化工学报 ›› 2022, Vol. 73 ›› Issue (4): 1436-1454.DOI: 10.11949/0438-1157.20211571
收稿日期:2021-11-05
修回日期:2022-02-18
出版日期:2022-04-05
发布日期:2022-04-25
通讯作者:
薛文东
作者简介:胡华坤(1997—),男,硕士研究生,基金资助:
Huakun HU( ),Wendong XUE(
),Wendong XUE( ),Sida HUO,Yong LI,Peng JIANG
),Sida HUO,Yong LI,Peng JIANG
Received:2021-11-05
Revised:2022-02-18
Online:2022-04-05
Published:2022-04-25
Contact:
Wendong XUE
摘要:
稳定的固体电解质界面(SEI)是提高锂离子电池电化学性能的关键,用电解液添加剂是改善锂离子电池性能最经济有效的方法之一。本文综述了近五年间包括不饱和酯化合物、含硫化合物、锂盐、无机化合物等作为电解液成膜添加剂在锂离子电池中的研究进展和作用机理,对它们的优缺点进行了评价,最后进行了总结和展望。未来成膜类添加剂的研究思路应该为:(1)应以有机物种为主,能够形成弹性模量小的SEI膜,便于适应阳极材料产生的膨胀行为。(2)添加剂要尽量保证形成的SEI膜与石墨等阳极材料产生良好的黏结,因此添加剂形成的聚合物的聚合度不能太小。(3)在没有性能极其优秀的成膜添加剂出现之前,添加剂的分子结构可以在现有的添加剂的基础上进行结构的优化或者官能团的设计。(4)重点攻关当前添加剂的应用的问题,提高添加剂的合成技术,降低合成成本。
中图分类号:
胡华坤, 薛文东, 霍思达, 李勇, 蒋朋. 锂离子电池电解液SEI成膜添加剂的研究进展[J]. 化工学报, 2022, 73(4): 1436-1454.
Huakun HU, Wendong XUE, Sida HUO, Yong LI, Peng JIANG. Review of SEI film forming additives for electrolyte of lithium ion battery[J]. CIESC Journal, 2022, 73(4): 1436-1454.
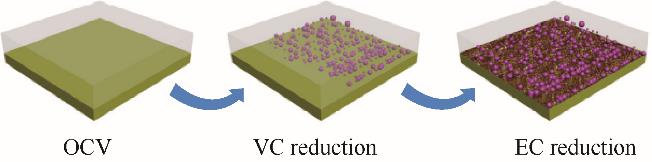
图2 在含有1%VC的1.0 mol/L LiPF6/EC/DMC中,HOPG电极上不同表面演化阶段的示意图[29]
Fig.2 The schematic of different surface evolution stage on HOPG electrode in 1.0 mol/L LiPF6/EC/DMC with 1% VC[29]

图3 向电解液中添加VC时表面保护层形成过程的示意图[34]
Fig.3 Schematic illustrations of the postulated formation process of surface protective layer when VC was added to the electrolyte[34]
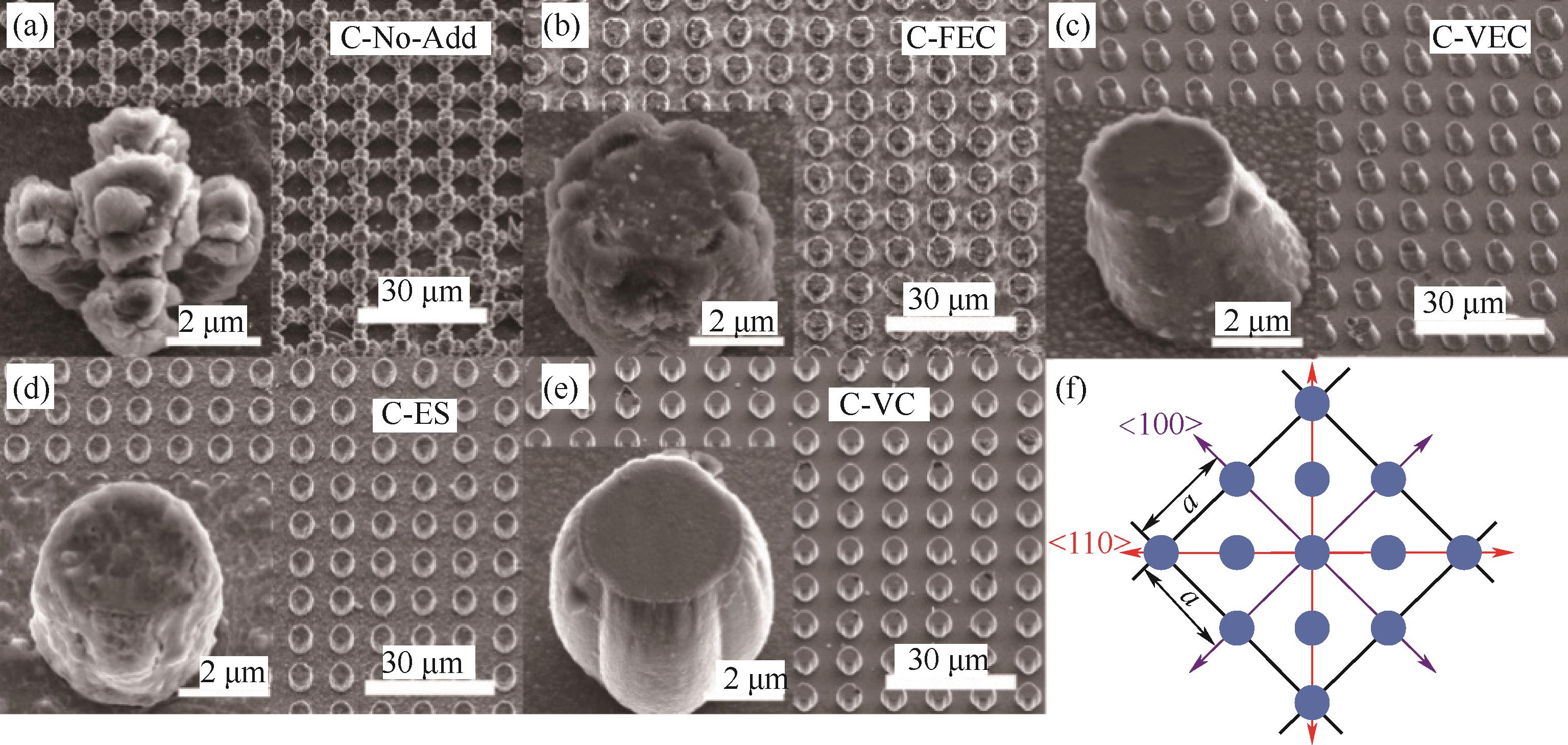
图6 五种电解液中10次充放电循环后硅微柱阵列电极的SEM图像[(a)~(e)]; 晶体Si〈100〉晶面原子分布示意图(f) [63]
Fig.6 SEM images of Si micropillar array electrodes after 10 charge/discharge cycles in five electrolytes[(a)~(e)]; Schematic diagram of the atomic distribution of crystal Si〈100〉 crystal plane (f) [63]
| 参数 | DTD | PS | SPA | PES | EC |
|---|---|---|---|---|---|
| dipole/D(1D = 3.33563 ×10-30 C·m) | 7.99 | 8.45 | 7.40 | 8.64 | 7.33 |
| HOMO/eV | -10.77 | -10.33 | -10.67 | -9.89 | -10.62 |
| LOMO/eV | 0.04 | 0.03 | -0.10 | -0.27 | 0.06 |
| IP/eV | 9.6 | 9.02 | 9.21 | 8.78 | 9.55 |
| EA/eV | 0.66 | 0.69 | 1.08 | 1.45 | 0.65 |
| η/eV | 4.48 | 4.16 | 4.06 | 3.67 | 4.36 |
| ΔG(Li+)(neutral)/(kJ/mol) | 3.76 | 0.85 | 3.70 | 1.20 | 0.45 |
| ΔG(Li+)(anion)/(kJ/mol) | -19.56 | -15.72 | -24.73 | -18.42 | -41.54 |
表1 偶极矩、HOMO和LUMO能级能量、电离势(IP)、电子亲和性(EA)、化学硬度(η)以及与锂离子相互作用的Gibbs自由能(ΔG)[95]
Table 1 Dipole moments, HOMO and LUMO level energies, ionization potentials (IP), electron affinities (EA), chemical hardnesses (η), and Gibbs free energies of interaction with lithium cation (ΔG)[95]
| 参数 | DTD | PS | SPA | PES | EC |
|---|---|---|---|---|---|
| dipole/D(1D = 3.33563 ×10-30 C·m) | 7.99 | 8.45 | 7.40 | 8.64 | 7.33 |
| HOMO/eV | -10.77 | -10.33 | -10.67 | -9.89 | -10.62 |
| LOMO/eV | 0.04 | 0.03 | -0.10 | -0.27 | 0.06 |
| IP/eV | 9.6 | 9.02 | 9.21 | 8.78 | 9.55 |
| EA/eV | 0.66 | 0.69 | 1.08 | 1.45 | 0.65 |
| η/eV | 4.48 | 4.16 | 4.06 | 3.67 | 4.36 |
| ΔG(Li+)(neutral)/(kJ/mol) | 3.76 | 0.85 | 3.70 | 1.20 | 0.45 |
| ΔG(Li+)(anion)/(kJ/mol) | -19.56 | -15.72 | -24.73 | -18.42 | -41.54 |
| Sample | After the 1st cycle | After the 50th cycle | After the 150th cycle | |||
|---|---|---|---|---|---|---|
| rSEI/Ω | rct/Ω | rSEI/Ω | rct/Ω | rSEI/Ω | rct/Ω | |
| no additive | 14.4 | 8.6 | 1.7 | 30.2 | 4.6 | 162.5 |
| 1% LiPO2F2 | 10.3 | 5.7 | 1.9 | 3.1 | 2.8 | 3.8 |
表2 无添加剂和1.0%LiPO2F2样品的EIS拟合结果[118]
Table 2 EIS fitting results of samples with no additive and 1.0% LiPO2F2[118]
| Sample | After the 1st cycle | After the 50th cycle | After the 150th cycle | |||
|---|---|---|---|---|---|---|
| rSEI/Ω | rct/Ω | rSEI/Ω | rct/Ω | rSEI/Ω | rct/Ω | |
| no additive | 14.4 | 8.6 | 1.7 | 30.2 | 4.6 | 162.5 |
| 1% LiPO2F2 | 10.3 | 5.7 | 1.9 | 3.1 | 2.8 | 3.8 |
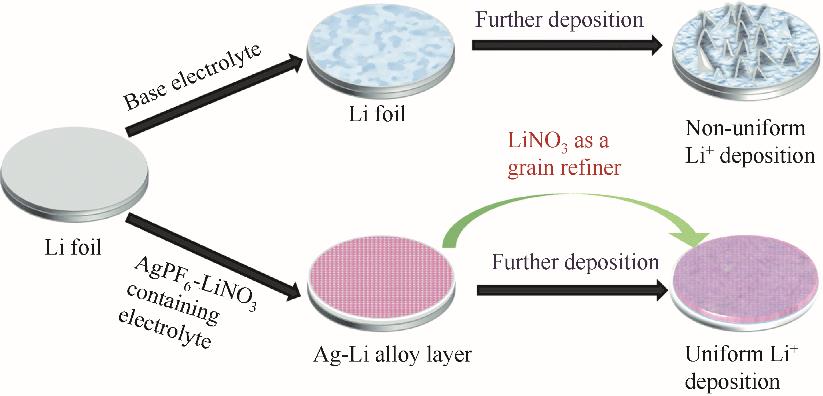
图14 裸锂箔和均匀Ag-Li合金层上Li形核和生长过程的示意图[135]
Fig.14 Schematic diagrams of the Li nucleation and growth process on the bare Li foil and uniform Ag-Li alloy layer[135]
| 118 | Zhao W M, Zheng G R, Lin M, et al. Toward a stable solid-electrolyte-interfaces on nickel-rich cathodes: LiPO2F2 salt-type additive and its working mechanism for LiNi0.5Mn0.25Co0.25O2 cathodes[J]. Journal of Power Sources, 2018, 380: 149-157. |
| 119 | Liu B, Zhou H M, Yin C J, et al. Enhanced electrochemical performance of LiNi0.5Mn1.5O4 cathode by application of LiPF2O2 for lithium difluoro(oxalate)borate electrolyte[J]. Electrochimica Acta, 2019, 321: 134690. |
| 120 | Yang B W, Zhang H, Yu L, et al. Lithium difluorophosphate as an additive to improve the low temperature performance of LiNi0.5Co0.2Mn0.3O2/graphite cells[J]. Electrochimica Acta, 2016, 221: 107-114. |
