化工学报 ›› 2024, Vol. 75 ›› Issue (4): 1317-1332.DOI: 10.11949/0438-1157.20231293
收稿日期:2023-12-04
修回日期:2024-02-07
出版日期:2024-04-25
发布日期:2024-06-06
通讯作者:
邢蕊蕊,闫学海
作者简介:常蕊(1994—),女,博士后,changrui@ipe.ac.cn
基金资助:
Rui CHANG1( ), Ruirui XING1,2(
), Ruirui XING1,2( ), Xuehai YAN1,2(
), Xuehai YAN1,2( )
)
Received:2023-12-04
Revised:2024-02-07
Online:2024-04-25
Published:2024-06-06
Contact:
Ruirui XING, Xuehai YAN
摘要:
作为一种生物分子构建单元,肽具有生物相容性、可降解性、多功能性和序列可调性等特性。利用肽分子的非共价化学,可以实现具有多尺度结构的新型材料的可控构建,为开发生态友好、生物可降解和生物再利用的绿色生物可循环材料提供了新策略。然而,如何利用肽非共价化学策略进行单分子设计,并实现绿色生物可循环材料的可控构建和功能化应用,面临着重要挑战。本文从肽分子间的弱相互作用力协同角度,介绍肽非共价化学的策略,并详细阐述实现绿色生物可循环肽材料可控构建的单分子设计策略。最后,对基于非共价化学的绿色生物可循环肽材料在仿生光合成和光催化、药物递送和疾病诊疗以及可加工材料等领域的应用进行综述。
中图分类号:
