化工学报 ›› 2024, Vol. 75 ›› Issue (2): 647-658.DOI: 10.11949/0438-1157.20231088
收稿日期:2023-10-24
修回日期:2023-12-24
出版日期:2024-02-25
发布日期:2024-04-10
通讯作者:
张兰河
作者简介:贾艳萍(1973—),女,博士,教授,jiayanping1111@sina.com
基金资助:
Yanping JIA( ), Dongxu YIN, Jingyi XU, Haifeng ZHANG, Lanhe ZHANG(
), Dongxu YIN, Jingyi XU, Haifeng ZHANG, Lanhe ZHANG( )
)
Received:2023-10-24
Revised:2023-12-24
Online:2024-02-25
Published:2024-04-10
Contact:
Lanhe ZHANG
摘要:
盐酸土霉素常被用于治疗畜禽疾病,但是它不能被畜禽完全代谢,残留的盐酸土霉素进入水体危害水环境的健康。铁锰作为常见的过渡金属,通常以二价态活化亚硫酸盐来降解有机污染物,反应条件温和、操作简单,但是单独的二价铁与二价锰氧化还原电势低,活化亚硫酸盐效果较差。本研究采用Fe2+/Mn2+共活化Na2SO3降解水中的盐酸土霉素,考察药剂用量、pH、溶解氧、氯离子、碳酸根及腐殖酸对Fe2+/Mn2+/Na2SO3体系降解盐酸土霉素的影响;通过焦磷酸盐实验、自由基淬灭实验和EPR实验分析Fe2+/Mn2+/Na2SO3体系中的活性物种;利用紫外可见光谱、傅里叶红外光谱、气相色谱-质谱联用仪识别盐酸土霉素的官能团及其降解中间产物的变化,推断盐酸土霉素的降解途径。结果表明:当Fe2+/Mn2+/Na2SO3浓度比为1∶4∶20(浓度分别为0.1、0.4和2 mmol/L)时,在反应45 min、pH为9.0条件下,盐酸土霉素的去除率和矿化率最高,分别达到94%和49%。随着溶解氧从9 mg/L下降至1.89 mg/L,盐酸土霉素去除率从94%下降至17%;氯离子、腐殖酸和碳酸根均对盐酸土霉素的降解产生抑制作用。Mn(Ⅲ)和
中图分类号:
贾艳萍, 阴东旭, 徐静仪, 张海丰, 张兰河. Fe2+/Mn2+活化亚硫酸盐降解盐酸土霉素的机理研究[J]. 化工学报, 2024, 75(2): 647-658.
Yanping JIA, Dongxu YIN, Jingyi XU, Haifeng ZHANG, Lanhe ZHANG. Mechanism study of oxytetracycline hydrochloride degradation through activating sulfite by Fe2+/Mn2+[J]. CIESC Journal, 2024, 75(2): 647-658.

