化工学报 ›› 2023, Vol. 74 ›› Issue (9): 3628-3639.DOI: 10.11949/0438-1157.20230531
陈美思( ), 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋(
), 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋( ), 张志炳(
), 张志炳( )
)
收稿日期:2023-05-31
修回日期:2023-07-18
出版日期:2023-09-25
发布日期:2023-11-20
通讯作者:
张锋,张志炳
作者简介:陈美思(1996—),女,博士研究生,chenmeisi1995@163.com
基金资助:
Meisi CHEN( ), Weida CHEN, Xinyao LI, Shangyu LI, Youting WU, Feng ZHANG(
), Weida CHEN, Xinyao LI, Shangyu LI, Youting WU, Feng ZHANG( ), Zhibing ZHANG(
), Zhibing ZHANG( )
)
Received:2023-05-31
Revised:2023-07-18
Online:2023-09-25
Published:2023-11-20
Contact:
Feng ZHANG, Zhibing ZHANG
摘要:
解决工业过程污染气体的过量排放问题,具有重要的科学和环境意义。离子液体(ILs)作为室温呈液态的绿色溶剂,在气体捕集转化方面具有独特的优势,但天然的高黏度特性严重阻碍了其工业应用。本团队基于多年研究发现,不执拗于大幅降低离子液体的黏度,而顺其自然,通过“微颗粒化”技术,实现离子液体于准静止状态的高效利用,是离子液体适应工业化的有效路径之一。鉴于此,综述了以二氧化硅(SiO2)为介质的离子液体微颗粒,及其衍生的离子液体纳-微界面反应单元在气体捕集(VOCs和CO2)和CO2转化方面的应用研究进展,探讨了微颗粒化离子液体体系较传统体系的特性优势,并分析了离子液体“微颗粒化”的应用前景及工业可行性。
中图分类号:
陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639.
Meisi CHEN, Weida CHEN, Xinyao LI, Shangyu LI, Youting WU, Feng ZHANG, Zhibing ZHANG. Advances in silicon-based ionic liquid microparticle enhanced gas capture and conversion[J]. CIESC Journal, 2023, 74(9): 3628-3639.
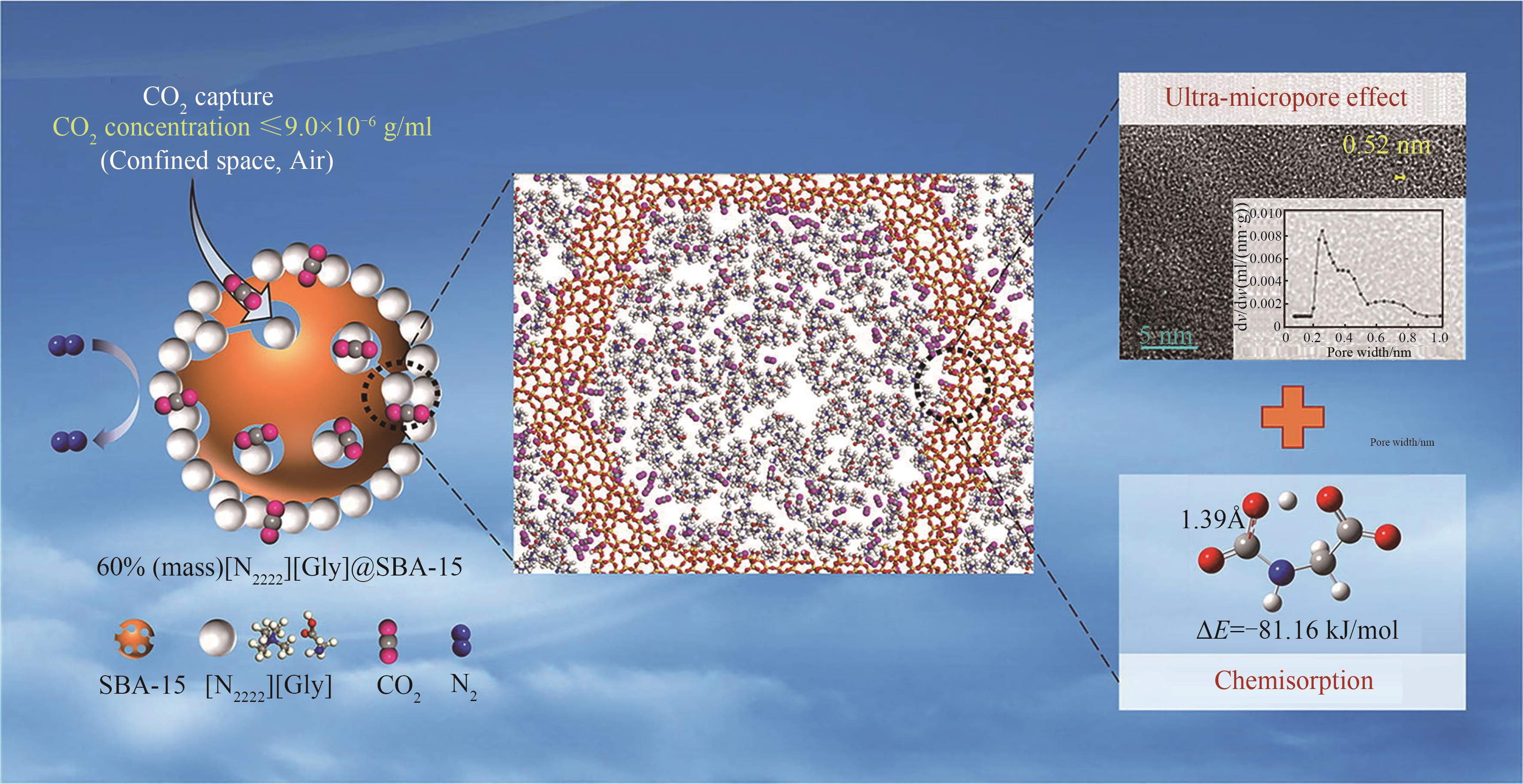
图4 [N2222][Gly]@SBA-15硅基离子液体微颗粒通过化学协同作用形成超微孔结构[45] (1 Å=10-10 m)
Fig. 4 [N2222][Gly]@SBA-15 silica-based microparticles form ultra-microporous structures through chemical synergy[45]
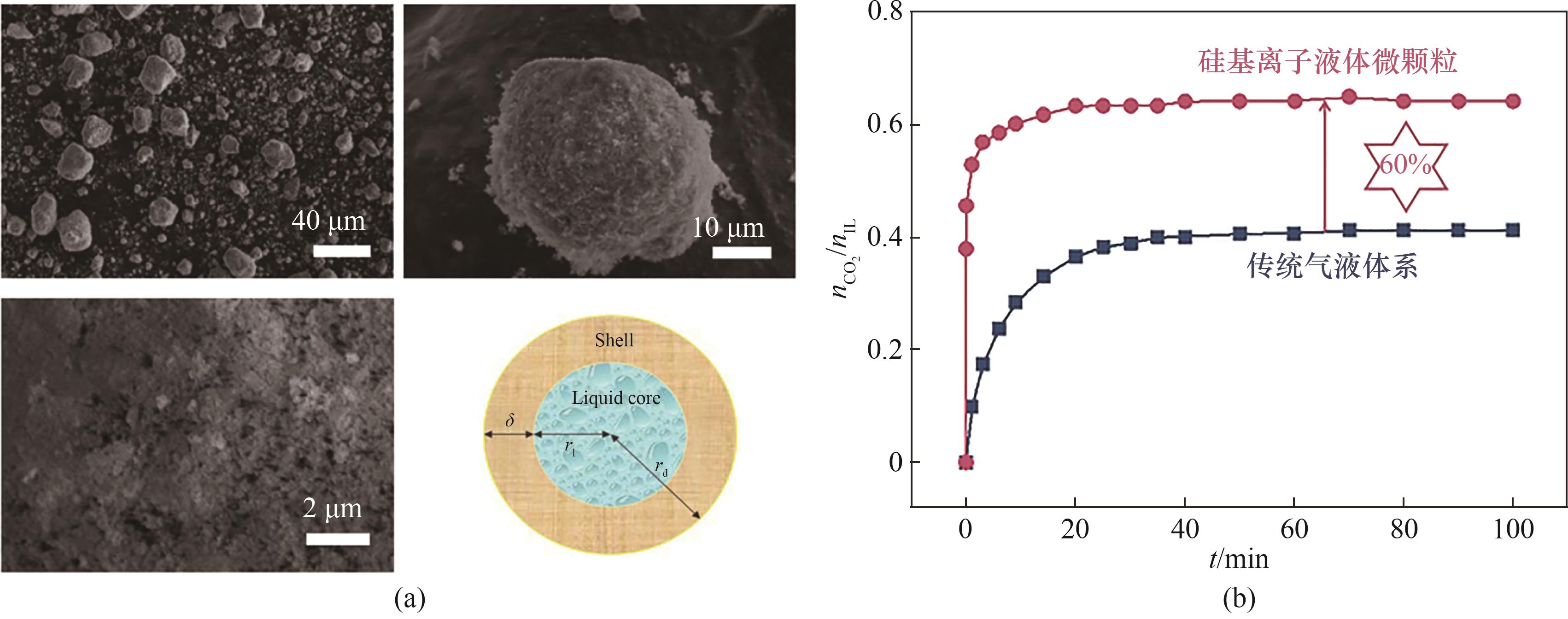
图5 [N1111][Gly]-EG硅基离子液体微颗粒形貌特征及其优异的捕集性能[48]
Fig.5 The morphological characteristics of [N1111][Gly]-EG silica-based microparticles and its capture rate[48]
| 吸收剂 | 吸收量/(mol/kg) | 吸收条件 | 文献 |
|---|---|---|---|
| [Bmim][HSO4] | 0.3 | CO2: 12 ml/min;25℃ | [ |
| [Bmim][HSO4]-SiO2 | 0.53 | ||
| [Bmim][BF4] | 0.24 | CO2: 12 ml/min;25℃ | [ |
| [Bmim][BF4]-SiO2 | 0.51 | ||
| [Bmim][PF6] | 0.09 | ||
| [Bmim][PF6]-SiO2 | 0.36 | ||
| [Bmim][TfO] | 0.28 | ||
| [Bmim][TfO]-SiO2 | 0.43 | ||
| [Bmim][Tf2N] | 0.12 | ||
| [Bmim][Tf2N]-SiO2 | 0.45 |
表1 硅基离子液体微颗粒与纯离子液体CO2吸收量的比较
Table1 Comparison of CO2 uptake between silica-based ionic liquid microparticles and pure ionic liquid
| 吸收剂 | 吸收量/(mol/kg) | 吸收条件 | 文献 |
|---|---|---|---|
| [Bmim][HSO4] | 0.3 | CO2: 12 ml/min;25℃ | [ |
| [Bmim][HSO4]-SiO2 | 0.53 | ||
| [Bmim][BF4] | 0.24 | CO2: 12 ml/min;25℃ | [ |
| [Bmim][BF4]-SiO2 | 0.51 | ||
| [Bmim][PF6] | 0.09 | ||
| [Bmim][PF6]-SiO2 | 0.36 | ||
| [Bmim][TfO] | 0.28 | ||
| [Bmim][TfO]-SiO2 | 0.43 | ||
| [Bmim][Tf2N] | 0.12 | ||
| [Bmim][Tf2N]-SiO2 | 0.45 |
| 反应底物 | 反应条件 | 转化率/%,转化时间/h | |
|---|---|---|---|
| 硅基[C1C6Im][HCO3]微颗粒 | [C1C6Im][HCO3]体系 | ||
