化工学报 ›› 2023, Vol. 74 ›› Issue (8): 3522-3532.DOI: 10.11949/0438-1157.20230513
收稿日期:2023-05-29
修回日期:2023-07-31
出版日期:2023-08-25
发布日期:2023-10-18
通讯作者:
孙文寿
作者简介:李锦潼(1998—),男,硕士研究生,2632950395@qq.com
基金资助:
Jintong LI( ), Shun QIU, Wenshou SUN(
), Shun QIU, Wenshou SUN( )
)
Received:2023-05-29
Revised:2023-07-31
Online:2023-08-25
Published:2023-10-18
Contact:
Wenshou SUN
摘要:
以N2、O2、SO2三种气体模拟烟气,在1 L的光反应器中,研究了主要因素对煤砷浸出、As(Ⅲ) 氧化以及SO2脱除的影响规律,分析了过程机理,确定了浸出过程速率控制步骤。结果表明,紫外线照射和草酸均能有效提高砷浸出率,180 min时,在遮光和pH为2.5的条件下,加入1.0 mmol/L草酸能使砷浸出率提高32.9%,在紫外线照射下,可使砷浸出率再提高21.3%,且浸出液中的As(Ⅲ) 占总砷之比小于13%,还能提高SO2脱除率。低pH有利于煤中砷的浸出,但对SO2的脱除不利。煤砷浸出率和SO2脱除率均随温度的提高而显著增大。动力学分析计算得出煤砷浸出过程的表观活化能为4.9 kJ/mol,说明砷浸出过程受已反应固体层内的扩散过程控制。自由基猝灭实验表明,硫酸根自由基和羟基自由基是紫外线照射下草酸强化煤砷浸出体系中的主要自由基。ATR-FTIR与XPS表征结果进一步证实了浸出液的测定结果。
中图分类号:
李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532.
Jintong LI, Shun QIU, Wenshou SUN. Oxalic acid and UV enhanced arsenic leaching from coal in flue gas desulfurization by coal slurry[J]. CIESC Journal, 2023, 74(8): 3522-3532.

图1 实验流程图1—气体钢瓶;2—开关阀;3—减压阀;4—稳压阀;5—转子流量计;6—气体混合瓶;7—皂膜流量计;8—斜管压差计;9—搅拌桨;10—紫外灯;11—温度计;12—反应器;13—加热套;14—冷却器;15—电子皂膜流量计;16—尾气吸收装置
Fig.1 Schematic diagram of the experimental apparatus1—gas cylinder; 2—switch valve; 3—pressure reducing valve; 4—pressure maintaining valve; 5—rotor flow meter; 6—gas mixing bottle; 7—soap film flow meter; 8—inclined tube manometer; 9—agitator; 10—ultraviolet lamp; 11—thermometer; 12—reactor; 13—heating jacket; 14—water cooler; 15—electronic soap film flowmeter; 16—absorption vessel
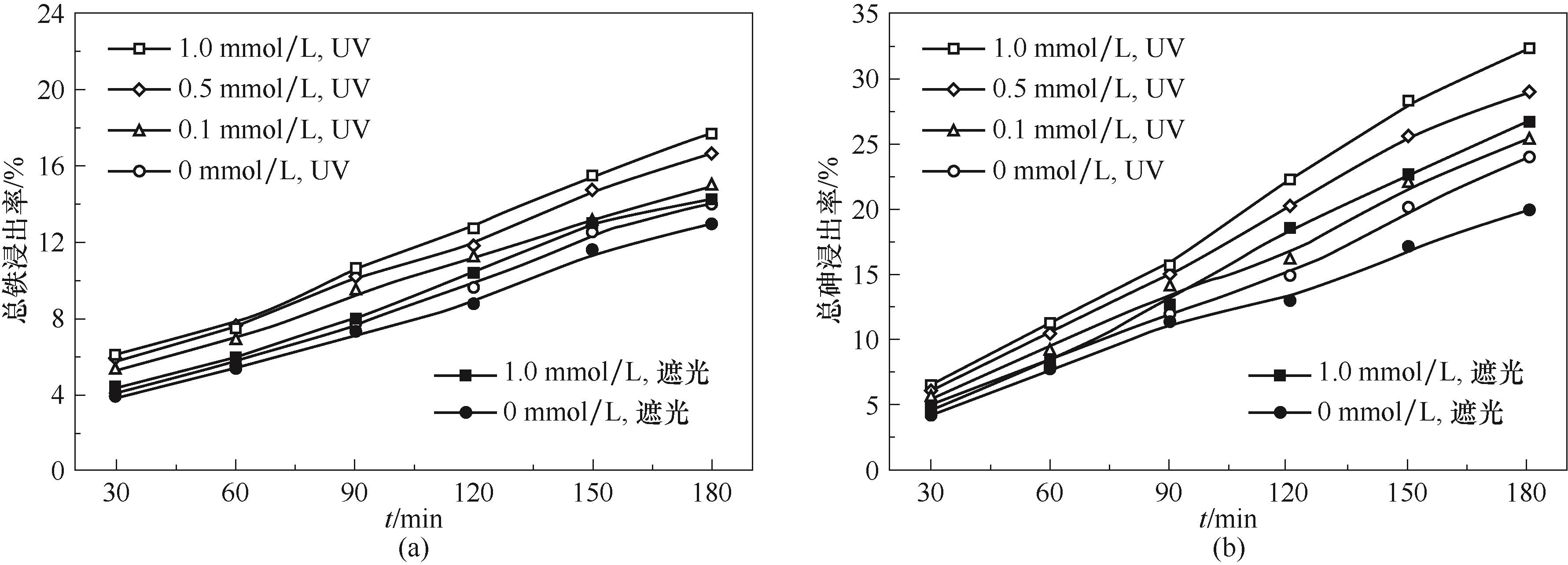
图2 紫外线照射和草酸浓度对煤中铁浸出率的影响(a)和对砷浸出率的影响(b) (pH=2.5,50℃)
Fig.2 Effect of UV irradiation and oxalic acid concentration on extraction ratio of iron (a) and on extraction ratio of arsenic from coal (b)

图3 紫外线照射及草酸浓度对As(Ⅲ)/As(total)的影响(a)和对烟气脱硫率的影响(b) (pH=2.5,50℃)
Fig.3 Effects of UV irradiation and oxalic acid concentration on As(Ⅲ)/As(total) ratio (a) and on flue gas desulfurization efficiency (b)
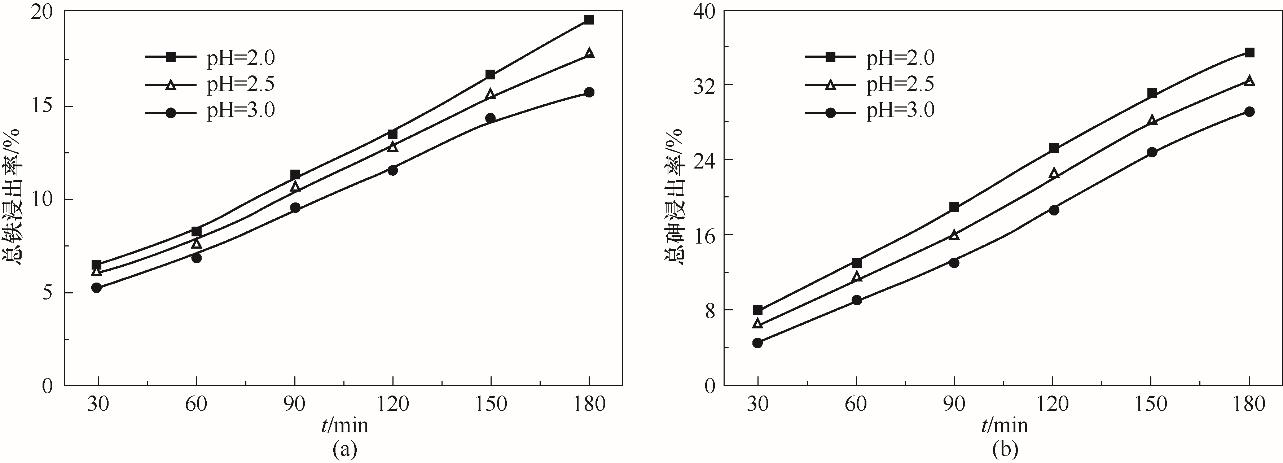
图4 pH对煤中铁浸出率的影响(a)和对砷浸出率的影响(b) (UV,1.0 mmol/L草酸,50℃)
Fig.4 Effect of pH on extraction ratio of iron (a) and on extraction ratio of arsenic from coal (b)
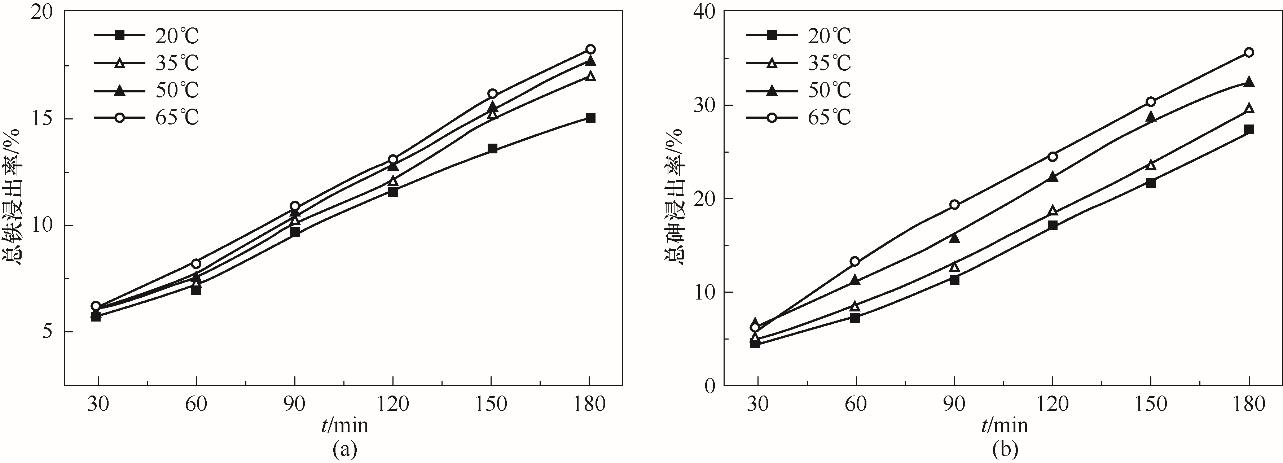
图6 温度对煤中铁浸出率的影响(a)和对砷浸出率的影响(b) (UV,1.0 mmol/L草酸,pH=2.5)
Fig.6 Effect of temperature on extraction ratio of iron (a) and on extraction ratio of arsenic from coal (b)