图3 在不同反应体系中盐酸土霉素浓度、pH和矿化率的变化
Fig.3 The changes of oxytetracycline hydrochloride concentration, pH and mineralization rate in different reaction systems
| 产物 | 保留 时间/min | m/z | 分子式 | 分子结构 | 产物 | 保留 时间/min | m/z | 分子式 | 分子结构 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.295 | 106 | C8H10 | 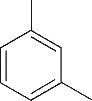 | 10 | 9.585 | 138 | C8H10O2 | 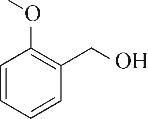 |
| 2 | 4.900 | 108 | C7H8O | 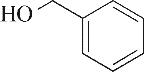 | 11 | 13.525 | 206 | C14H22O | 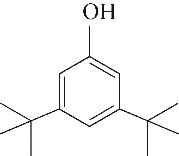 |
| 3 | 5.955 | 144 | C8H16O2 | 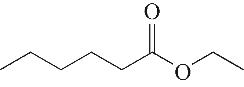 | 12 | 18.705 | 278 | C16H22O4 | 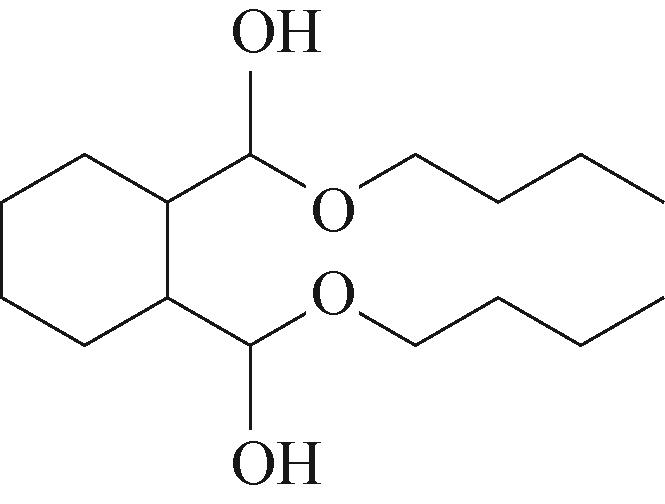 |
| 4 | 6.385 | 134 | C10H14 | 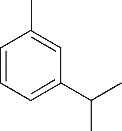 | 13 | 20.050 | 196 | C10H12O4 | 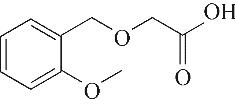 |
| 5 | 7.260 | 108 | C7H8O | 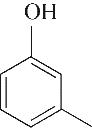 | 14 | 23.385 | 154 | C9H14O2 | 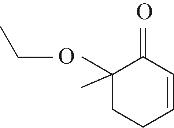 |
| 6 | 7.360 | 136 | C10H16 | 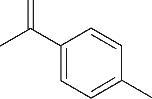 | 15 | 24.215 | 424 | C29H44O2 | 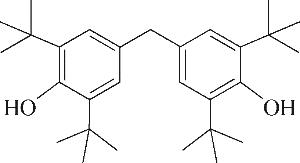 |
| 7 | 7.940 | 152 | C10H16O | 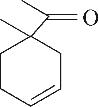 | 16 | 24.640 | 208 | C13H20O2 | 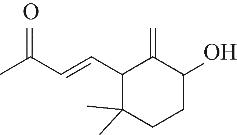 |
| 8 | 8.975 | 134 | C9H10O | 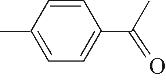 | 17 | 26.645 | 180 | C11H16O2 | 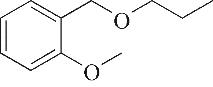 |
| 9 | 9.150 | 138 | C9H14O | 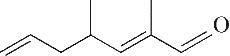 |
表1 OTH降解过程的中间产物
Table 1 Intermediate products in the degradation process of OTH
| 产物 | 保留 时间/min | m/z | 分子式 | 分子结构 | 产物 | 保留 时间/min | m/z | 分子式 | 分子结构 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.295 | 106 | C8H10 |  | 10 | 9.585 | 138 | C8H10O2 |  |
| 2 | 4.900 | 108 | C7H8O |  | 11 | 13.525 | 206 | C14H22O |  |
| 3 | 5.955 | 144 | C8H16O2 |  | 12 | 18.705 | 278 | C16H22O4 |  |
| 4 | 6.385 | 134 | C10H14 |  | 13 | 20.050 | 196 | C10H12O4 |  |
| 5 | 7.260 | 108 | C7H8O |  | 14 | 23.385 | 154 | C9H14O2 |  |
| 6 | 7.360 | 136 | C10H16 |  | 15 | 24.215 | 424 | C29H44O2 |  |
| 7 | 7.940 | 152 | C10H16O |  | 16 | 24.640 | 208 | C13H20O2 |  |
| 8 | 8.975 | 134 | C9H10O |  | 17 | 26.645 | 180 | C11H16O2 |  |
| 9 | 9.150 | 138 | C9H14O |  |
| 1 | Moudgil P, Bedi J S, Aulakh R S, et al. Validation of HPLC multi-residue method for determination of fluoroquinolones, tetracycline, sulphonamides and chloramphenicol residues in bovine milk[J]. Food Analytical Methods, 2019, 12(2): 338-346. |
| 2 | Petković H, Lukežič T, Šušković J. Biosynthesis of oxytetracycline by Streptomyces rimosus: past, present and future directions in the development of tetracycline antibiotics[J]. Food Technology and Biotechnology, 2017, 55(1): 3-13. |
| 3 | 展海银, 周启星. 环境中四环素类抗生素污染处理技术研究进展[J]. 环境工程技术学报, 2021, 11(3): 571-581. |
| Zhan H Y, Zhou Q X. Research progress on treatment technology of tetracycline antibiotics pollution in the environment[J]. Journal of Environmental Engineering Technology, 2021, 11(3): 571-581. | |