| 氧化苯乙烯 | 60 ℃ 0.4 MPa | 96.08%,22.5 h | 96.08%,65 h |
| 环氧氯丙烷 | 97%,10 h | 97%,23.3 h | |
| 烯丙基缩水甘油醚 | 95%,14.5 h | 95%,31.2 h | |
| 环氧己烷 | 69%,26.5 h | 51%,26.5 h | |
| 环氧环己烷 | 9.1%,25 h | 4%,25 h | |
表2 不同底物参与环加成反应的动力学比较
Table 2 Comparison of the kinetics of different substrates involved in cycloaddition reactions
| 反应底物 | 反应条件 | 转化率/%,转化时间/h | |
|---|---|---|---|
| 硅基[C1C6Im][HCO3]微颗粒 | [C1C6Im][HCO3]体系 | ||
| 氧化苯乙烯 | 60 ℃ 0.4 MPa | 96.08%,22.5 h | 96.08%,65 h |
| 环氧氯丙烷 | 97%,10 h | 97%,23.3 h | |
| 烯丙基缩水甘油醚 | 95%,14.5 h | 95%,31.2 h | |
| 环氧己烷 | 69%,26.5 h | 51%,26.5 h | |
| 环氧环己烷 | 9.1%,25 h | 4%,25 h | |
| 反应底物 | 反应温度 | 转化率/%,转化时间/h,CO2分压 | |
|---|---|---|---|
| 硅基[C1C6Im][HCO3]微颗粒 | [C3C4Im][HCO3]-SiO2微颗粒 | ||
| 氧化苯乙烯 | 60℃ | 96.08%,22.5 h,0.4 MPa | 95%,12 h,0.3 MPa |
| 环氧氯丙烷 | 97%,10 h,0.4 MPa | 95.3%,2.5 h,0.3 MPa | |
| 烯丙基缩水甘油醚 | 95%,14.5 h,0.4 MPa | 93.4%,10.2 h,0.3 MPa | |
表3 环加成反应中硅基离子液体微颗粒的催化性能
Table 3 Catalytic performance of silica-based IL microparticles in cycloaddition reactions
| 反应底物 | 反应温度 | 转化率/%,转化时间/h,CO2分压 | |
|---|---|---|---|
| 硅基[C1C6Im][HCO3]微颗粒 | [C3C4Im][HCO3]-SiO2微颗粒 | ||
| 氧化苯乙烯 | 60℃ | 96.08%,22.5 h,0.4 MPa | 95%,12 h,0.3 MPa |
| 环氧氯丙烷 | 97%,10 h,0.4 MPa | 95.3%,2.5 h,0.3 MPa | |
| 烯丙基缩水甘油醚 | 95%,14.5 h,0.4 MPa | 93.4%,10.2 h,0.3 MPa | |
| 1 | Chiang Y C, Chiang P C, Huang C P. Effects of pore structure and temperature on VOC adsorption on activated carbon[J]. Carbon, 2001, 39(4): 523-534. |
| 2 | Jarraya I, Fourmentin S, Benzina M, et al. VOC adsorption on raw and modified clay materials[J]. Chemical Geology, 2010, 275(1): 1-8. |
| 3 | Kim K J, Ahn H G. The effect of pore structure of zeolite on the adsorption of VOCs and their desorption properties by microwave heating[J]. Microporous&Mesoporous Materials, 2012, 152(152): 78-83. |
| 4 | Li R, Xu J, Wang L J, et al. Reduction of VOC emissions by a membrane-based gas absorption process[J]. Journal of Environmental Sciences, 2009, 21(8): 1096-1102. |
| 5 | White C M, Strazisar B R, Granite E J, et al. Separation and capture of CO2 from large stationary sources and sequestration in geological formations—coalbeds and deep saline aquifers[J]. Journal of the Air & Waste Management Association, 2003, 53(6): 645-715. |
| 6 | Rao A B, Rubin E S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control[J]. Environmental Science & Technology, 2002, 36(20): 4467-4475. |
| 7 | Greer A J, Jacquemin J, Hardacre C J M. Industrial applications of ionic liquids[J]. Molecules, 2020, 25(21): 5207. |
| 8 | Pei Y C, Zhang Y X, Ma J, et al. Ionic liquids for advanced materials[J]. Materials Today Nano, 2022, 17: 100159. |