| 121 | Chen H X, Liu B, Wang Y, et al. Insight into wide temperature electrolyte based on lithiumdifluoro(oxalate)borate for high voltage lithium-ion batteries[J]. Journal of Alloys and Compounds, 2021, 876: 159966. |
| 122 | Qin Y, Chen Z H, Liu J, et al. Lithium tetrafluoro oxalato phosphate as electrolyte additive for lithium-ion cells[J]. Electrochemical and Solid-State Letters, 2010, 13(2): A11. |
| 123 | Song G, Yi Z L, Su F Y, et al. New insights into the mechanism of LiDFBOP for improving the low-temperature performance via the rational design of an interphase on a graphite anode[J]. ACS Applied Materials & Interfaces, 2021, 13(33): 40042-40052. |
| 124 | Wang J, Zhao D N, Cong Y Y, et al. Analyzing the mechanism of functional groups in phosphate additives on the interface of LiNi0.8Co0.15Al0.05O2 cathode materials[J]. ACS Applied Materials & Interfaces, 2021, 13(14): 16939-16951. |
| 125 | Wang S C, Hu H P, Yu P F, et al. Effect of electrolyte additives on high-temperature cycling performance of spinel LiMn2O4 cathode[J]. Journal of Applied Electrochemistry, 2018, 48(11): 1221-1230. |
| 126 | Sharova V, Moretti A, Diemant T, et al. Comparative study of imide-based Li salts as electrolyte additives for Li-ion batteries[J]. Journal of Power Sources, 2018, 375: 43-52. |
| 127 | Aurbach D, Ein-Eli Y, Chusid O, et al. The correlation between the surface chemistry and the performance of Li-carbon intercalation anodes for rechargeable ‘rocking-chair’ type batteries[J]. Journal of the Electrochemical Society, 1994, 141(3): 603-611. |
| 128 | Krause L J, Chevrier V L, Jensen L D, et al. The effect of carbon dioxide on the cycle life and electrolyte stability of Li-ion full cells containing silicon alloy[J]. Journal of the Electrochemical Society, 2017, 164(12): A2527-A2533. |
| 129 | Schwenke K U, Solchenbach S, Demeaux J, et al. The impact of CO2 evolved from VC and FEC during formation of graphite anodes in lithium-ion batteries[J]. Journal of the Electrochemical Society, 2019, 166(10): A2035-A2047. |
| 1 | Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries[J]. Chemical Reviews, 2004, 104(10): 4303-4417. |
| 2 | Xu K. Electrolytes and interphases in Li-ion batteries and beyond[J]. Chemical Reviews, 2014, 114(23): 11503-11618. |
| 3 | Goodenough J B, Kim Y. Challenges for rechargeable Li batteries[J]. Chemistry of Materials, 2010, 22(3): 587-603. |
| 4 | Zhu J E, Wierzbicki T, Li W. A review of safety-focused mechanical modeling of commercial lithium-ion batteries[J]. Journal of Power Sources, 2018, 378: 153-168. |
| 5 | Zheng Y J, Ouyang M G, Han X B, et al. Investigating the error sources of the online state of charge estimation methods for lithium-ion batteries in electric vehicles[J]. Journal of Power Sources, 2018, 377: 161-188. |
| 6 | IEA.Global EV Outlook 2021[EB/OL]. [2012-04]. . |
| 7 | Zhang S S. A review on electrolyte additives for lithium-ion batteries[J]. Journal of Power Sources, 2006, 162(2): 1379-1394. |
| 8 | Chen Z H, Amine K. Bifunctional electrolyte additive for lithium-ion batteries[J]. Electrochemistry Communications, 2007, 9(4): 703-707. |
| 9 | 张文林, 霍宇, 李功伟, 等. 离子液体作为电解液添加剂用于高压锂离子电池[J]. 化工学报, 2019, 70(6): 2334-2342. |
| Zhang W L, Huo Y, Li G W, et al. Ionic liquids as electrolyte additives for high-voltage lithium-ion batteries[J]. CIESC Journal, 2019, 70(6): 2334-2342. | |
| 10 | Peled E, Golodnitsky D, Menachem C, et al. An advanced tool for the selection of electrolyte components for rechargeable lithium batteries[J]. Journal of the Electrochemical Society, 1998, 145(10): 3482-3486. |
| 11 | Tiedemann W H, Bennion D N. Chemical and electrochemical behavior of lithium electrodes in dimethyl sulfite, electrolytic solutions[J]. Journal of the Electrochemical Society, 1973, 120(12): 1624. |
| 12 | Peled E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—the solid electrolyte interphase model[J]. Journal of the Electrochemical Society, 1979, 126(12): 2047-2051. |
| 13 | Fong R, von Sacken U, Dahn J R. Studies of lithium intercalation into carbons using nonaqueous electrochemical cells[J]. Journal of the Electrochemical Society, 1990, 137(7): 2009-2013. |
| 14 | Hou C, Han J H, Liu P, et al. Operando observations of SEI film evolution by mass-sensitive scanning transmission electron microscopy[J]. Advanced Energy Materials, 2019, 9(45): 1902675. |
| 15 | Shinagawa C, Ushiyama H, Yamashita K. Multiscale simulations for lithium-ion batteries: SEI film growth and capacity fading[J]. Journal of the Electrochemical Society, 2017, 164(13): A3018-A3024. |