图7 紫外线照射下1.0 mmol/L草酸强化过程中1-(1-F2)1/3随时间t的变化(a)和1-3(1-F2)2/3+2(1-F2)随时间t的变化(b)
Fig.7 Variation of 1-(1-F2)1/3 with time (a) and variation of 1-3(1-F2)2/3+2(1-F2)with time (b) during 1.0 mmol/L oxalic acid enhanced process under UV irradiation
| 煤样 | 组分 | 结合能/ eV | 半峰宽/ eV | 积分面积 | 面积 占比/% |
|---|---|---|---|---|---|
| 原煤样 | 黄铁矿铁 | 707.96 | 2.00 | 1016.03 | 34.16 |
| Fe2+ | 710.70 | 2.00 | 1269.06 | 42.66 | |
| Fe3+ | 712.78 | 2.00 | 689.56 | 23.18 | |
| 浸出后煤样 | 黄铁矿铁 | 707.01 | 2.00 | 219.83 | 20.70 |
| Fe2+ | 710.82 | 2.00 | 446.60 | 42.06 | |
| Fe3+ | 713.07 | 2.00 | 395.30 | 37.24 | |
| 原煤样 | As(Ⅲ) | 44.04 | 1.00 | 87.44 | 70.94 |
| As(Ⅴ) | 45.63 | 1.00 | 35.82 | 29.06 | |
| 浸出后煤样 | As(Ⅲ) | 44.21 | 1.00 | 17.57 | 26.37 |
| As(Ⅴ) | 45.72 | 1.00 | 49.07 | 73.63 | |
| 原煤样 | 黄铁矿硫 | 162.97 | 1.70 | 309.19 | 30.59 |
| 有机硫 | 165.79 | 1.70 | 149.74 | 14.82 | |
| 硫酸盐硫 | 168.29 | 1.70 | 551.68 | 54.59 | |
| 浸出后煤样 | 黄铁矿硫 | 162.22 | 1.70 | 57.85 | 7.89 |
| 有机硫 | 164.80 | 1.70 | 62.66 | 8.54 | |
| 硫酸盐硫 | 168.73 | 1.70 | 612.92 | 83.57 |
表1 原煤样与浸出后煤样的XPS谱图参数
Table 1 XPS parameters of raw coal and coal residue
| 煤样 | 组分 | 结合能/ eV | 半峰宽/ eV | 积分面积 | 面积 占比/% |
|---|---|---|---|---|---|
| 原煤样 | 黄铁矿铁 | 707.96 | 2.00 | 1016.03 | 34.16 |
| Fe2+ | 710.70 | 2.00 | 1269.06 | 42.66 | |
| Fe3+ | 712.78 | 2.00 | 689.56 | 23.18 | |
| 浸出后煤样 | 黄铁矿铁 | 707.01 | 2.00 | 219.83 | 20.70 |
| Fe2+ | 710.82 | 2.00 | 446.60 | 42.06 | |
| Fe3+ | 713.07 | 2.00 | 395.30 | 37.24 | |
| 原煤样 | As(Ⅲ) | 44.04 | 1.00 | 87.44 | 70.94 |
| As(Ⅴ) | 45.63 | 1.00 | 35.82 | 29.06 | |
| 浸出后煤样 | As(Ⅲ) | 44.21 | 1.00 | 17.57 | 26.37 |
| As(Ⅴ) | 45.72 | 1.00 | 49.07 | 73.63 | |
| 原煤样 | 黄铁矿硫 | 162.97 | 1.70 | 309.19 | 30.59 |
| 有机硫 | 165.79 | 1.70 | 149.74 | 14.82 | |
| 硫酸盐硫 | 168.29 | 1.70 | 551.68 | 54.59 | |
| 浸出后煤样 | 黄铁矿硫 | 162.22 | 1.70 | 57.85 | 7.89 |
| 有机硫 | 164.80 | 1.70 | 62.66 | 8.54 | |
| 硫酸盐硫 | 168.73 | 1.70 | 612.92 | 83.57 |
| 1 | Wang C B, Liu H M, Zhang Y, et al. Review of arsenic behavior during coal combustion: volatilization, transformation, emission and removal technologies[J]. Progress in Energy and Combustion Science, 2018, 68: 1-28. |
| 2 | Liu H M, Wang C B, Sun X, et al. Volatilization of arsenic in coal during isothermal oxy-fuel combustion[J]. Energy & Fuels, 2016, 30(4): 3479-3487. |