| 4 | 陈明如, 马可可, 周律. 基于亚硫酸盐的高级氧化活化方法最新进展[J]. 工业水处理, 2022, 42(6): 109-115, 124. |
| Chen M R, Ma K K, Zhou L. Recent progress in sulfite advanced oxidation activation approaches[J]. Industrial Water Treatment, 2022, 42(6): 109-115, 124. | |
| 5 | Yuan Y N, Luo T, Xu J, et al. Enhanced oxidation of aniline using Fe(Ⅲ)-S(Ⅳ) system: role of different oxysulfur radicals[J]. Chemical Engineering Journal, 2019, 362: 183-189. |
| 6 | 李阳, 关小红, 董红钰. Fe(Ⅱ)活化亚硫酸盐降解卡马西平的动力学及机制研究[J]. 土木与环境工程学报(中英文), 2021, 43(6): 165-171. |
| Li Y, Guan X H, Dong H Y. Kinetics and mechanism of carbamazepine degradation through activating sulfite by Fe(Ⅱ)[J]. Journal of Civil and Environmental Engineering, 2021, 43(6): 165-171. | |
| 7 | Grgić I, Hudnik V, Bizjak M, et al. Aqueous S(Ⅳ) oxidation(Ⅰ): Catalytic effects of some metal ions[J]. Atmospheric Environment. Part A. General Topics, 1991, 25(8): 1591-1597. |
| 8 | Berglund J, Elding L I. Manganese-catalysed autoxidation of dissolved sulfur dioxide in the atmospheric aqueous phase[J]. Atmospheric Environment, 1995, 29(12): 1379-1391. |
| 9 | Zhao X D, Wu W J, Yan Y G. Efficient abatement of an iodinated X-ray contrast media iohexol by Co(Ⅱ) or Cu(Ⅱ) activated sulfite autoxidation process[J]. Environmental Science and Pollution Research, 2019, 26(24): 24707-24719. |
| 10 | Podkrajšek B, Berčič G, Turšič J, et al. Aqueous oxidation of sulfur(Ⅳ) catalyzed by manganese(Ⅱ): a generalized simple kinetic model[J]. Journal of Atmospheric Chemistry, 2004, 47(3): 287-303. |
| 11 | Guo Y G, Lou X Y, Fang C L, et al. Novel photo-sulfite system: toward simultaneous transformations of inorganic and organic pollutants[J]. Environmental Science & Technology, 2013, 47(19): 11174-11181. |
| 12 | Ling L, Zhang D P, Fan C, et al. A Fe(Ⅱ)/citrate/UV/PMS process for carbamazepine degradation at a very low Fe(Ⅱ)/PMS ratio and neutral pH: the mechanisms[J]. Water Research, 2017, 124: 446-453. |
| 13 | Berglund J, Werndrup P, Elding L I. Kinetics and mechanism for the redox reaction between hexaaquathallium(Ⅲ) and sulfur dioxide in acidic aqueous solution[J]. Journal of the Chemical Society, Dalton Transactions, 1994(9): 1435-1439. |
| 14 | Fronaeus S, Berglund J, Elding L I. Iron-manganese redox processes and synergism in the mechanism for manganese-catalyzed autoxidation of hydrogen sulfite[J]. Inorganic Chemistry, 1998, 37(19): 4939-4944. |
| 15 | Zhang J M, Ma J, Song H R, et al. Organic contaminants degradation from the S(Ⅳ) autoxidation process catalyzed by ferrous-manganous ions: a noticeable Mn(Ⅲ) oxidation process[J]. Water Research, 2018, 133: 227-235. |
| 16 | Zhang J M, Song H R, Liu Y L, et al. Remarkable enhancement of a photochemical Fenton-like system (UV-A/Fe(Ⅱ)/PMS) at near-neutral pH and low Fe(Ⅱ)/peroxymonosulfate ratio by three alpha hydroxy acids: mechanisms and influencing factors[J]. Separation and Purification Technology, 2019, 224: 142-151. |