常蕊, 邢蕊蕊, 闫学海. 基于非共价化学的绿色生物可循环肽材料[J]. 化工学报, 2024, 75(4): 1317-1332.
Rui CHANG, Ruirui XING, Xuehai YAN. Green and biorecyclable materials based on peptide noncovalent chemistry[J]. CIESC Journal, 2024, 75(4): 1317-1332.
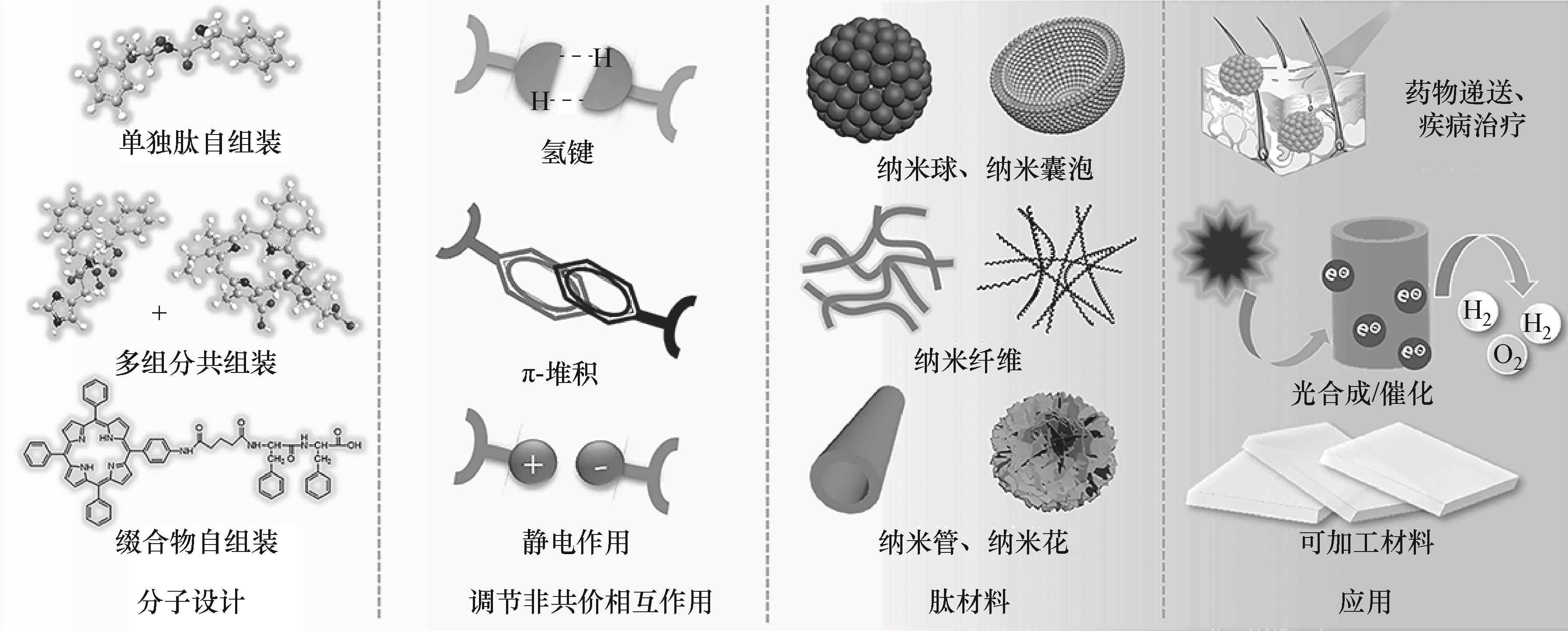
图1 基于肽非共价化学的从单分子设计到肽材料的可控构建及应用
Fig.1 Controlled construction and application of peptide materials from single molecule design based on peptide noncovalent chemistry
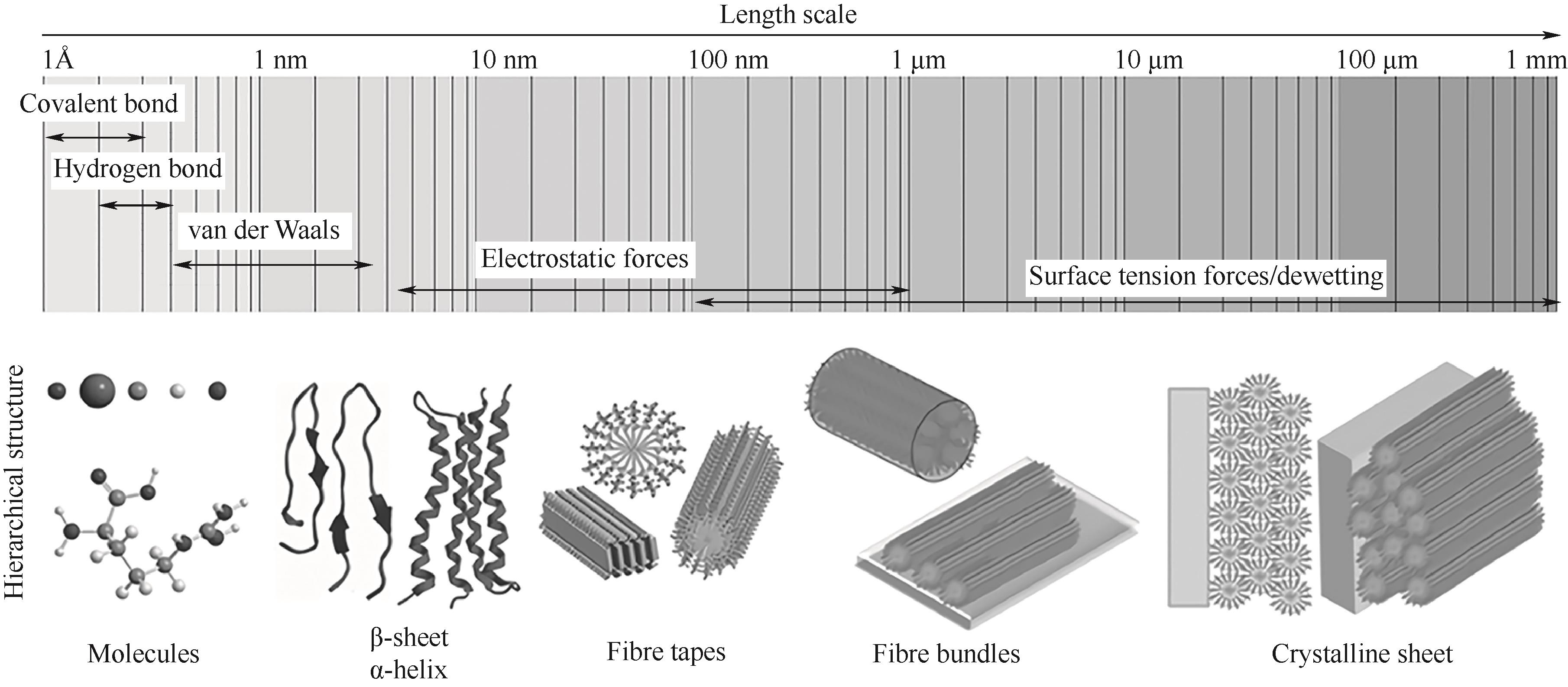
图2 操纵肽的非共价相互作用促进多尺度结构的形成[21](1 Å =0.1 nm)
Fig.2 Manipulating noncovalent interactions of peptides to promote the formation of multiscale structures[21]
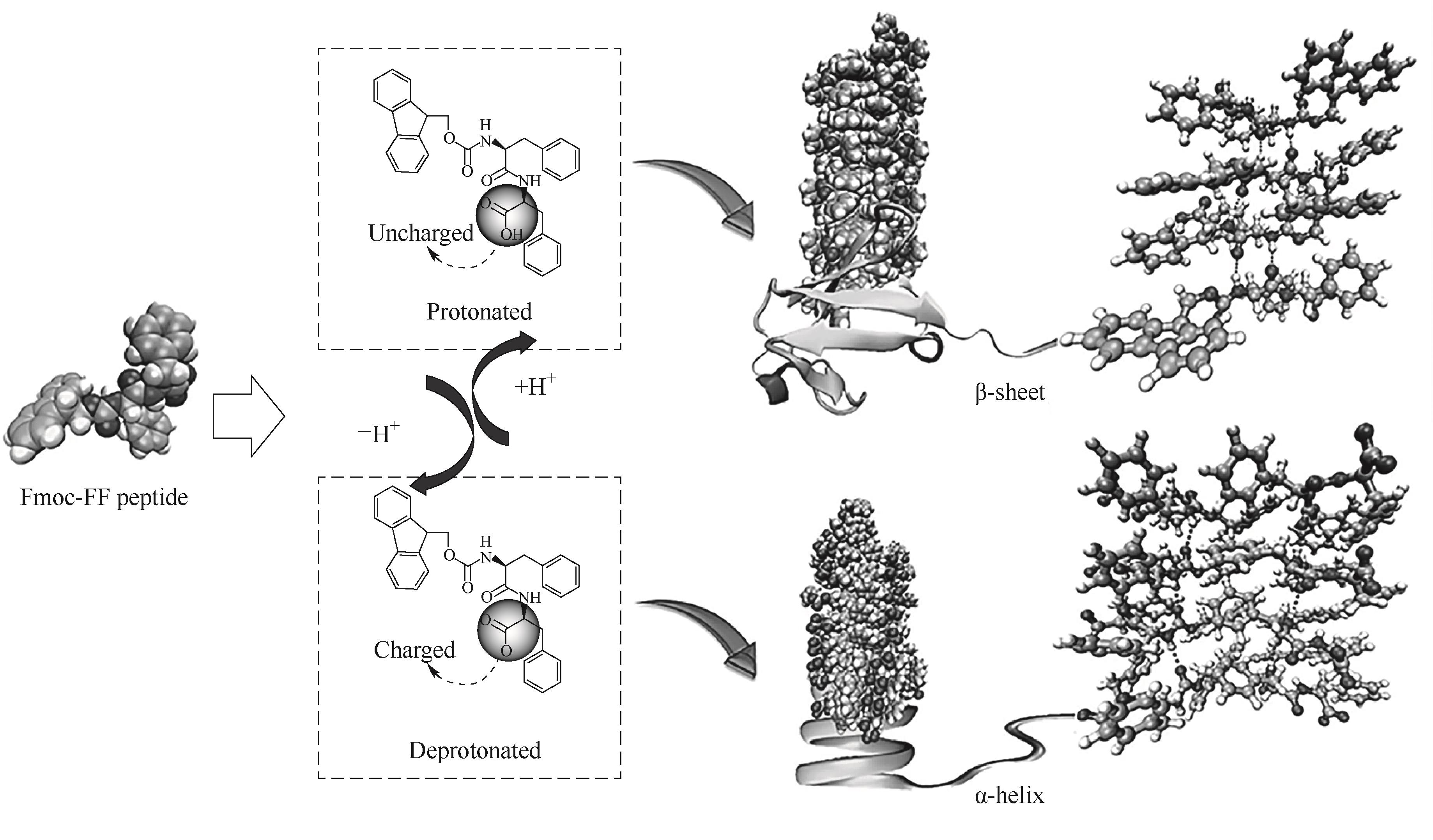
图3 Fmoc-FF自组装形成的β-折叠纳米纤维向α-螺旋纳米纤维转变的机理图[24]