| 3 | López-Antón M A, Díaz-Somoano M, Fierro J L G, et al. Retention of arsenic and selenium compounds present in coal combustion and gasification flue gases using activated carbons[J]. Fuel Processing Technology, 2007, 88(8): 799-805. |
| 4 | Zhang Y, Wang C B, Li W H, et al. Removal of gas-phase As2O3 by metal oxide adsorbents: effects of experimental conditions and evaluation of adsorption mechanism[J]. Energy & Fuels, 2015, 29(10): 6578-6585. |
| 5 | Kang Y, Liu G J, Chou C L, et al. Arsenic in Chinese coals: distribution, modes of occurrence, and environmental effects[J]. Science of the Total Environment, 2011, 412/413: 1-13. |
| 6 | Sundaram H P, Cho E H, Miller A. SO2 removal by leaching coal pyrite[J]. Energy & Fuels, 2001, 15(2): 470-476. |
| 7 | Black A, Craw D. Arsenic, copper and zinc occurrence at the Wangaloa coal mine, southeast Otago, New Zealand[J]. International Journal of Coal Geology, 2001, 45(2/3): 181-193. |
| 8 | Sun W S, Liu J C, Wang L C, et al. Aluminum-enhanced coal pyrite leaching during SO2 removal with coal slurry[J]. Water, Air, & Soil Pollution, 2016, 227(7): 221. |
| 9 | Moses C O, Kirk Nordstrom D, Herman J S, et al. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron[J]. Geochimica et Cosmochimica Acta, 1987, 51(6): 1561-1571. |
| 10 | 鲁真真, 孙文寿, 郭远峰, 等. 氧气摩尔分数和紫外光照射对烟气浸出煤中砷的影响[J]. 高校化学工程学报, 2019, 33(4): 989-997. |
| Lu Z Z, Sun W S, Guo Y F, et al. Effects of O2 mole fraction and ultraviolet irradiation on arsenic leaching from coal with flue gas[J]. Journal of Chemical Engineering of Chinese Universities, 2019, 33(4): 989-997. | |
| 11 | Wang W, Qu Y P, Yang B, et al. Lactate oxidation in pyrite suspension: a Fenton-like process in situ generating H2O2 [J]. Chemosphere, 2012, 86(4): 376-382. |
| 12 | Schoonen M A A, Harrington A D, Laffers R, et al. Role of hydrogen peroxide and hydroxyl radical in pyrite oxidation by molecular oxygen[J]. Geochimica et Cosmochimica Acta, 2010, 74(17): 4971-4987. |
| 13 | Santana-Casiano J M, González-Dávila M, Millero F J. The role of Fe(Ⅱ) species on the oxidation of Fe(Ⅱ) in natural waters in the presence of O2 and H2O2 [J]. Marine Chemistry, 2006, 99(1/2/3/4): 70-82. |
| 14 | Cohn C A, Mueller S, Wimmer E, et al. Pyrite-induced hydroxyl radical formation and its effect on nucleic acids[J]. Geochemical Transactions, 2006, 7: 3. |