| 16 | Agubra V A, Fergus J W. The formation and stability of the solid electrolyte interface on the graphite anode[J]. Journal of Power Sources, 2014, 268: 153-162. |
| 17 | He J, Wang H P, Zhou Q, et al. Unveiling the role of Li+ solvation structures with commercial carbonates in the formation of solid electrolyte interphase for lithium metal batteries[J]. Small Methods, 2021, 5(8): e2100441. |
| 18 | Aurbach D, Zaban A, Gofer Y, et al. Recent studies of the lithium-liquid electrolyte interface electrochemical, morphological and spectral studies of a few important systems[J]. Journal of Power Sources, 1995, 54(1): 76-84. |
| 19 | Wang A, Kadam S, Li H, et al. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries[J]. npj Computational Materials, 2018, 4: 15. |
| 20 | Malmgren S, Ciosek K, Lindblad R, et al. Consequences of air exposure on the lithiated graphite SEI[J]. Electrochimica Acta, 2013, 105: 83-91. |
| 21 | Gauthier M, Carney T J, Grimaud A, et al. Electrode-electrolyte interface in Li-ion batteries: current understanding and new insights[J]. The Journal of Physical Chemistry Letters, 2015, 6(22): 4653-4672. |
| 22 | Cheng X B, Zhang R, Zhao C Z, et al. A review of solid electrolyte interphases on lithium metal anode[J]. Advanced Science, 2015, 3(3): 1500213. |
| 23 | An S J, Li J L, Daniel C, et al. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling[J]. Carbon, 2016, 105: 52-76. |
| 24 | Zhang Q L, Pan J, Lu P, et al. Synergetic effects of inorganic components in solid electrolyte interphase on high cycle efficiency of lithium ion batteries[J]. Nano Letters, 2016, 16(3): 2011-2016. |
| 25 | Borodin O, Ren X M, Vatamanu J, et al. Modeling insight into battery electrolyte electrochemical stability and interfacial structure[J]. Accounts of Chemical Research, 2017, 50(12): 2886-2894. |
| 26 | Chu Y L, Shen Y B, Guo F, et al. Advanced characterizations of solid electrolyte interphases in lithium-ion batteries[J]. Electrochemical Energy Reviews, 2020, 3(1): 187-219. |
| 27 | Chung G C, Kim H J, Yu S I, et al. Origin of graphite exfoliation an investigation of the important role of solvent cointercalation[J]. Journal of the Electrochemical Society, 2000, 147(12): 4391. |
| 28 | Ota H, Sakata Y, Inoue A, et al. Analysis of vinylene carbonate derived SEI layers on graphite anode[J]. Journal of the Electrochemical Society, 2004, 151(10): A1659-A1669. |
| 29 | Lin L P, Yang K, Chen H B, et al. In situ atomic force microscope observing the effect of vinylene carbonate on the formation of solid-electrolyte interphase layer during the initial cycle[J]. Functional Materials Letters, 2017, 10(5): 1750052. |
| 30 | Guo Q P, Han Y, Wang H, et al. Design of ionic liquid-based hybrid electrolytes with additive for lithium insertion in graphite effectively and their effects on interfacial properties[J]. Ionics, 2018, 24(9): 2601-2609. |
| 31 | Petibon R, Henry E C, Burns J C, et al. Comparative study of vinyl ethylene carbonate (VEC) and vinylene carbonate (VC) in LiCoO2/graphite pouch cells using high precision coulometry and electrochemical impedance spectroscopy measurements on symmetric cells[J]. Journal of the Electrochemical Society, 2013, 161(1): A66-A74. |
| 130 | Yu H, Obrovac M N. Quantitative determination of carbon dioxide content in organic electrolytes by infrared spectroscopy[J]. Journal of the Electrochemical Society, 2019, 166(12): A2467-A2470. |
| 131 | Ein-Eli Y, Thomas S R, Koch V R. The role of SO2 as an additive to organic Li-ion battery electrolytes[J]. Journal of the Electrochemical Society, 1997, 144(4): 1159-1165. |
| 32 | Haruna H, Takahashi S, Tanaka Y. Accurate consumption analysis of vinylene carbonate as an electrolyte additive in an 18650 lithium-ion battery at the first charge-discharge cycle[J]. Journal of the Electrochemical Society, 2016, 164(1): A6278-A6280. |
| 33 | Pritzl D, Solchenbach S, Wetjen M, et al. Analysis of vinylene carbonate (VC) as additive in graphite/LiNi0.5Mn1.5O4 cells[J]. Journal of the Electrochemical Society, 2017, 164(12): A2625-A2635. |
| 34 | Lee W J, Prasanna K, Jo Y N, et al. Depth profile studies on nickel rich cathode material surfaces after cycling with an electrolyte containing vinylene carbonate at elevated temperature[J]. Physical Chemistry Chemical Physics: PCCP, 2014, 16(32): 17062-17071. |
| 35 | Deshpande R D, Ridgway P, Fu Y B, et al. The limited effect of VC in graphite/NMC cells[J]. Journal of the Electrochemical Society, 2014, 162(3): A330-A338. |