Fig.3 Mechanism diagram of the transition from β-sheet nanofibers to α-helix nanofibers formed by Fmoc-FF self-assembly[24]
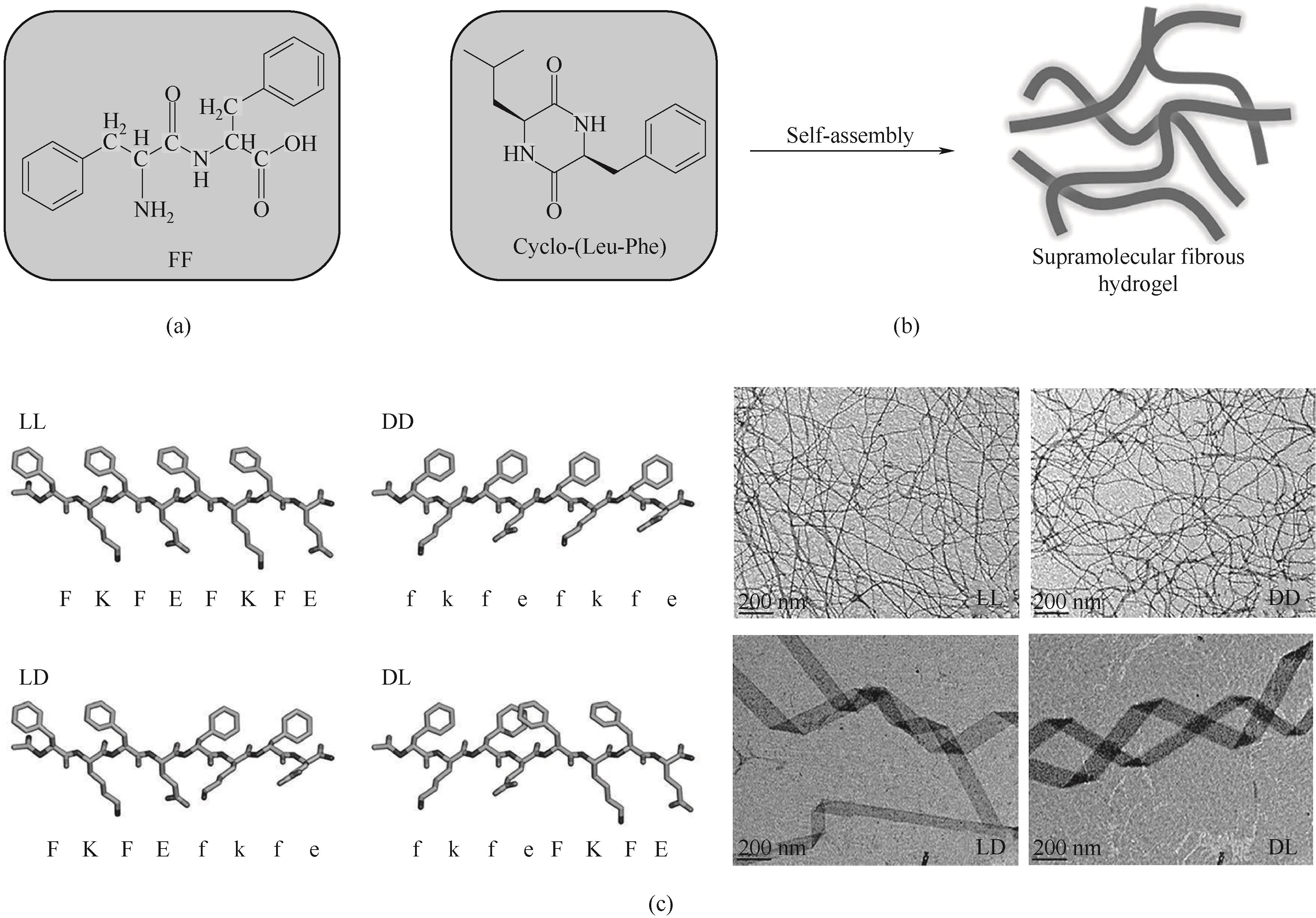
图4 (a)FF的分子结构;(b)Cyclo-(Leu-Phe)自组装形成超分子纤维状水凝胶示意图[35];(c)两亲肽分子KFE8的嵌段异手性类似物及形成组装体的负染透射电子显微镜(TEM)图像[36]
Fig.4 (a) Molecular structure of FF; (b) Schematic diagram of supramolecular fibrous hydrogel formed by self-assembly of Cyclo-(Leu-Phe)[35]; (c) Negative-stain transmission electron microscopy (TEM) images of block heterochiral analogs of the amphipathic peptide KFE8 and the formation of assemblies[36]
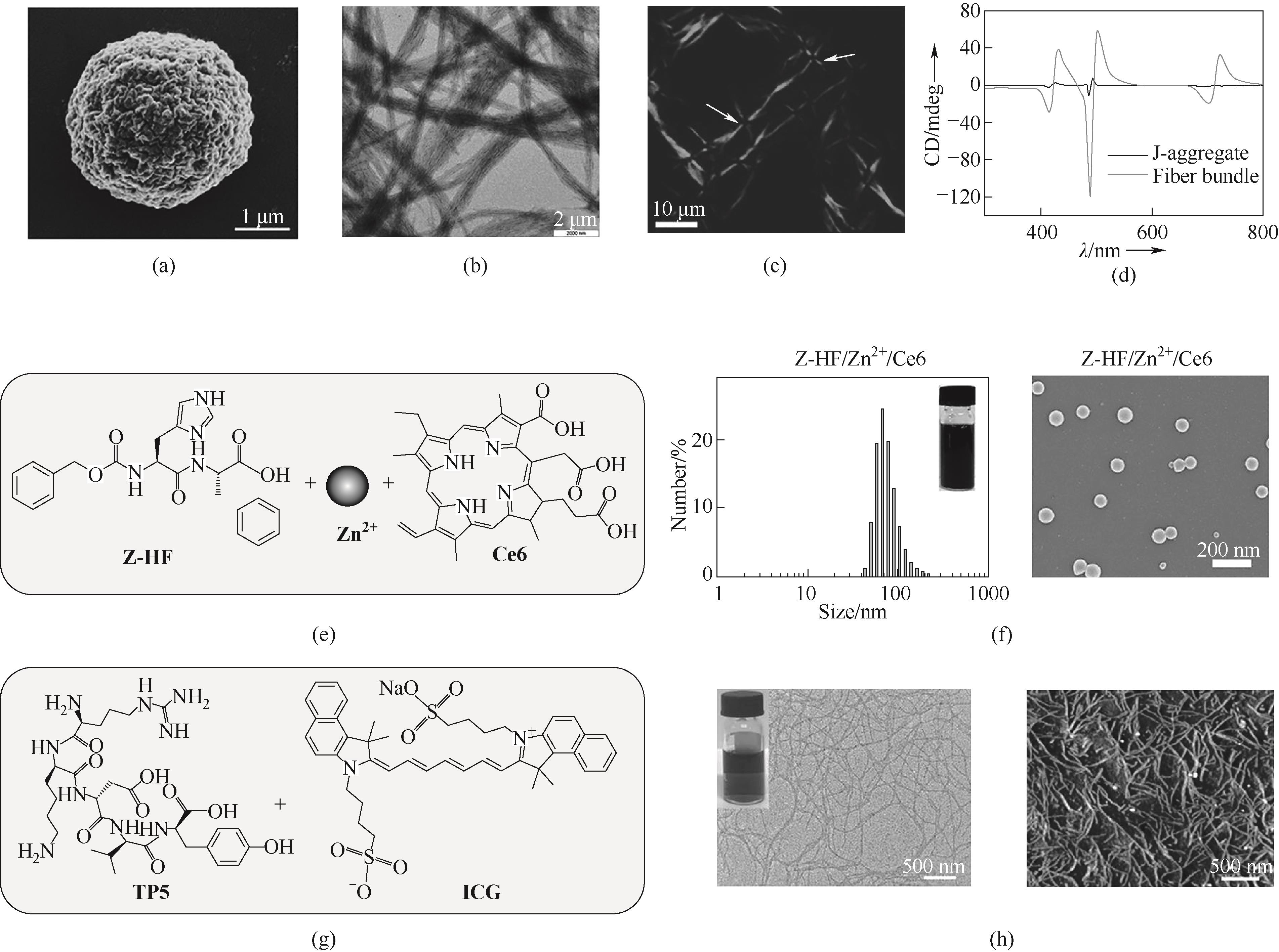
图5 (a)微球结构的扫描电子显微镜(SEM)图[39];(b)纤维束的TEM图[40];(c)交叉偏振器下正交光纤维束交叉点处各向异性光致发光的消光(如箭头所示)[40];(d)纤维束和J聚集体的圆二色谱[40];(e)Z-HF、Zn2+和Ce6分子结构;(f)Z-HF/Zn2+/Ce6纳米颗粒的粒径和SEM图[42];(g)TP5和ICG的分子结构;(h)纳米纤维的TEM图和原子力显微镜(AFM)图[43]