| 15 | Rimstidt J D, Vaughan D J. Pyrite oxidation: a state-of-the-art assessment of the reaction mechanism[J]. Geochimica et Cosmochimica Acta, 2003, 67(5): 873-880. |
| 16 | Zhang Y, Zhou J T, Li C Y, et al. Reaction kinetics and mechanism of iron(Ⅱ)-induced catalytic oxidation of sulfur(Ⅳ) during wet desulfurization[J]. Industrial & Engineering Chemistry Research, 2012, 51(3): 1158-1165. |
| 17 | Emett M T, Khoe G H. Photochemical oxidation of arsenic by oxygen and iron in acidic solutions[J]. Water Research, 2001, 35(3): 649-656. |
| 18 | Zhou L, Zheng W, Ji Y F, et al. Ferrous-activated persulfate oxidation of arsenic(Ⅲ) and diuron in aquatic system[J]. Journal of Hazardous Materials, 2013, 263: 422-430. |
| 19 | Ren H T, Ji Z Y, Wu S H, et al. Photoreductive dissolution of schwertmannite induced by oxalate and the mobilization of adsorbed As(V)[J]. Chemosphere, 2018, 208: 294-302. |
| 20 | Sundman A, Karlsson T, Sjöberg S, et al. Complexation and precipitation reactions in the ternary As(V)-Fe(Ⅲ)-OM (organic matter) system[J]. Geochimica et Cosmochimica Acta, 2014, 145: 297-314. |
| 21 | Mangiante D M, Schaller R D, Zarzycki P, et al. Mechanism of ferric oxalate photolysis[J]. ACS Earth and Space Chemistry, 2017, 1(5): 270-276. |
| 22 | Xu T Y, Zhu R L, Shang H, et al. Photochemical behavior of ferrihydrite-oxalate system: interfacial reaction mechanism and charge transfer process[J]. Water Research, 2019, 159: 10-19. |
| 23 | Sun J, Bostick B C, Mailloux B J, et al. Effect of oxalic acid treatment on sediment arsenic concentrations and lability under reducing conditions[J]. Journal of Hazardous Materials, 2016, 311: 125-133. |
| 24 | 王明仕, 杨娜娜, 钦凡, 等. 贵州省某村煤中砷含量及赋存状态[J]. 煤炭转化, 2010, 33(4): 1-4. |
| Wang M S, Yang N N, Qin F, et al. Content and occurrence of arsenic in coal of a village in Guizhou Province[J]. Coal Conversion, 2010, 33(4): 1-4. | |
| 25 | 刘恩栋, 杨宁光. 一种新的方便的测定天然水中三价砷的方法[J]. 交通环保, 1998(6): 21-22. |
| Liu E D, Yang N G. A new and convenient method for the determination of trivalent arsenic in natural water[J]. Environmental Protection in Transportation, 1998(6): 21-22. | |
| 26 | Nogueira A A, Souza B M, Dezotti M W C, et al. Ferrioxalate complexes as strategy to drive a photo-FENTON reaction at mild pH conditions: a case study on levofloxacin oxidation[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2017, 345: 109-123. |