| 9 | Buettner C S, Cognigni A, Schröder C, et al. Surface-active ionic liquids: a review[J]. Journal of Molecular Liquids, 2022, 347: 118160. |
| 10 | Nordness O, Brennecke J F. Ion dissociation in ionic liquids and ionic liquid solutions[J]. Chemical reviews, 2020, 120(23): 12873-12902. |
| 11 | Cho C W, Pham T P T, Zhao Y, et al. Review of the toxic effects of ionic liquids[J]. Science of The Total Environment, 2021, 786: 147309. |
| 12 | Xiong W, Shi M, Peng L, et al. Low viscosity superbase protic ionic liquids for the highly efficient simultaneous removal of H2S and CO2 from CH4 [J]. Separation and Purification Technology, 2021, 263: 118417. |
| 13 | Amith W D, Araque J C, Margulis C J. A pictorial view of viscosity in ionic liquids and the link to nanostructural heterogeneity[J]. The Journal of Physical Chemistry Letters, 2020, 11(6): 2062-2066. |
| 14 | Bates E D, Mayton R D, Ntai I, et al. CO2 capture by a task-specific ionic liquid[J]. Journal of the American Chemical Society, 2002, 124(6): 926-927. |
| 15 | Yu G R, Zhang S J, Yao X Q, et al. Design of task-specific ionic liquids for capturing CO2: a molecular orbital study[J]. Industrial & Engineering Chemistry Research, 2006, 45(8): 2875-2880. |
| 16 | Cui G K, Liu J X, Lyu S Z, et al. Efficient and reversible SO2 absorption by environmentally friendly task-specific deep eutectic solvents of PPZBr+Gly[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(16): 14236-14246. |
| 17 | Zhang N, Huang Z H, Zhang H M, et al. Highly efficient and reversible CO2 capture by task-specific deep eutectic solvents[J]. Industrial & Engineering Chemistry Research, 2019, 58(29): 13321-13329. |
| 18 | Shi M Z, Xiong W K, Zhang X M, et al. Highly efficient and selective H2S capture by task-specific deep eutectic solvents through chemical dual-site absorption[J]. Separation and Purification Technology, 2022, 283: 120167. |
| 19 | Tamilarasan P, Ramaprabhu S. Task-specific functionalization of graphene for use as a cathode catalyst support for carbon dioxide conversion[J]. Journal of Materials Chemistry A, 2015, 3(2): 797-804. |
| 20 | Chen F F, Huang K, Fan J P, et al. Chemical solvent in chemical solvent: a class of hybrid materials for effective capture of CO2 [J]. AIChE Journal, 2018, 64(2): 632-639. |
| 21 | Chen M S, Li M J, Zhang L, et al. Intensification of amino acid ionic liquids with different additives for CO2 capture[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(37): 12082-12089. |
| 22 | Li A L, Tian Z Q, Yan T Y, et al. Anion-functionalized task-specific ionic liquids: molecular origin of change in viscosity upon CO2 capture[J]. The Journal of Physical Chemistry B, 2014, 118(51): 14880-14887. |