| 17 | Morgan J J. Kinetics of reaction between O2 and Mn(Ⅱ) species in aqueous solutions[J]. Geochimica et Cosmochimica Acta, 2005, 69(1): 35-48. |
| 18 | Springer S D, Butler A. Magnetic susceptibility of Mn(Ⅲ) complexes of hydroxamate siderophores[J]. Journal of Inorganic Biochemistry, 2015, 148: 22-26. |
| 19 | Wang Z M, Xiong W, Tebo B M, et al. Oxidative UO2 dissolution induced by soluble Mn(Ⅲ)[J]. Environmental Science & Technology, 2014, 48(1): 289-298. |
| 20 | Feng J W, Zheng Z, Sun Y B, et al. Degradation of diuron in aqueous solution by dielectric barrier discharge[J]. Journal of Hazardous Materials, 2008, 154(1/2/3): 1081-1089. |
| 21 | Klewicki J K, Morgan J J. Kinetic behavior of Mn(Ⅲ) complexes of pyrophosphate, EDTA, and citrate[J]. Environmental Science and Technology, 1998, 32(19): 2916-2922. |
| 22 | Xie P C, Zhang L, Chen J H, et al. Enhanced degradation of organic contaminants by zero-valent iron/sulfite process under simulated sunlight irradiation[J]. Water Research, 2019, 149: 169-178. |
| 23 | Luo C W, Jiang J, Ma J, et al. Oxidation of the odorous compound 2, 4, 6-trichloroanisole by UV activated persulfate: kinetics, products, and pathways[J]. Water Research, 2016, 96: 12-21. |
| 24 | Sun P Z, Lee W N, Zhang R C, et al. Degradation of DEET and caffeine under UV/chlorine and simulated sunlight/chlorine conditions[J]. Environmental Science & Technology, 2016, 50(24): 13265-13273. |
| 25 | Fang G D, Dionysiou D D, Wang Y, et al. Sulfate radical-based degradation of polychlorinated biphenyls: effects of chloride ion and reaction kinetics[J]. Journal of Hazardous Materials, 2012, 227/228: 394-401. |
| 26 | Wu Z H, Guo K H, Fang J Y, et al. Factors affecting the roles of reactive species in the degradation of micropollutants by the UV/chlorine process[J]. Water Research, 2017, 126: 351-360. |
| 27 | Chen Y, Liu Z Z, Wang Z P, et al. Photodegradation of propranolol by Fe(Ⅲ)-citrate complexes: kinetics, mechanism and effect of environmental media[J]. Journal of Hazardous Materials, 2011, 194: 202-208. |
| 28 | Wang X H, Yao J Y, Wang S Y, et al. Phototransformation of estrogens mediated by Mn(Ⅲ), not by reactive oxygen species, in the presence of humic acids[J]. Chemosphere, 2018, 201: 224-233. |
| 29 | Xu K, Ben W W, Ling W C, et al. Impact of humic acid on the degradation of levofloxacin by aqueous permanganate: kinetics and mechanism[J]. Water Research, 2017, 123: 67-74. |
| 30 | Yang Y, Jiang J, Lu X L, et al. Production of sulfate radical and hydroxyl radical by reaction of ozone with peroxymonosulfate: a novel advanced oxidation process[J]. Environmental Science & Technology, 2015, 49(12): 7330-7339. |
| 31 | Feng M B, Sharma V K. Enhanced oxidation of antibiotics by ferrate ( Ⅵ ) - s u l f u r ( Ⅳ ) system: elucidating multi-oxidant mechanism[J]. Chemical Engineering Journal, 2018, 341: 137-145. |