Fig.5 (a) Scanning electron microscopy (SEM) image of microsphere structure[39]; (b) TEM image of fiber bundles[40]; (c) Extinction of anisotropic photoluminescence (as denoted by arrows) at the cross-points of orthogonal fiber bundles under crossed polarizers[40]; (d) Circular dichroism of fiber bundles and J-aggregates[40]; (e) Molecular structure of Z-HF, Zn2+, and Ce6; (f) Particle size and SEM image of Z-HF/Zn2+/Ce6 nanoparticles[42]; (g) Molecular structure of TP5 and ICG; (h) TEM image and atomic force microscopy (AFM) image of nanofibers[43]
| 策略名称 | 构筑基元 | 优势 | 不足 | 应用范围 |
|---|---|---|---|---|
| 特定肽序列的自组装 | 肽序列 | 简单、易操作 | 受限于自身具备的性质及结构 赋予的性质 | 疾病相关蛋白纤维化的机理研究、生物光学和光电设备、3D组织培养 |
| 其他分子调控肽自组装 | 其他分子、肽序列 | 增加新功能和 结构 | 易泄漏、装封率不可控 | 光催化、成像、光动力/热治疗、免疫 治疗 |
| 其他分子与肽生成缀合物的自组装 | 其他分子-肽缀合物 | 增加稳定性、可控装封率 | 缀合物需分离提纯,或需引用 连接剂,过程复杂 | 光动力/热治疗、成像、载药、化疗 |
表1 基于非共价化学设计肽材料的三种策略的优缺点及应用范围
Table 1 Advantages, disadvantages, and application scope of three strategies for designing peptide materials based on noncovalent chemistry
| 策略名称 | 构筑基元 | 优势 | 不足 | 应用范围 |
|---|---|---|---|---|
| 特定肽序列的自组装 | 肽序列 | 简单、易操作 | 受限于自身具备的性质及结构 赋予的性质 | 疾病相关蛋白纤维化的机理研究、生物光学和光电设备、3D组织培养 |
| 其他分子调控肽自组装 | 其他分子、肽序列 | 增加新功能和 结构 | 易泄漏、装封率不可控 | 光催化、成像、光动力/热治疗、免疫 治疗 |
| 其他分子与肽生成缀合物的自组装 | 其他分子-肽缀合物 | 增加稳定性、可控装封率 | 缀合物需分离提纯,或需引用 连接剂,过程复杂 | 光动力/热治疗、成像、载药、化疗 |
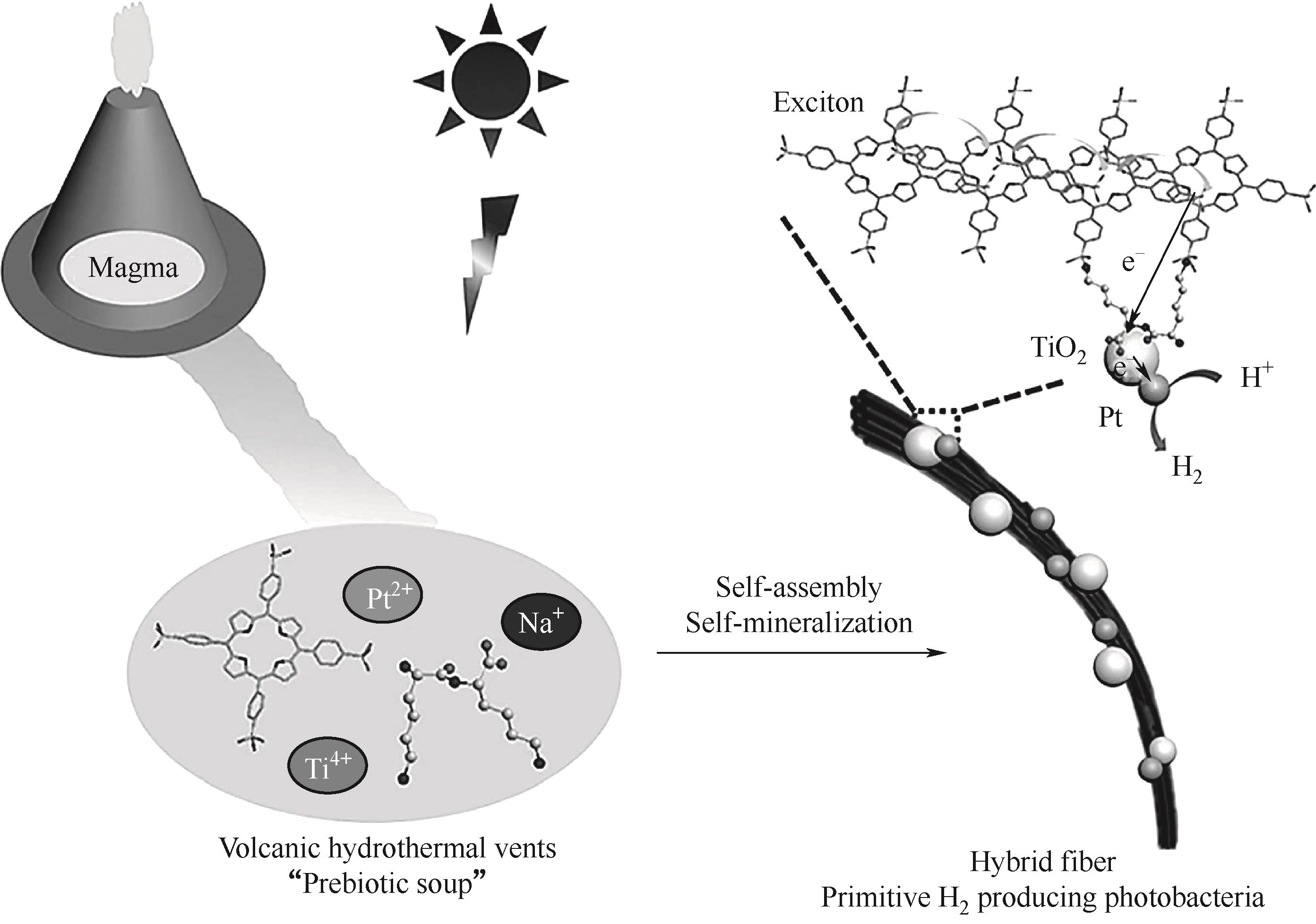
图7 集光分子卟啉和肽在“益生元汤”中的分子演变示意图[55]
