchar-fb(实验值) | 105.0 | 0.088 | 4.23 |
| NMHcoal/NRPchar -fb(加权平均值) | 148.3 | 0.093 | 4.86 |
| 峰 | 碳类型 | 符号 | 峰化学位移 | 摩尔分取/% | ||
|---|---|---|---|---|---|---|
| NMHcoal-fb | NMHcoal/NRPchar-fb | NMHcoal-fb | NMHcoal/NRPchar-fb | |||
| 脂肪碳 | ||||||
| 1 | 脂肪甲基 | 12.2 | 11.0 | 1.64 | 0.74 | |
| 2 | 芳环甲基 | 20.2 | 20.3 | 3.13 | 1.24 | |
| 3 | 亚甲基 | 30.2 | 29.2 | 1.36 | 0.99 | |
| 4 | 次甲基 | 36.8 | 36.1 | 1.88 | 1.67 | |
| 5 | 季碳 | 45.5 | 45.9 | 2.23 | 2.81 | |
| 6 | 连氧脂肪碳 | 55.5 67.6 86.0 | 55.0 66.7 88.0 | 15.12 | 16.98 | |
| 总 | 25.36 | 24.43 | ||||
| 芳碳 | ||||||
| 7 | 质子化邻氧芳碳 | 108.9 | 108 | 5.49 | 5.96 | |
| 8 | 烷基化邻氧芳碳 | 116.1 | 116.8 | 4.78 | 7.64 | |
| 9 | 质子化芳碳 | 122 | 122.3 | 7.13 | 7.86 | |
| 10 | 桥头芳碳 | 129 | 128.6 | 23.61 | 24.29 | |
| 11 | 烷基取代芳碳 | 138.9 147.1 | 138.8 147.8 | 13.13 | 11.06 | |
| 12 | 连氧芳碳 | 155 165 | 155 165 | 6.51 | 4.45 | |
| 总 | 60.65 | 61.26 | ||||
| 羰基 | ||||||
| 13 | 羧基、酯的羰基碳 | 178.5 | 176.3 | 1.55 | 1.02 | |
| 14 | 酮、醛的碳 | 199.3 | 197.3 | 12.41 | 13.27 | |
| 总 | 13.96 | 14.29 | ||||
表6 NMHcoal-fb和NMHcoal /NRPchar(5∶5)-fb半焦的碳分布
Table 6 Distribution of carbon types in NMHcoal-fb and NMHcoal /NRPchar(5∶5)-fb
| 峰 | 碳类型 | 符号 | 峰化学位移 | 摩尔分取/% | ||
|---|---|---|---|---|---|---|
| NMHcoal-fb | NMHcoal/NRPchar-fb | NMHcoal-fb | NMHcoal/NRPchar-fb | |||
| 脂肪碳 | ||||||
| 1 | 脂肪甲基 | 12.2 | 11.0 | 1.64 | 0.74 | |
| 2 | 芳环甲基 | 20.2 | 20.3 | 3.13 | 1.24 | |
| 3 | 亚甲基 | 30.2 | 29.2 | 1.36 | 0.99 | |
| 4 | 次甲基 | 36.8 | 36.1 | 1.88 | 1.67 | |
| 5 | 季碳 | 45.5 | 45.9 | 2.23 | 2.81 | |
| 6 | 连氧脂肪碳 | 55.5 67.6 86.0 | 55.0 66.7 88.0 | 15.12 | 16.98 | |
| 总 | 25.36 | 24.43 | ||||
| 芳碳 | ||||||
| 7 | 质子化邻氧芳碳 | 108.9 | 108 | 5.49 | 5.96 | |
| 8 | 烷基化邻氧芳碳 | 116.1 | 116.8 | 4.78 | 7.64 | |
| 9 | 质子化芳碳 | 122 | 122.3 | 7.13 | 7.86 | |
| 10 | 桥头芳碳 | 129 | 128.6 | 23.61 | 24.29 | |
| 11 | 烷基取代芳碳 | 138.9 147.1 | 138.8 147.8 | 13.13 | 11.06 | |
| 12 | 连氧芳碳 | 155 165 | 155 165 | 6.51 | 4.45 | |
| 总 | 60.65 | 61.26 | ||||
| 羰基 | ||||||
| 13 | 羧基、酯的羰基碳 | 178.5 | 176.3 | 1.55 | 1.02 | |
| 14 | 酮、醛的碳 | 199.3 | 197.3 | 12.41 | 13.27 | |
| 总 | 13.96 | 14.29 | ||||
| 1 | Wang N, Yu J L, Tahmasebi A, et al. Experimental study on microwave pyrolysis of an Indonesian low-rank coal[J]. Energy & Fuels, 2014, 28(1): 254-263. |
| 2 | 袁帅, 陈雪莉, 李军, 等. 煤快速热解固相和气相产物生成规律[J]. 化工学报, 2011, 62(5): 1382-1388. |
| Yuan S, Chen X L, Li J, et al. Formations of solid and gas phase products during rapid pyrolysis of coal[J]. CIESC Journal, 2011, 62(5): 1382-1388. | |
| 3 | Wang Z Q, Bai Z Q, Li W, et al. Quantitative study on cross-linking reactions of oxygen groups during liquefaction of lignite by a new model system[J]. Fuel Processing Technology, 2010, 91(4): 410-413. |
| 4 | Ibarra J V, Cervero I, García M, et al. Influence of cross-linking on tar formation during pyrolysis of low-rank coals[J]. Fuel Processing Technology, 1990, 24: 19-25. |
| 5 | 王志青, 白宗庆, 李文, 等. 吡啶预处理抑制煤热解过程中交联反应的研究[J]. 燃料化学学报, 2008, 36(6): 641-645. |
| Wang Z Q, Bai Z Q, Li W, et al. Suppressing cross-linking reactions during pyrolysis of lignite pretreated by pyridine[J]. Journal of Fuel Chemistry and Technology, 2008, 36(6): 641-645. | |