| 27 | Dong H Y, Wei G F, Yin D Q, et al. Mechanistic insight into the generation of reactive oxygen species in sulfite activation with Fe(Ⅲ) for contaminants degradation[J]. Journal of Hazardous Materials, 2020, 384: 121497. |
| 28 | Romero A, Santos A, Vicente F, et al. Diuron abatement using activated persulphate: effect of pH, Fe(Ⅱ) and oxidant dosage[J]. Chemical Engineering Journal, 2010, 162(1): 257-265. |
| 29 | 韩东晖, 李瑛, 李开明, 等. UV强化草酸络合Fe3+活化过硫酸盐氧化降解苯胺[J]. 环境科学, 2018, 39(9): 4257-4264. |
| Han D H, Li Y, Li K M, et al. Enhanced degradation of aniline by PS oxidation in the presence of UV and ferric oxalate[J]. Environmental Science, 2018, 39(9): 4257-4264. | |
| 30 | Hislop K A, Bolton J R. The photochemical generation of hydroxyl radicals in the UV-vis/ferrioxalate/H2O2 system[J]. Environmental Science & Technology, 1999, 33(18): 3119-3126. |
| 31 | 梁柱, 罗平, 殷井云, 等. UV-vis/H2O2/草酸铁络合物法调理对污泥脱水性能的影响[J]. 绿色科技, 2017(6): 1-4. |
| Liang Z, Luo P, Yin J Y, et al. Effect of UV-vis/H2O2/ferrioxalate complex method on the dewatering performance of sludge[J]. Journal of Green Science and Technology, 2017(6): 1-4. | |
| 32 | Neppolian B, Celik E, Choi H. Photochemical oxidation of a r s e n i c ( Ⅲ ) to arsenic(V) using peroxydisulfate ions as an oxidizing agent[J]. Environmental Science & Technology, 2008, 42(16): 6179-6184. |
| 33 | 廖斌,衷水平.砷钙渣稳定化技术研究[J]. 矿产保护与利用, 2013(3): 51-54. |
| Liao B, Zhong S P. Research on calcium arsenate residue stabilization technology[J]. Conservation and Utilization of Mineral Resources, 2013(3): 51-54. | |
| 34 | Kuo D T F, Kirk D W, Jia C Q. The chemistry of aqueous S(Ⅳ)-Fe-O2 system: state of the art[J]. Journal of Sulfur Chemistry, 2006, 27(5): 461-530. |
| 35 | 易平贵, 俞庆森, 宗汉兴. 黄铁矿化学脱硫的热力学分析[J]. 煤炭转化, 1999, 22(1): 47-52. |
| Yi P G, Yu Q S, Zong H X. Thermodynamic analysis for chemical desulfurization of pyrite in coal[J]. Coal Conversion, 1999, 22(1): 47-52. | |
| 36 | Daggupati V N, Naterer G F, Gabriel K S. Diffusion of gaseous products through a particle surface layer in a fluidized bed reactor[J]. International Journal of Heat and Mass Transfer, 2010, 53(11/12): 2449-2458. |
| 37 | Wang H H, Li G Q, Zhao D, et al. Dephosphorization of high phosphorus oolitic hematite by acid leaching and the leaching kinetics[J]. Hydrometallurgy, 2017, 171: 61-68. |