| 23 | Chen Y, Mutelet F, Jaubert J-N. Modeling the solubility of carbon dioxide in imidazolium-based ionic liquids with the PC-SAFT equation of state[J]. The Journal of Physical Chemistry B, 2012, 116(49): 14375-14388. |
| 24 | Zhang X, Bao D, Huang Y, et al. Gas-liquid mass-transfer properties in CO2 absorption system with ionic liquids[J]. AIChE Journal, 2014, 60(8): 2929-2939. |
| 25 | Xu F, Gao H, Dong H, et al. Solubility of CO2 in aqueous mixtures of monoethanolamine and dicyanamide-based ionic liquids[J]. Fluid Phase Equilibria, 2014, 365: 80-87. |
| 26 | Binks B P, Murakami R. Phase inversion of particle-stabilized materials from foams to dry water[J]. Nature Materials, 2006, 5(11): 865-869. |
| 27 | Hasenzahl S, Gray A, Walzer E, et al. Dry water for the skin[J]. SÖFW-Journal, 2005, 131(3): 2-8. |
| 28 | Shirato K, Satoh M. "Dry ionic liquid" as a newcomer to "dry matter"[J]. Soft Matter, 2011, 7(16): 7191-7193. |
| 29 | Saleh K, Forny L, Guigon P, et al. Dry water: from physico-chemical aspects to process-related parameters[J]. Chemical Engineering Research and Design, 2011, 89(5): 537-544. |
| 30 | Ding A, Yang L, Fan S, et al. Reversible methane storage in porous hydrogel supported clathrates[J]. Chemical Engineering Science, 2013, 96: 124-130. |
| 31 | Shi B H, Fan S S, Lou X. Application of the shrinking-core model to the kinetics of repeated formation of methane hydrates in a system of mixed dry-water and porous hydrogel particulates[J]. Chemical Engineering Science, 2014, 109: 315-325. |
| 32 | Li C H, Gao K X, Meng Y N, et al. Solution thermodynamics of imidazolium-based ionic liquids and volatile organic compounds: benzene and acetone[J]. Journal of Chemical & Engineering Data, 2015, 60(6): 1600-1607. |
| 33 | Bedia J, Ruiz E, de Riva J, et al. Optimized ionic liquids for toluene absorption[J]. AIChE Journal, 2013, 59(5): 1648-1656. |
| 34 | Wang W, Ma X, Grimes S, et al. Study on the absorbability, regeneration characteristics and thermal stability of ionic liquids for VOCs removal[J]. Chemical Engineering Journal, 2017, 328: 353-359. |
| 35 | Salar-García M, Ortiz-Martínez V, Hernández-Fernández F, et al. Ionic liquid technology to recover volatile organic compounds(VOCs)[J]. Journal of Hazardous Materials, 2017, 321: 484-499. |
| 36 | Hirota Y, Maeda Y, Yamamoto Y, et al. Organosilica membrane with ionic liquid properties for separation of toluene/H2 mixture[J]. Materials, 2017, 10(8): 901. |
| 37 | Zhou M, Li S, Chai S, et al. Hydrophobically modified mesoporous silica supported Pt as a dual-function adsorbent buffer-catalyst for toluene removal under low-temperature[J]. New Journal of Chemistry, 2023, 47: 1767-1776. |