| 6 | Li J G, Zhu J L, Hu H Q, et al. Co-pyrolysis of Baiyinhua lignite and pine in an infrared-heated fixed bed to improve tar yield[J]. Fuel, 2020, 272: 117739. |
| 7 | Chen Y Y, Mastalerz M, Schimmelmann A. Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy[J]. International Journal of Coal Geology, 2012, 104: 22-33. |
| 8 | Haykiri-Acma H, Yaman S. Interaction between biomass and different rank coals during co-pyrolysis[J]. Renewable Energy, 2010, 35(1): 288-292. |
| 9 | 王刚, 李爱民, 全翠. 生物质与聚乳酸塑料共热解特性[J]. 化工学报, 2009, 60(7): 1787-1792. |
| Wang G, Li A M, Quan C. Thermal decomposition of biomass/polylactic acid during co-pyrolysis[J]. Journal of Chemical Industry and Engineering(China), 2009, 60(7): 1787-1792. | |
| 10 | Zhang Y, Zhang X Q, Zhong Q F, et al. Structural differences of spontaneous combustion prone inertinite-rich Chinese lignite coals: insights from XRD, solid-state 13C NMR, LDIMS, and HRTEM[J]. Energy & Fuels, 2019, 33(5): 4575-4584. |
| 11 | Jing Z H, Rodrigues S, Strounina E, et al. Use of FTIR, XPS, NMR to characterize oxidative effects of NaClO on coal molecular structures[J]. International Journal of Coal Geology, 2019, 201: 1-13. |
| 12 | Solum M S, Pugmire R J, Grant D M. 13C solid-state NMR of Argonne-premium coals[J]. Energy & Fuels, 1989, 3(2): 187-193. |
| 13 | Cao S Q, Wang D C, Wang M Y, et al. In‐situ upgrading of coal pyrolysis tar with steam catalytic cracking over Ni/Al2O3 catalysts[J]. ChemistrySelect, 2020, 5(16): 4905-4912. |
| 14 | Zhu J L, Jin L J, Luo Y W, et al. Fast co-pyrolysis of a massive Naomaohu coal and cedar mixture using rapid infrared heating[J]. Energy Conversion and Management, 2020, 205: 112442. |
| 15 | 陈宗定, 王永刚, 许德平, 等. 褐煤热解过程中半焦重整催化剂性质的变化[J]. 燃料化学学报, 2017, 45(8): 908-915. |
| Chen Z D, Wang Y G, Xu D P, et al. Changes in char properties after catalytic reforming volatiles from pyrolysis of brown coal[J]. Journal of Fuel Chemistry and Technology, 2017, 45(8): 908-915. | |
| 16 | Mae K, Maki T, Miura K. A new method for estimating the cross-linking reaction during the pyrolysis of brown coal[J]. Journal of Chemical Engineering of Japan, 2002, 35(8): 778-785. |
| 17 | Qiu S X, Zhang S F, Zhou X H, et al. Thermal behavior and organic functional structure of poplar-fat coal blends during co-pyrolysis[J]. Renewable Energy, 2019, 136: 308-316. |
| 18 | Fletcher T H, Hardesty D R. Compilation of Sandia coal devolatilization data: milestone report[R]. Livermore, CA, USA: Sandia National Laboratories, 1992. |
| 19 | Solomon P R, Serio M A, Carangelo R M, et al. Very rapid coal pyrolysis[J]. Fuel, 1986, 65(2): 182-194. |
| 20 | Niu Z Y, Liu G J, Yin H, et al. In-situ FTIR study of reaction mechanism and chemical kinetics of a Xundian lignite during non-isothermal low temperature pyrolysis[J]. Energy Conversion and Management, 2016, 124: 180-188. |
| 21 | Xiong G, Li Y S, Jin L J, et al. In situ FT-IR spectroscopic studies on thermal decomposition of the weak covalent bonds of brown coal[J]. Journal of Analytical and Applied Pyrolysis, 2015, 115: 262-267. |
| 22 | Iglesias M J, del Rı́o J C, Laggoun-Défarge F, et al. Control of the chemical structure of perhydrous coals; FTIR and Py-GC/MS investigation[J]. Journal of Analytical and Applied Pyrolysis, 2002, 62(1): 1-34. |