| 32 | Neta P, Huie R E. Free-radical chemistry of sulfite[J]. Environmental Health Perspectives, 1985, 64: 209-217. |
| 33 | Neta P, Huie R E, Ross A B. Rate constants for reactions of inorganic radicals in aqueous solution[J]. Journal of Physical and Chemical Reference Data, 1988, 17(3): 1027-1284. |
| 34 | Chen L, Peng X Z, Liu J H, et al. Decolorization of orange Ⅱ in aqueous solution by an F e ( Ⅱ ) /sulfite system: replacement of persulfate[J]. Industrial & Engineering Chemistry Research, 2012, 51(42): 13632-13638. |
| 35 | Anipsitakis G P, Dionysiou D D. Radical generation by the interaction of transition metals with common oxidants[J]. Environmental Science & Technology, 2004, 38(13): 3705-3712. |
| 36 | Zou J, Ma J, Chen L W, et al. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting F e ( Ⅲ ) / F e ( Ⅱ ) cycle with hydroxylamine[J]. Environmental Science & Technology, 2013, 47(20): 11685-11691. |
| 37 | 孙绍芳, 李佳龙, 邱琪, 等. Fe(Ⅵ)/Na2SO3体系降解阿特拉津效能[J]. 中国环境科学, 2021, 41(1): 192-198. |
| Sun S F, Li J L, Qiu Q, et al. Degradation efficiency of atrazine by Fe(Ⅵ)/Na2SO3 system[J]. China Environmental Science, 2021, 41(1): 192-198. | |
| 38 | 唐海, 张昊楠, 段升飞, 等. S O 3 2 - 活化 S 2 O 8 2 - 降解偶氮染料废水的机制研究[J]. 中国环境科学, 2018, 38(3): 959-967. |
| Tang H, Zhang H N, Duan S F, et al. Mechanism research for degradation of azo dying wastewater based on persulfate activated by sulphite[J]. China Environmental Science, 2018, 38(3): 959-967. | |
| 39 | 于怀东, 方茹, 陈士明, 等. 锰离子参与的类Fenton反应的HPLC和ESR波谱研究[J]. 化学学报, 2005, 63(14): 1357-1360, 1244. |
| Yu H D, Fang R, Chen S M, et al. Manganous participation in Fenton like reaction studied by HPLC and ESR spectroscopy[J]. Acta Chimica Sinica, 2005, 63(14): 1357-1360, 1244. | |
| 40 | Rao D D, Sun Y K, Shao B B, et al. Activation of oxygen with sulfite for enhanced removal of Mn(Ⅱ): the involvement of S O 4 • - [J]. Water Research, 2019, 157: 435-444. |
| 41 | Sun S F, Pang S Y, Jiang J, et al. The combination of ferrate(Ⅵ) and sulfite as a novel advanced oxidation process for enhanced degradation of organic contaminants[J]. Chemical Engineering Journal, 2018, 333: 11-19. |
| 42 | Wang Y, Zhang H, Chen L. Ultrasound enhanced catalytic ozonation of tetracycline in a rectangular air-lift reactor[J]. Catalysis Today, 2011, 175(1): 283-292. |
| 43 | Wang Y, Zhang H, Zhang J H, et al. Degradation of tetracycline in aqueous media by ozonation in an internal loop-lift reactor[J]. Journal of Hazardous Materials, 2011, 192(1): 35-43. |
| 44 | Zhou J, Hou M F, Pan D Y. Degradation of oxytetracycline by the iron wire/H2O2 system[C]//Proceedings of the 2016 International Conference on Biological Engineering and Pharmacy (BEP 2016). Paris, France: Atlantis Press, 2017. |
| 45 | Zhu X D, Wang Y J, Sun R J, et al. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2 [J]. Chemosphere, 2013, 92(8): 925-932. |
| 46 | Chen B L, Zhou D D, Zhu L Z. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures[J]. Environmental Science & Technology, 2008, 42(14): 5137-5143. |