| 36 | Brown Z L, Jurng S, Lucht B L. Investigation of the lithium solid electrolyte interphase in vinylene carbonate electrolytes using Cu||LiFePO4 cells[J]. Journal of the Electrochemical Society, 2017, 164(9): A2186-A2189. |
| 37 | Lim T W, Park C W, White S R, et al. Time release of encapsulated additives for enhanced performance of lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(46): 40244-40251. |
| 38 | 邓邦为, 孙大明, 万琦, 等. 锂离子电池三元正极材料电解液添加剂的研究进展[J]. 化学学报, 2018, 76(4): 30-48. |
| Deng B W, Sun D M, Wan Q, et al. Review of electrolyte additives for ternary cathode lithium-ion battery[J]. Acta Chimica Sinica, 2018, 76(4): 30-48. | |
| 39 | Lee Y, Kim S O, Mun J, et al. Influence of salt, solvents, and additives on the thermal stability of delithiated cathodes in lithium-ion batteries[J]. Journal of Electroanalytical Chemistry, 2017, 807: 174-180. |
| 40 | Han G S, Li B, Ye Z Q, et al. The cooperative effect of vinylene carbonate and 1, 3-propane sultone on the elevated temperature performance of lithium ion batteries[J]. International Journal of Electrochemical Science, 2012, 7(12): 12963-12973. |
| 41 | Oh J, Kim J, Lee Y M, et al. Effects of vinylene carbonate and 1, 3-propane sultone on high-rate cycle performance and surface properties of high-nickel layered oxide cathodes[J]. Materials Research Bulletin, 2020, 132: 111008. |
| 42 | 蒋志敏, 王莉, 沈旻, 等. 锂离子电池正极界面修饰用电解液添加剂[J]. 化学进展, 2019, 31(5): 699-713. |
| Jiang Z M, Wang L, Shen M, et al. Electrolyte additives for interfacial modification of cathodes in lithium ion battery[J]. Progress in Chemistry, 2019, 31(5): 699-713. | |
| 43 | Wang Q S, Jiang L H, Yu Y, et al. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect[J]. Nano Energy, 2019, 55: 93-114. |
| 44 | Liu S F, Ji X, Piao N, et al. An inorganic-rich solid electrolyte interphase for advanced lithium-metal batteries in carbonate electrolytes[J]. Angewandte Chemie-International Edition, 2021, 60(7): 3661-3671. |
| 45 | Liu H, Li T, Xu X Q, et al. Stable interfaces constructed by concentrated ether electrolytes to render robust lithium metal batteries[J]. Chinese Journal of Chemical Engineering, 2021, 37: 152-158. |
| 46 | Zhang X Q, Chen X, Cheng X B, et al. Highly stable lithium metal batteries enabled by regulating the solvation of lithium ions in nonaqueous electrolytes[J]. Angewandte Chemie-International Edition, 2018, 57(19): 5301-5305. |
| 47 | Lee J H, Vuong T H L, Hwang S M, et al. Controlling a lithium surface with an alkyl halide nucleophile exchange[J]. Journal of Energy Chemistry, 2021, 62: 617-626. |
| 48 | Hou T Z, Fong K D, Wang J Y, et al. The solvation structure, transport properties and reduction behavior of carbonate-based electrolytes of lithium-ion batteries[J]. Chemical Science, 2021, 12(44): 14740-14751. |
| 49 | Yang S J, Xu X Q, Cheng X B, et al. Columnar lithium metal deposits: the role of non-aqueous electrolyte additive[J]. Acta Physico Chimica Sinica, 2020: 2007058. |
| 50 | Xu Y, Liu J L, Zhou L, et al. FEC as the additive of 5 V electrolyte and its electrochemical performance for LiNi0.5Mn1.5O4 [J]. Journal of Electroanalytical Chemistry, 2017, 791: 109-116. |
| 51 | Liu D Q, Qian K, He Y B, et al. Positive film-forming effect of fluoroethylene carbonate (FEC) on high-voltage cycling with three-electrode LiCoO2/Graphite pouch cell[J]. Electrochimica Acta, 2018, 269: 378-387. |
| 52 | Zhang X Q, Cheng X B, Chen X, et al. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries[J]. Advanced Functional Materials, 2017, 27(10): 1605989. |
| 53 | Meng F H, Zhu S, Gao J H, et al. Effect of electrolyte additives on the performance of lithium-ion batteries[J]. Ionics, 2021, 27(9): 3821-3827. |
| 54 | Hou T Z, Yang G, Rajput N N, et al. The influence of FEC on the solvation structure and reduction reaction of LiPF6/EC electrolytes and its implication for solid electrolyte interphase formation[J]. Nano Energy, 2019, 64: 103881. |
| 55 | Jaumann T, Balach J, Langklotz U, et al. Lifetime vs. rate capability: understanding the role of FEC and VC in high-energy Li-ion batteries with nano-silicon anodes[J]. Energy Storage Materials, 2017, 6: 26-35. |
| 56 | Kitz P G, Lacey M J, Novák P, et al. Operando investigation of the solid electrolyte interphase mechanical and transport properties formed from vinylene carbonate and fluoroethylene carbonate[J]. Journal of Power Sources, 2020, 477: 228567. |
| 57 | Arnot D J, Allcorn E, Harrison K L. Effect of temperature and FEC on silicon anode heat generation measured by isothermal microcalorimetry[J]. Journal of the Electrochemical Society, 2021, 168(11): 110509. |