| 38 | Karimi B, Khorasani M, Bakhshandeh R F, et al. Tungstate supported on periodic mesoporous organosilica with imidazolium framework as an efficient and recyclable catalyst for the selective oxidation of sulfides[J]. ChemPlusChem, 2015, 80(6): 990-999. |
| 39 | Rostamnia S, Doustkhah E, Bulgar R, et al. Supported palladium ions inside periodic mesoporous organosilica with ionic liquid framework(Pd@IL-PMO) as an efficient green catalyst for S-arylation coupling[J]. Microporous and Mesoporous Materials, 2016, 225: 272-279. |
| 40 | Li X Y, Liu X M, Yu Y S, et al. Preparation and characterization of nanometre silicon-based ionic liquid micro-particle materials[J]. Journal of Molecular Liquids, 2020, 311: 113327. |
| 41 | 曲江源, 齐娜娜, 关彦军, 等. 湿法烟气脱硫塔内传递与化学反应过程CFD模拟[J]. 化工学报, 2019, 70(6): 2117-2128. |
| Qu J Y, Qi N N, Guan Y J, et al. CFD simulation of transfer and chemical reaction process in wet flue gas desulfurization tower[J]. CIESC Journal, 2019, 70(6): 2117-2128. | |
| 42 | 张志炳, 田洪舟, 张锋, 等. 多相反应体系的微界面强化简述[J]. 化工学报, 2018, 69(1): 44-49. |
| Zhang Z B, Tian H Z, Zhang F, et al. Overview of microinterface intensification in multiphase reaction systems[J]. CIESC Journal, 2018, 69(1): 44-49. | |
| 43 | Romanos G E, Schulz P S, Bahlmann M, et al. CO2 capture by novel supported ionic liquid phase systems consisting of silica nanoparticles encapsulating amine-functionalized ionic liquids[J]. The Journal of Physical Chemistry C, 2014, 118(42): 24437-24451. |
| 44 | Mirzaei M, Mokhtarani B, Badiei A, et al. Improving physical adsorption of CO2 by ionic liquids-loaded mesoporous silica[J]. Chemical Engineering & Technology, 2018, 41(7): 1272-1281. |
| 45 | Zheng S, Zeng S J, Li G L, et al. Superior selective adsorption of trace CO2 induced by chemical interaction and created ultra-micropores of ionic liquid composites[J]. Chemical Engineering Journal, 2023, 451: 138736. |
| 46 | Pohako-Esko K, Bahlmann M, Schulz P S, et al. Chitosan containing supported ionic liquid phase materials for CO2 absorption[J]. Industrial & Engineering Chemistry Research, 2016, 55(25): 7052-7059. |
| 47 | Duczinski R, Polesso B B, Bernard F L, et al. Enhancement of CO2/N2 selectivity and CO2 uptake by tuning concentration and chemical structure of imidazolium-based ILs immobilized in mesoporous silica[J]. Journal of Environmental Chemical Engineering, 2020, 8(3): 103740. |
| 48 | Chen M, Wang X, Liu X, et al. Anhydrous "dry ionic liquids": a promising absorbent for CO2 capture[J]. Journal of Molecular Liquids, 2020, 305: 112810. |