| 38 | Ashraf M, Zafar Z I, Ansari T M. Selective leaching kinetics and upgrading of low-grade calcareous phosphate rock in succinic acid[J]. Hydrometallurgy, 2005, 80(4): 286-292. |
| 39 | Mendive C B, Bredow T, Blesa M A, et al. ATR-FTIR measurements and quantum chemical calculations concerning the adsorption and photoreaction of oxalic acid on TiO2 [J]. Physical Chemistry Chemical Physics, 2006, 8(27): 3232-3247. |
| 40 | Kim E J, Batchelor B. Macroscopic and X-ray photoelectron spectroscopic investigation of interactions of arsenic with synthesized pyrite[J]. Environmental Science & Technology, 2009, 43(8): 2899-2904. |
| 41 | Zhang X S, Song X D, Wang J F, et al. CO2 gasification of Yangchangwan coal catalyzed by iron-based waste catalyst from indirect coal-liquefaction plant[J]. Fuel, 2021, 285: 119228. |
| 42 | Yamashita T, Hayes P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials[J]. Applied Surface Science, 2008, 254(8): 2441-2449. |
| 43 | Wang X Q, Wang B, Chen Y F, et al. Fe2P nanoparticles embedded on Ni2P nanosheets as highly efficient and stable bifunctional electrocatalysts for water splitting[J]. Journal of Materials Science & Technology, 2022, 105: 266-273. |
| 44 | Zoroufchi Benis K, Soltan J, McPhedran K N. Electrochemically modified adsorbents for treatment of aqueous arsenic: pore diffusion in modified biomass vs. biochar[J]. Chemical Engineering Journal, 2021, 423: 130061. |
| 45 | Zhang B, Yan G H, Zhao Y M, et al. Coal pyrite microwave magnetic strengthening and electromagnetic response in magnetic separation desulfurization process[J]. International Journal of Mineral Processing, 2017, 168: 136-142. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [3] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [4] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [5] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [6] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [7] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [8] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [11] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [12] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [13] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [14] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [15] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号