| 58 | Zhang X Q, Li T, Li B Q, et al. A sustainable solid electrolyte interphase for high-energy-density lithium metal batteries under practical conditions[J]. Angewandte Chemie-International Edition, 2020, 59(8): 3252-3257. |
| 59 | Zhu Z Q, Tang Y X, Lv Z S, et al. Fluoroethylene carbonate enabling a robust LiF-rich solid electrolyte interphase to enhance the stability of the MoS2 anode for lithium-ion storage[J]. Angewandte Chemie-International Edition, 2018, 57(14): 3656-3660. |
| 60 | Wu Q, Tang X, Qian Y, et al. Enhancing the cycling stability for lithium-metal batteries by localized high-concentration electrolytes with 2-fluoropyridine additive[J]. ACS Applied Energy Materials, 2021, 4(9): 10234-10243. |
| 61 | Jin Y T, Kneusels N J H, Marbella L E, et al. Understanding fluoroethylene carbonate and vinylene carbonate based electrolytes for Si anodes in lithium ion batteries with NMR spectroscopy[J]. Journal of the American Chemical Society, 2018, 140(31): 9854-9867. |
| 62 | Aupperle F, von Aspern N, Berghus D, et al. The role of electrolyte additives on the interfacial chemistry and thermal reactivity of Si-anode-based Li-ion battery[J]. ACS Applied Energy Materials, 2019, 2(9): 6513-6527. |
| 63 | Hu F R, Zhang M Y, Qi W B, et al. Silicon micropillar electrodes of lithiumion batteries used for characterizing electrolyte additives[J]. Chinese Physics B, 2021, 30(6): 068202. |
| 64 | Xu S D, Zhuang Q C, Wang J, et al. New insight into vinylethylene carbonate as a film forming additive to ethylene carbonate-based electrolytes for lithium-ion batteries[J]. International Journal of Electrochemical Science, 2013, 8(6): 8058-8076. |
| 65 | Li B, Wang Y Q, Lin H B, et al. Improving high voltage stability of lithium cobalt oxide/graphite battery via forming protective films simultaneously on anode and cathode by using electrolyte additive[J]. Electrochimica Acta, 2014, 141: 263-270. |
| 66 | Zuo X X, Wu J H, Zhao M K, et al. Vinyl ethylene carbonate as an electrolyte additive for high-voltage LiNi0.4Mn0.4Co0.2O2/graphite Li-ion batteries[J]. Ionics, 2016, 22(2): 201-208. |
| 67 | Zuo X X, Xu M Q, Li W S, et al. Electrochemical reduction of 1, 3-propane sultone on graphite electrodes and its application in Li-ion batteries[J]. Electrochemical and Solid-State Letters, 2006, 9(4): A196. |
| 68 | Lee H, Choi S, Choi S, et al. SEI layer-forming additives for LiNi0.5Mn1.5O4/graphite 5 V Li-ion batteries[J]. Electrochemistry Communications, 2007, 9(4): 801-806. |
| 69 | Park G, Nakamura H, Lee Y, et al. The important role of additives for improved lithium ion battery safety[J]. Journal of Power Sources, 2009, 189(1): 602-606. |
| 70 | Yoon S R, Jeong S K. Effects of organic additives on electrochemical properties of SiO x electrodes in lithium secondary batteries[J]. Applied Mechanics and Materials, 2016, 835: 121-125. |
| 71 | Li X X, Liu L, Li S M, et al. Improving cyclic stability of LiMn2O4/graphite battery under elevated temperature by using 1, 3-propane sultone as electrolyte additive[J]. Frontiers in Materials, 2020, 7: 263. |
| 72 | Birrozzi A, Laszczynski N, Hekmatfar M, et al. Beneficial effect of propane sultone and tris(trimethylsilyl) borate as electrolyte additives on the cycling stability of the lithium rich nickel manganese cobalt (NMC) oxide[J]. Journal of Power Sources, 2016, 325: 525-533. |
| 73 | Xu D W, Kang Y Y, Wang J, et al. Exploring synergetic effects of vinylene carbonate and 1,3-propane sultone on LiNi0.6Mn0.2Co0.2O2/ graphite cells with excellent high-temperature performance[J]. Journal of Power Sources, 2019, 437: 226929. |
| 74 | Lin F W, Tran N T T, Hsu W D. Effect of 1, 3-propane sultone on the formation of solid electrolyte interphase at Li-ion battery anode surface: a first-principles study[J]. ACS Omega, 2020, 5(23): 13541-13547. |
| 75 | Xu M Q, Li W S, Lucht B L. Effect of propane sultone on elevated temperature performance of anode and cathode materials in lithium-ion batteries[J]. Journal of Power Sources, 2009, 193(2): 804-809. |
| 76 | Jung H M, Park S H, Jeon J, et al. Fluoropropane sultone as an SEI-forming additive that outperforms vinylene carbonate[J]. Journal of Materials Chemistry A, 2013, 1(38): 11975. |
| 77 | Li B, Xu M Q, Li T T, et al. Prop-1-ene-1, 3-sultone as SEI formation additive in propylene carbonate-based electrolyte for lithium ion batteries[J]. Electrochemistry Communications, 2012, 17: 92-95. |
| 78 | Self J, Hall D S, Madec L, et al. The role of prop-1-ene-1, 3-sultone as an additive in lithium-ion cells[J]. Journal of Power Sources, 2015, 298: 369-378. |