| 47 | Jia M Y, Wang F, Bian Y R, et al. Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar[J]. Bioresource Technology, 2013, 136: 87-93. |
| 48 | 徐惟馨, 夏静静, 韦芸, 等. 红外光谱对牛预混料中违禁添加盐酸土霉素的快速定量[J]. 光谱学与光谱分析, 2023, 43(3): 842-847. |
| Xu W X, Xia J J, Wei Y, et al. Rapid determination of oxytetracycline hydrochloride illegally added in cattle premix by ATR-FTIR[J]. Spectroscopy and Spectral Analysis, 2023, 43(3): 842-847. | |
| 49 | Shi Z Y, Jin C, Zhang J, et al. Insight into mechanism of arsanilic acid degradation in permanganate-sulfite system: role of reactive species[J]. Chemical Engineering Journal, 2019, 359: 1463-1471. |
| 50 | Anipsitakis G P, Dionysiou D D, Gonzalez M A. Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions[J]. Environmental Science & Technology, 2006, 40(3): 1000-1007. |
| 51 | 黄智辉, 纪志永, 陈希, 等. 过硫酸盐高级氧化降解水体中有机污染物研究进展[J]. 化工进展, 2019, 38(5): 2461-2470. |
| Huang Z H, Ji Z Y, Chen X, et al. Degradation of organic pollutants in water by persulfate advanced oxidation[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2461-2470. | |
| 52 | 吴文瞳, 张玲玲, 李子富, 等. 高级氧化技术降解抗生素及去除耐药性的研究进展[J]. 化工进展, 2021, 40(8): 4551-4561. |
| Wu W T, Zhang L L, Li Z F, et al. Research progress of advanced oxidation technology in degradation of antibiotics and removal of antibiotic resistance[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4551-4561. | |
| 53 | 叶林静, 关卫省, 李宇亮. 高级氧化技术降解双酚A的研究进展[J]. 化工进展, 2013, 32(4): 909-918. |
| Ye L J, Guan W S, Li Y L. Research advances in bisphenol A degraded by advanced oxidation processes[J]. Chemical Industry and Engineering Progress, 2013, 32(4): 909-918. | |
| 54 | Hu E D, Zhang Y, Wu S Y, et al. Role of dissolved Mn(Ⅲ) in transformation of organic contaminants: non-oxidative versus oxidative mechanisms[J]. Water Research, 2017, 111: 234-243. |
| [1] | 朱芝, 许恒杰, 陈维, 毛文元, 邓强国, 孙雪剑. 超临界二氧化碳螺旋槽干气密封热流耦合润滑临界阻塞特性研究[J]. 化工学报, 2024, 75(2): 604-615. |
| [2] | 孙瑞, 田华, 吴子睿, 孙孝存, 舒歌群. 二氧化碳混合工质临界参数计算模型对比研究[J]. 化工学报, 2024, 75(2): 439-449. |
| [3] | 张泽欣, 郑伟中, 徐益升, 胡冬冬, 卓欣宇, 宗原, 孙伟振, 赵玲. 超临界二氧化碳介质中晶圆清洗与选择性刻蚀研究进展[J]. 化工学报, 2024, 75(1): 110-119. |
| [4] | 赵若晗, 黄蒙蒙, 朱春英, 付涛涛, 高习群, 马友光. 缩口T型微通道内纳米流体吸收CO2的流动与传质研究[J]. 化工学报, 2024, 75(1): 221-230. |
| [5] | 张强, 王宪飞, 王凯, 骆广生, 路忠凯. 非金属催化剂在环氧化物和环状酸酐共聚中的研究进展[J]. 化工学报, 2024, 75(1): 60-73. |
| [6] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [7] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [8] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [9] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [10] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [11] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [12] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [13] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [14] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [15] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号