| 49 | Mirzaei M, Badiei A R, Mokhtarani B, et al. Experimental study on CO2 sorption capacity of the neat and porous silica supported ionic liquids and the effect of water content of flue gas[J]. Journal of Molecular Liquids, 2017, 232: 462-470. |
| 50 | Zhai Q G, Xie S J, Fan W Q, et al. Photocatalytic conversion of carbon dioxide with water into methane: platinum and copper(Ⅰ) oxide co-catalysts with a core-shell structure[J]. Angewandte Chemie International Edition, 2013, 52(22): 5776-5779. |
| 51 | Kuhl K P, Hatsukade T, Cave E R, et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces[J]. Journal of the American Chemical Society, 2014, 136(40): 14107-14113. |
| 52 | Bae K L, Kim J, Lim C K, et al. Colloidal zinc oxide-copper(Ⅰ) oxide nanocatalysts for selective aqueous photocatalytic carbon dioxide conversion into methane[J]. Nature Communications, 2017, 8(1): 1156. |
| 53 | Obert R, Dave B C. Enzymatic conversion of carbon dioxide to methanol: enhanced methanol production in silica sol-gel matrices[J]. Journal of the American Chemical Society, 1999, 121(51): 12192-12193. |
| 54 | Riduan S N, Zhang Y, Ying J Y. Conversion of carbon dioxide into methanol with silanes over N-heterocyclic carbene catalysts[J]. Angewandte Chemie International Edition, 2009, 48(18): 3322-3325. |
| 55 | Kuk S K, Singh R K, Nam D H, et al. Photoelectrochemical reduction of carbon dioxide to methanol through a highly efficient enzyme cascade[J]. Angewandte Chemie International Edition, 2017, 56(14): 3827-3832. |
| 56 | Maeda C, Taniguchi T, Ogawa K, et al. Bifunctional catalysts based on m-phenylene-bridged porphyrin dimer and trimer platforms: synthesis of cyclic carbonates from carbon dioxide and epoxides[J]. Angewandte Chemie International Edition, 2015, 54(1): 134-138. |
| 57 | Kathalikkattil A C, Babu R, Tharun J, et al. Advancements in the conversion of carbon dioxide to cyclic carbonates using metal organic frameworks as catalysts[J]. Catalysis Surveys from Asia, 2015, 19(4): 223-235. |
| 58 | Wu X, North M. A Bimetallic aluminium(salphen) complex for the synthesis of cyclic carbonates from epoxides and carbon dioxide[J]. ChemSusChem, 2017, 10(1): 74-78. |
| 59 | Sakakura T, Saito Y, Okano M, et al. Selective conversion of carbon dioxide to dimethyl carbonate by molecular catalysis[J]. The Journal of Organic Chemistry, 1998, 63(20): 7095-7096. |
| 60 | Tamboli A H, Chaugule A A, Kim H. Catalytic developments in the direct dimethyl carbonate synthesis from carbon dioxide and methanol[J]. Chemical Engineering Journal, 2017, 323: 530-544. |