Fig.7 Schematic diagram of molecular evolution of light harvesting molecular porphyrin and peptides in “prebiotic soup”[55]
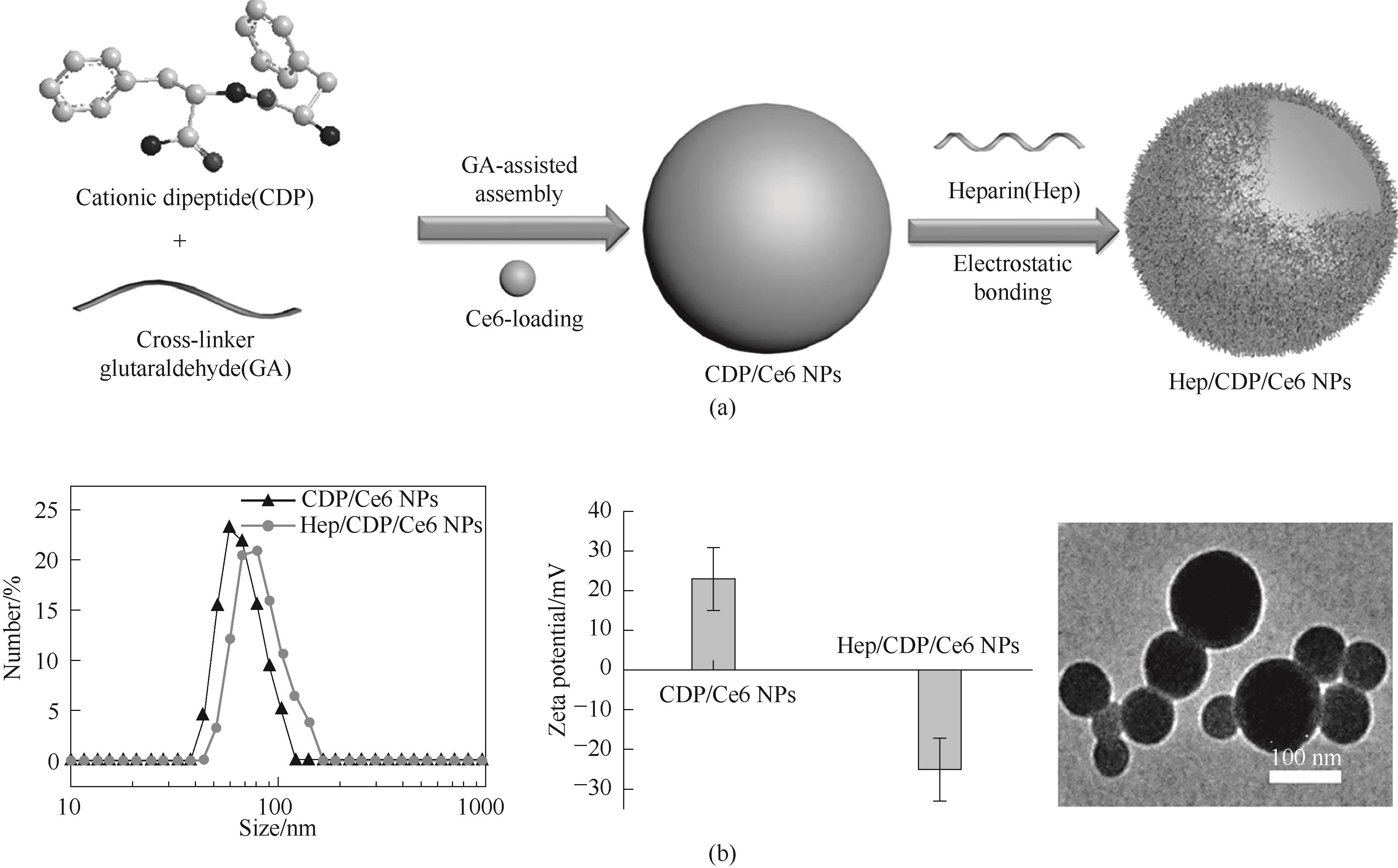
图8 (a)戊二醛(GA)辅助CDP交联自组装过程示意图;(b)肽基纳米球的粒径、电位和TEM图[61]
Fig.8 (a) Schematic diagram of glutaraldehyde (GA)-assisted CDP cross-linking self-assembly process; (b) Particle size, potential, and TEM image of peptide based nanospheres[61]
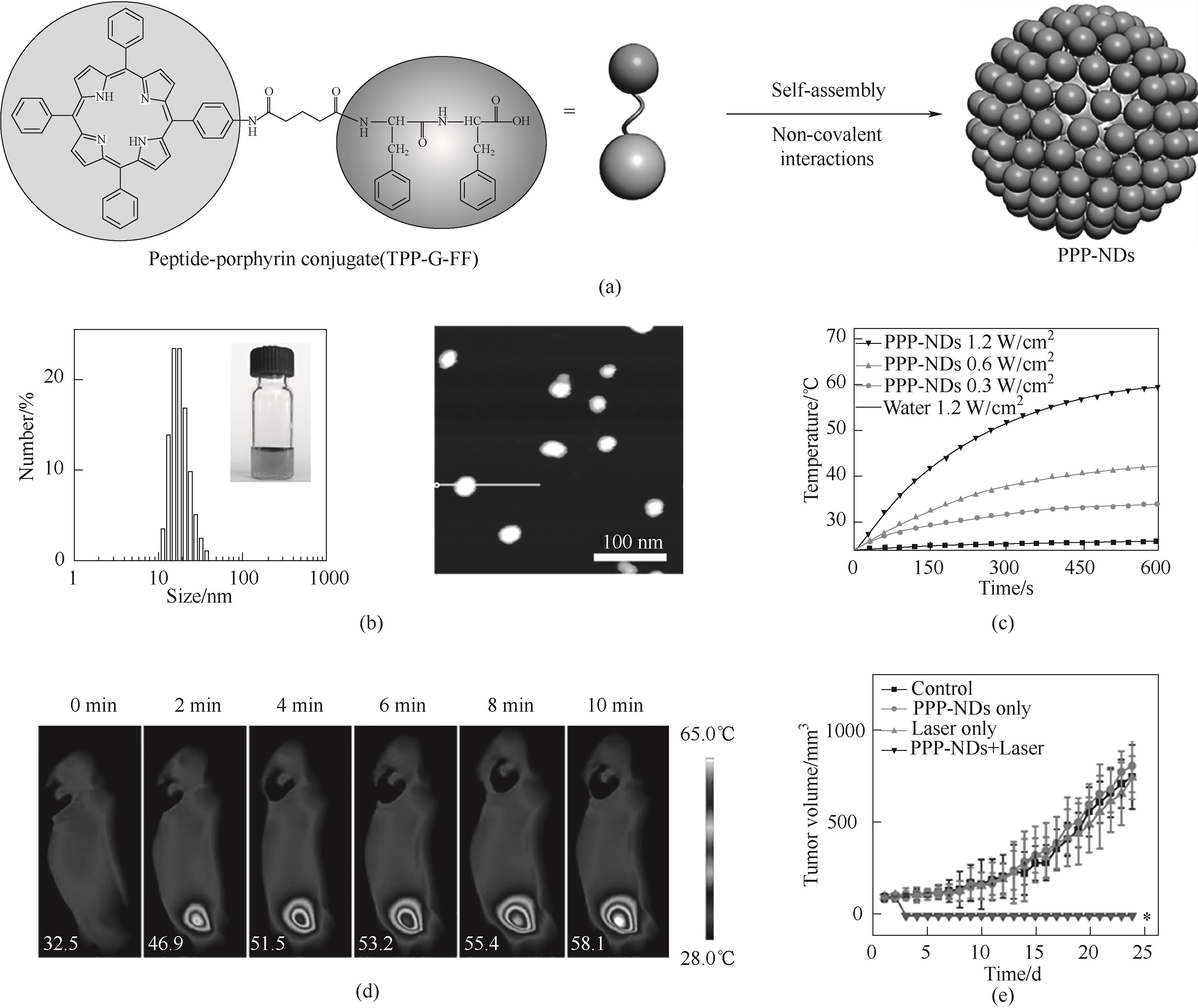
图9 (a)TPP-G-FF自组装形成纳米点示意图;(b)纳米点的粒径和AFM图;(c)纳米点在不同激光功率照射下的温升曲线;(d)激光连续照射10 min内小鼠肿瘤部位的红外热成像图;(e)不同组小鼠的肿瘤体积变化[46]
Fig.9 (a) Schematic diagram of TPP-G-FF self-assembled into nanodots; (b) Particle size and AFM image of nanodots; (c) Temperature elevation curves of nanodots under different laser power