| 23 | 商铁成. 热解温度对低阶煤热解性能影响研究[J]. 洁净煤技术, 2014, 20(6): 28-31. |
| Shang T C. Influence of temperature on pyrolysis properties of low rank coal[J]. Clean Coal Technology, 2014, 20(6): 28-31. | |
| 24 | Liang L T, Huang W, Gao F X, et al. Mild catalytic depolymerization of low rank coals: a novel way to increase tar yield[J]. RSC Advances, 2015, 5(4): 2493-2503. |
| 25 | 杨亚慧. 淖毛湖煤慢速热解过程化学渗透热解模型研究[D]. 大连: 大连理工大学, 2021. |
| Yang Y H. Study on chemical percolation devolatilization model for slow pyrolysis process of NMH coal[D]. Dalian: Dalian University of Technology, 2021. | |
| 26 | Wu Y F, Zhu J L, Zhao S, et al. Co-pyrolysis behaviors of low-rank coal and polystyrene with in-situ pyrolysis time-of-flight mass spectrometry[J]. Fuel, 2021, 286: 119461. |
| 27 | 陈霞, 徐艳梅, 陆人春, 等. 淖毛湖煤及显微组分热解半焦微观结构分析[J]. 高校化学工程学报, 2020, 34(3): 831-837. |
| Chen X, Xu Y M, Lu R C, et al. Microstructure analysis of char from pyrolysis of Naomaohu coal and macerals[J]. Journal of Chemical Engineering of Chinese Universities, 2020, 34(3): 831-837. | |
| 28 | 韩峰, 张衍国, 蒙爱红, 等. 云南褐煤结构的FTIR分析[J]. 煤炭学报, 2014, 39(11): 2293-2299. |
| Han F, Zhang Y G, Meng A H, et al. FTIR analysis of Yunnan lignite[J]. Journal of China Coal Society, 2014, 39(11): 2293-2299. | |
| 29 | Peng C N, Zhang G Y, Yue J R, et al. Pyrolysis of black liquor for phenols and impact of its inherent alkali[J]. Fuel Processing Technology, 2014, 127: 149-156. |
| 30 | Yang F, Hou Y C, Wu W Z, et al. A new insight into the structure of Huolinhe lignite based on the yields of benzene carboxylic acids[J]. Fuel, 2017, 189: 408-418. |
| 31 | Xiong Y K, Jin L J, Li Y, et al. Structural features and pyrolysis behaviors of extracts from microwave-assisted extraction of a low-rank coal with different solvents[J]. Energy & Fuels, 2019, 33(1): 106-114. |
| 32 | Li L, Fan H J, Hu H Q. A theoretical study on bond dissociation enthalpies of coal based model compounds[J]. Fuel, 2015, 153: 70-77. |
| [1] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [2] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [3] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [4] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [5] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [6] | 何晓崐, 刘锐, 薛园, 左然. MOCVD生长AlN单晶薄膜的气相和表面化学反应综述[J]. 化工学报, 2023, 74(7): 2800-2813. |
| [7] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [8] | 朱风, 陈凯琳, 黄小凤, 鲍银珠, 李文斌, 刘嘉鑫, 吴玮强, 高王伟. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| [9] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [10] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [11] | 李瑞康, 何盈盈, 卢维鹏, 王园园, 丁皓东, 骆勇名. 电化学强化钴基阴极活化过一硫酸盐的研究[J]. 化工学报, 2023, 74(5): 2207-2216. |
| [12] | 衣思敏, 马亚丽, 刘伟强, 张金帅, 岳岩, 郑强, 贾松岩, 李雪. 微晶菱镁矿蒸氨及水化动力学研究[J]. 化工学报, 2023, 74(4): 1578-1586. |
| [13] | 吴选军, 王超, 曹子健, 蔡卫权. 数据与物理信息混合驱动的固定床吸附穿透深度学习模型[J]. 化工学报, 2023, 74(3): 1145-1160. |
| [14] | 杨庆云, 李青松, 陈泽铭, 邓靖, 李玉瑛, 杨帆, 陈国元, 李国新. UV/PMS、UV/PDS、UV/SPC工艺降解尼泊金甲酯[J]. 化工学报, 2023, 74(3): 1322-1331. |
| [15] | 陈瑞哲, 程磊磊, 顾菁, 袁浩然, 陈勇. 纤维增强树脂复合材料化学回收技术研究进展[J]. 化工学报, 2023, 74(3): 981-994. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号