| 61 | Zhao T, Hu X, Wu D, et al. Direct synthesis of dimethyl carbonate from carbon dioxide and methanol at room temperature using imidazolium hydrogen carbonate ionic liquid as a recyclable catalyst and dehydrant[J]. ChemSusChem, 2017, 10(9): 2046-2052. |
| 62 | Liu A H, Yu B, He L N. Catalytic conversion of carbon dioxide to carboxylic acid derivatives[J]. Greenhouse Gases: Science and Technology, 2015, 5(1): 17-33. |
| 63 | Banerjee A, Dick G R, Yoshino T, et al. Carbon dioxide utilization via carbonate-promoted C—H carboxylation[J]. Nature, 2016, 531(7593): 215-219. |
| 64 | Schaefer A, Saak W, Haase D, et al. Silyl cation mediated conversion of CO2 into benzoic acid, formic acid, and methanol[J]. Angewandte Chemie-International Edition, 2012, 51(12): 2981-2984. |
| 65 | Yu B, Zhao Y F, Zhang H Y, et al. Pd/C-catalyzed direct formylation of aromatic iodides to aryl aldehydes using carbon dioxide as a C1 resource[J]. Chemical Communications, 2014, 50(18): 2330-2333. |
| 66 | He X, Li X Y, Song Y, et al. Synthesis of urea derivatives using carbon dioxide as carbonylation reagent in ionic liquids[J]. Current Organocatalysis, 2017, 4(2): 112-121. |
| 67 | Kember M R, Williams C K. Efficient magnesium catalysts for the copolymerization of epoxides and CO2; Using water to synthesize polycarbonate polyols[J]. Journal of the American Chemical Society, 2012, 134(38): 15676-15679. |
| 68 | Chapman A M, Keyworth C, Kember M R, et al. Adding value to power station captured CO2: tolerant Zn and Mg homogeneous catalysts for polycarbonate polyol production[J]. ACS Catalysis, 2015, 5(3): 1581-1588. |
| 69 | Xu B H, Wang J Q, Sun J, et al. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: a multi-scale approach[J]. Green Chemistry, 2015, 17(1): 108-122. |
| 70 | Kawanami H, Ikushima Y. Chemical fixation of carbon dioxide to styrene carbonate under supercritical conditions with DMF in the absence of any additional catalysts[J]. Chemical Communications, 2000(21): 2089-2090. |
| 71 | Decortes A, Belmonte M M, Benet-Buchholz J, et al. Efficient carbonate synthesis under mild conditions through cycloaddition of carbon dioxide to oxiranes using a Zn(salphen) catalyst[J]. Chemical Communications, 2010, 46(25): 4580-4582. |
| 72 | Fevre M, Pinaud J, Leteneur A, et al. Imidazol(in)ium hydrogen carbonates as a genuine source of N-heterocyclic carbenes(NHCs): applications to the facile preparation of NHC metal complexes and to NHC-organocatalyzed molecular and macromolecular syntheses[J]. Journal of the American Chemical Society, 2012, 134(15): 6776-6784. |
| 73 | 彭家建, 邓友全. 室温离子液体催化合成碳酸丙烯酯[J]. 催化学报, 2001, 22(6): 598-600. |