| 79 | Han Y K, Yoo J, Jung J. Reductive decomposition mechanism of prop-1-ene-1, 3-sultone in the formation of a solid–electrolyte interphase on the anode of a lithium-ion battery[J]. The Journal of Physical Chemistry C, 2016, 120(50): 28390-28397. |
| 80 | Song W F. Effect of prop-1-ene-1, 3-sultone on the performances of lithium cobalt oxide/graphite battery operating over a wide temperature range[J]. International Journal of Electrochemical Science, 2017: 10749-10762. |
| 81 | Yu X Y, Wang Y M, Cai H, et al. Enhancing the stability of high-voltage lithium-ion battery by using sulfur-containing electrolyte additives[J]. Ionics, 2019, 25(4): 1447-1457. |
| 82 | Leggesse E G, Jiang J C. Theoretical study of the reductive decomposition of ethylene sulfite: a film-forming electrolyte additive in lithium ion batteries[J]. The Journal of Physical Chemistry A, 2012, 116(45): 11025-11033. |
| 83 | Bhatt M D, O'Dwyer C. The role of carbonate and sulfite additives in propylene carbonate-based electrolytes on the formation of SEI layers at graphitic Li-ion battery anodes[J]. Journal of the Electrochemical Society, 2014, 161(9): A1415-A1421. |
| 132 | Zhang Y, Wang L Z, Feng H, et al. Effect of SO2 and CO2 additives on the cycle performances of commercial lithium-ion batteries[J]. Ionics, 2011, 17(8): 677-682. |
| 133 | 李佳, 曹茹, 侯涛, 等. 添加剂Na2CO3对石墨电极性能的影响[J]. 电池, 2012, 42(3): 119-122. |
| Li J, Cao R, Hou T, et al. Effects of electrolyte additive Na2CO3 on the performance of graphite electrode[J]. Battery Bimonthly, 2012, 42(3): 119-122. | |
| 134 | Zhuang Q C, Li J, Tian L L. Potassium carbonate as film forming electrolyte additive for lithium-ion batteries[J]. Journal of Power Sources, 2013, 222: 177-183. |
| 135 | Wu L N, Peng J, Han F M, et al. Suppressing lithium dendrite growth by a synergetic effect of uniform nucleation and inhibition[J]. Journal of Materials Chemistry A, 2020, 8(8): 4300-4307. |
| 136 | 郑洪河, 王显军, 李苞, 等. 钾盐添加剂改善天然石墨负极的嵌脱锂性质[J]. 无机材料学报, 2006, 21(5): 1109-1113. |
| Zheng H H, Wang X J, Li B, et al. Potassium salts as electrolyte additives for enhancing electrochemical performances of natural graphite anodes[J]. Journal of Inorganic Materials, 2006, 21(5): 1109-1113. | |
| 137 | Wu B R, Ren Y H, Mu D B, et al. Effect of sodium chloride as electrolyte additive on the performance of mesocarbon microbeads electrode[J]. International Journal of Electrochemical Science, 2013, 8(1): 670-677. |
| 84 | Li A J, Du P, Chen Z J, et al. Effects of ethylene sulfite as a supplementary film-forming additive on the electrochemical performance of graphite anode in EC-based electrolyte[J]. Ionics, 2015, 21(9): 2431-2438. |
| 85 | Lin L P, Yang K, Tan R, et al. Effect of sulfur-containing additives on the formation of a solid-electrolyte interphase evaluated by in situ AFM and ex situ characterizations[J]. Journal of Materials Chemistry A, 2017, 5(36): 19364-19370. |
| 86 | Ren F C, Zuo W H, Yang X R, et al. Comprehensive understanding of reduction mechanisms of ethylene sulfite in EC-based lithium-ion batteries[J]. The Journal of Physical Chemistry C, 2019, 123(10): 5871-5880. |
| 87 | Tong B, Song Z Y, Wan H H, et al. Sulfur-containing compounds as electrolyte additives for lithium-ion batteries[J]. InfoMat, 2021, 3(12): 1364-1392. |
| 88 | Yang T X, Wang W L, Li S, et al. Sulfur-containing C2H2O8S2 molecules as an overall-functional electrolyte additive for high-voltage LiNi0.5Co0.2Mn0.3O2/graphite batteries with enhanced performance[J]. Journal of Power Sources, 2020, 470: 228462. |
| 89 | Xia L, Xia Y G, Liu Z P.A novel fluorocyclophosphazene as bifunctional additive for safer lithium-ion batteries[J]. Journal of Power Sources, 2015,278:190-196. |
| 90 | Madec L, Xia J, Petibon R, et al. Effect of sulfate electrolyte additives on LiNi1/3Mn1/3Co1/3O2/graphite pouch cell lifetime: correlation between XPS surface studies and electrochemical test results[J]. The Journal of Physical Chemistry C, 2014, 118(51): 29608-29622. |
| 91 | Chen J X, Zhang X Q, Li B Q, et al. The origin of sulfuryl-containing components in SEI from sulfate additives for stable cycling of ultrathin lithium metal anodes[J]. Journal of Energy Chemistry, 2020, 47: 128-131. |
| 92 | Li X C, Yin Z L, Li X H, et al. Ethylene sulfate as film formation additive to improve the compatibility of graphite electrode for lithium-ion battery[J]. Ionics, 2014, 20(6): 795-801. |
| 93 | Janssen P, Schmitz R, Müller R, et al. 1, 3, 2-Dioxathiolane-2, 2-dioxide as film-forming agent for propylene carbonate based electrolytes for lithium-ion batteries[J]. Electrochimica Acta, 2014, 125: 101-106. |