| Peng J J, Deng Y Q. Formation of propylene carbonate catalyzed by room temperature ionic liquids[J]. Chinese Journal of Catalysis, 2001, 22(6): 598-600. | |
| 74 | Sun J, Fujita S I, Zhao F, et al. Synthesis of styrene carbonate from styrene oxide and carbon dioxide in the presence of zinc bromide and ionic liquid under mild conditions[J]. Green Chemistry, 2004, 6(12): 613-616. |
| 75 | Wang J Q, Dong K, Cheng W G, et al. Insights into quaternary ammonium salts-catalyzed fixation carbon dioxide with epoxides[J]. Catalysis Science & Technology, 2012, 2(7): 1480-1484. |
| 76 | Zhou H, Zhang W Z, Liu C H, et al. CO2 adducts of N-heterocyclic carbenes: thermal stability and catalytic activity toward the coupling of CO2 with epoxides[J]. The Journal of Organic Chemistry, 2008, 73(20): 8039-8044. |
| 77 | Liu J, Yang G, Liu Y, et al. Efficient conversion of CO2 into cyclic carbonates at room temperature catalyzed by Al-salen and imidazolium hydrogen carbonate ionic liquids[J]. Green Chemistry,2020, 22(14): 4509-4515. |
| 78 | Li X, Wang Y, Liu X, et al. Micro interfacial effect: the stability of catalytic activity under gas-liquid reaction pressure changes[J]. Chemical Engineering Journal, 2022, 440: 135560. |
| 79 | Wang B R, Yang G Q, Tian H Z, et al. A new model of bubble Sauter mean diameter in fine bubble-dominated columns[J]. Chemical Engineering Journal, 2020, 393: 124673. |
| 80 | Qian H, Tian H, Yang G, et al. Microinterface intensification in hydrogenation and air oxidation processes[J]. Chinese Journal of Chemical Engineering, 2022, 50: 292-300. |
| 81 | Tian H Z, Pi S F, Feng Y C, et al. One-dimensional drift-flux model of gas holdup in fine-bubble jet reactor[J]. Chemical Engineering Journal, 2020, 386: 121222. |
| [1] | 苏伟, 马东旭, 金旭, 刘忠彦, 张小松. 表面润湿性对霜层传递特性影响可视化实验研究[J]. 化工学报, 2023, 74(S1): 122-131. |
| [2] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [3] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [4] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [5] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [6] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [7] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [8] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [9] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [10] | 王玉兵, 李杰, 詹宏波, 朱光亚, 张大林. R134a在菱形离散肋微小通道内的流动沸腾换热实验研究[J]. 化工学报, 2023, 74(9): 3797-3806. |
| [11] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [12] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [13] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [14] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [15] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号