| 94 | Zhang W J, Yang S M, Heng S, et al. Improved solid electrolyte interphase and Li-storage performance of Si/graphite anode with ethylene sulfate as electrolyte additive[J]. Functional Materials Letters, 2020, 13(7): 2051041. |
| 95 | Nelson K J, d'Eon G L, Wright A T B, et al. Studies of the effect of high voltage on the impedance and cycling performance of Li[Ni0.4Mn0.4Co0.2]O2/graphite lithium-ion pouch cells[J]. Journal of the Electrochemical Society, 2015, 162(6): A1046-A1054. |
| 96 | Hall D S, Allen J P, Glazier S L, et al. The solid-electrolyte interphase formation reactions of ethylene sulfate and its synergistic chemistry with prop-1-ene-1, 3-sultone in lithium-ion cells[J]. Journal of the Electrochemical Society, 2017, 164(14): A3445-A3453. |
| 97 | Li S, Li C H, Yang T X, et al. 3, 3-diethylene di-sulfite (DES) as a high-voltage electrolyte additive for 4.5 V LiNi0.8Co0.1Mn0.1O2/graphite batteries with enhanced performances[J]. ChemElectroChem, 2021, 8(4): 745-754. |
| 98 | Ding Z Y, Li X C, Wei T R, et al. Improved compatibility of graphite anode for lithium ion battery using sulfuric esters[J]. Electrochimica Acta, 2016, 196: 622-628. |
| 99 | Xu W, Angell C A. LiBOB and its derivatives: weakly coordinating anions, and the exceptional conductivity of their nonaqueous solutions [J]. Electrochemical and Solid-State Letters, 2001, 4(3): L3. |
| 100 | An Y X, Zuo P J, Cheng X Q, et al. The effects of LiBOB additive for stable SEI formation of PP13TFSI-organic mixed electrolyte in lithium ion batteries[J]. Electrochimica Acta, 2011, 56(13): 4841-4848. |
| 101 | Xiong S Z, Kai X, Hong X B, et al. Effect of LiBOB as additive on electrochemical properties of lithium-sulfur batteries[J]. Ionics, 2012, 18(3): 249-254. |
| 102 | Lian F, Li Y, He Y, et al. Preparation of LiBOB via rheological phase method and its application to mitigate voltage fade of Li1.16[Mn0.75Ni0.25]0.84O2 cathode[J]. RSC Advances, 2015, 5(105): 86763-86770. |
| 103 | 姜雪, 史楠楠, 张莹, 等. LiBOB对Li1.15Ni0.68Mn1.32O4电极电化学行为的影响[J]. 高等学校化学学报, 2015, 36(4): 739-744. |
| Jiang X, Shi N N, Zhang Y, et al. Influence of LiBOB on the electrochemical performance of Li1.15Ni0.68Mn1.32O4 electrode[J]. Chemical Journal of Chinese Universities, 2015, 36(4): 739-744. | |
| 104 | Kranz S, Kranz T, Jaegermann A G, et al. Is the solid electrolyte interphase in lithium-ion batteries really a solid electrolyte? Transport experiments on lithium bis(oxalato)borate-based model interphases[J]. Journal of Power Sources, 2019, 418: 138-146. |
| 105 | Ha S Y, Han J G, Song Y M, et al. Using a lithium bis(oxalato) borate additive to improve electrochemical performance of high-voltage spinel LiNi0.5Mn1.5O4 cathodes at 60℃[J]. Electrochimica Acta, 2013, 104: 170-177. |
| 106 | Xiao Z, Liu J D, Fan G L, et al. Lithium bis(oxalate)borate additive in the electrolyte to improve Li-rich layered oxide cathode materials[J]. Materials Chemistry Frontiers, 2020, 4(6): 1689-1696. |
| 107 | Zhu Y, Li Y, Bettge M, et al. Positive electrode passivation by LiDFOB electrolyte additive in high-capacity lithium-ion cells[J]. Journal of the Electrochemical Society, 2012, 159(12): A2109-A2117. |
| 108 | Bhatt M D, O'Dwyer C. Solid electrolyte interphases at Li-ion battery graphitic anodes in propylene carbonate (PC)-based electrolytes containing FEC, LiBOB, and LiDFOB as additives[J]. Chemical Physics Letters, 2015, 618: 208-213. |
| 109 | Liu M H, Dai F, Ma Z R, et al. Improved electrolyte and its application in LiNi1/3Mn1/3Co1/3O2-graphite full cells[J]. Journal of Power Sources, 2014, 268: 37-44. |
| 110 | Gao X M, Qu Q T, Zhu G B, et al. Piperidinium-based ionic liquid electrolyte with linear solvent and LiODFB for LiFePO4/Li cells at room and high temperature[J]. RSC Adv., 2017, 7(79): 50135-50142. |
| 111 | Aravindan V, Gnanaraj J, Madhavi S, et al. Lithium-ion conducting electrolyte salts for lithium batteries[J]. Chemistry-A European Journal, 2011, 17(51): 14326-14346. |
| 112 | Zhao Q P, Zhang Y, Tang F J, et al. Mixed salts of lithium difluoro (oxalate) borate and lithium tetrafluorobotate electrolyte on low-temperature performance for lithium-ion batteries[J]. Journal of the Electrochemical Society, 2017, 164(9): A1873-A1880. |
| 113 | Gu Y X, Fang S H, Yang L, et al. A non-flammable electrolyte for long-life lithium ion batteries operating over a wide-temperature range[J]. Journal of Materials Chemistry A, 2021, 9(27): 15363-15372. |
| 114 | Ma L, Ellis L, Glazier S L, et al. LiPO2F2 as an electrolyte additive in Li[Ni0.5Mn0.3Co0.2]O2/graphite pouch cells[J]. Journal of the Electrochemical Society, 2018, 165(5): A891-A899. |
| 115 | Kim K E, Jang J Y, Park I, et al. A combination of lithium difluorophosphate and vinylene carbonate as reducible additives to improve cycling performance of graphite electrodes at high rates[J]. Electrochemistry Communications, 2015, 61: 121-124. |
| 116 | Yang G H, Shi J L, Shen C, et al. Improving the cyclability performance of lithium-ion batteries by introducing lithium difluorophosphate (LiPO2F2) additive[J]. RSC Advances, 2017, 7(42): 26052-26059. |
| 117 | Hong S, Hong B, Song W F, et al. Communication—lithium difluorophosphate as an electrolyte additive to improve the high voltage performance of LiNi0.5Co0.2Mn0.3O2/graphite cell[J]. Journal of the Electrochemical Society, 2018, 165(2): A368-A370. |
| 138 | Takeuchi S, Fukutsuka T, Miyazaki K, et al. Electrochemical preparation of a lithium-graphite-intercalation compound in a dimethyl sulfoxide-based electrolyte containing calcium ions[J]. Carbon, 2013, 57: 232-238. |
| 139 | Lee T J, Lee J B, Yoon T, et al. Tris(pentafluorophenyl)silane as a solid electrolyte interphase (SEI)-forming agent for graphite electrodes[J]. Journal of the Electrochemical Society, 2017, 164(9): A1887-A1892. |
| 140 | Wang J P, Zhang L, Zhang H T. Effects of electrolyte additive on the electrochemical performance of Si/C anode for lithium-ion batteries[J]. Ionics, 2018, 24(11): 3691-3698. |
| 141 | Yue H Y, Yang Y G, Xiao Y, et al. Boron additive passivated carbonate electrolytes for stable cycling of 5 V lithium-metal batteries[J]. Journal of Materials Chemistry A, 2019, 7(2): 594-602. |
| 142 | Phiri I, Ko S, Kim S, et al. Zwitterionic osmolyte-inspired additives as scavengers and low temperature performance enhancers for lithium ion batteries[J]. Materials Letters, 2021, 288: 129366. |
| 143 | Shi C G, Shen C H, Peng X X, et al. A special enabler for boosting cyclic life and rate capability of LiNi0.8Co0.1Mn0.1O2: green and simple additive[J]. Nano Energy, 2019, 65: 104084. |
| 144 | Wang P P, Yang F J, Bai J, et al. Isatin anhydride as multifunctional film-forming additive to enhance cycle life of high-voltage Li-ion batteries at elevated temperature[J]. Journal of Power Sources, 2021, 509: 230361. |
| 145 | Zhang W L, Ma Q C, Liu X J, et al. Novel piperidinium-based ionic liquid as electrolyte additive for high voltage lithium-ion batteries[J]. RSC Advances, 2021, 11(25): 15091-15098. |
| 146 | Lee S H, Hwang J Y, Park S J, et al. Adiponitrile (C6H8 N2): a new bi-functional additive for high-performance Li-metal batteries[J]. Advanced Functional Materials, 2019, 29(30): 1902496. |
| 147 | Xiao D J, Li Q, Luo D, et al. Regulating the Li+-solvation structure of ester electrolyte for high-energy-density lithium metal batteries[J]. Small, 2020, 16(47): 2004688. |
| 148 | Yan C, Yao Y X, Cai W L, et al. The influence of formation temperature on the solid electrolyte interphase of graphite in lithium ion batteries[J]. Journal of Energy Chemistry, 2020, 49: 335-338. |
| 149 | Yan C, Yao Y X, Chen X, et al. Back cover: lithium nitrate solvation chemistry in carbonate electrolyte sustains high-voltage lithium metal batterie [J]. Angewandte Chemie-International Edition, 2018, 57(43): 14292. |
| 150 | Li S Y, Zhang W D, Wu Q, et al. Synergistic dual-additive electrolyte enables practical lithium-metal batteries[J]. Angewandte Chemie (English), 2020, 59(35): 14935-14941. |
| [1] | 邵苛苛, 宋孟杰, 江正勇, 张旋, 张龙, 高润淼, 甄泽康. 水平方向上冰中受陷气泡形成和分布实验研究[J]. 化工学报, 2023, 74(S1): 161-164. |
| [2] | 吴延鹏, 李晓宇, 钟乔洋. 静电纺丝纳米纤维双疏膜油性细颗粒物过滤性能实验分析[J]. 化工学报, 2023, 74(S1): 259-264. |
| [3] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [4] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [5] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [6] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [7] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [8] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [9] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [10] | 王志龙, 杨烨, 赵真真, 田涛, 赵桐, 崔亚辉. 搅拌时间和混合顺序对锂离子电池正极浆料分散特性的影响[J]. 化工学报, 2023, 74(7): 3127-3138. |
| [11] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [12] | 张贲, 王松柏, 魏子亚, 郝婷婷, 马学虎, 温荣福. 超亲水多孔金属结构驱动的毛细液膜冷凝及传热强化[J]. 化工学报, 2023, 74(7): 2824-2835. |
| [13] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| [14] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| [15] | 陈朝光, 贾玉香, 汪锰. 以低浓度废酸驱动中和渗析脱盐的模拟与验证[J]. 化工学报, 2023, 74(6): 2486-2494. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号