irradiation; (d) Infrared thermal images of mouse tumor sites within 10 min of continuous laser irradiation; (e) Changes in tumor volume in different groups of mice[46]
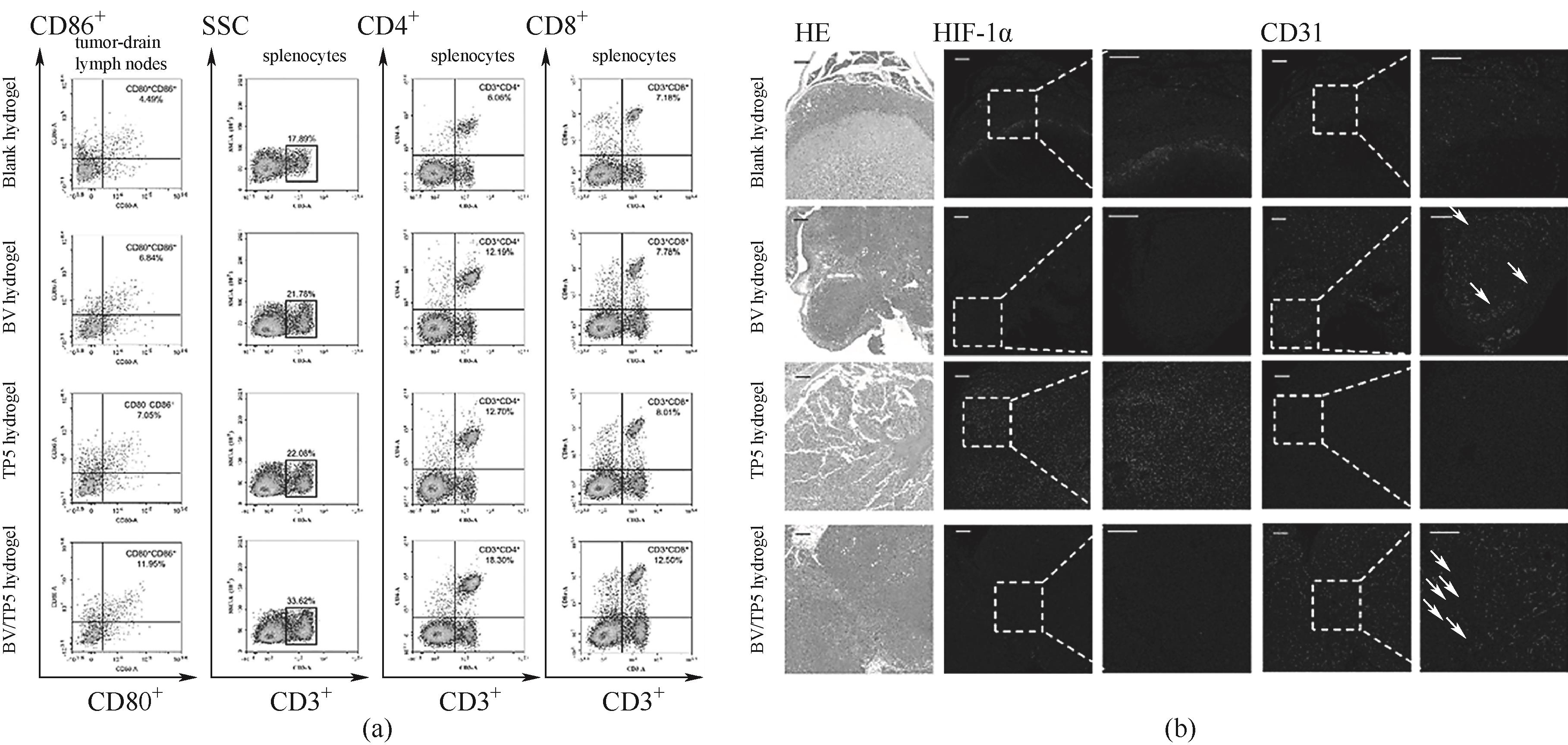
图10 (a)各治疗组肿瘤引流淋巴结成熟DCs (CD11c+CD80+CD86+)的比例及小鼠脾细胞中CD3+CD45+、CD3+CD4+、CD3+CD8+T细胞百分比的代表性流式细胞术图;(b)小鼠肿瘤中HIF-1α和CD31阳性区域的代表性H&E和IF染色图[62]
Fig.10 (a) Representative flow cytometry images of the ratio of mature DCs (CD11c+CD80+CD86+) in tumor-draining lymph nodes and the percentage of CD3+CD45+, CD3+CD4+, and CD3+CD8+T cells in mouse spleen cells in each treatment group; (b) Representative H&E and IF staining images of HIF-1α and CD31 positive areas in mouse tumor[62]

图11 (a)通过加热熔融和淬火冷却的方法制造生物玻璃示意图;(b)原位XRD谱图和原位拉曼光谱作为温度的函数;(c)3D打印机的示意图和印刷玻璃结构的照片;(d)玻璃珠在堆肥土壤中的降解图片;(e)玻璃珠在小鼠皮肤的解剖照片[75]
Fig.11 (a) Schematic diagram of manufacturing bioglass through heating, melting, and quenching and cooling methods; (b) In situ XRD and in situ Raman spectra as a function of temperature; (c) Schematic diagram of 3D printer and photos of printed glass architecture; (d) Image of degradation of glass beads in composting soil; (e) Anatomical photos of glass beads on mouse skin[75]
| 1 | Doane T, Burda C. Nanoparticle mediated non-covalent drug delivery[J]. Adv. Drug Delivery Rev., 2013, 65(5): 607-621. |
| 2 | Müller-Dethlefs K, Hobza P. Noncovalent interactions: a challenge for experiment and theory[J]. Chem. Rev., 2000, 100(1): 143-168. |
| 3 | Schreiber C L, Smith B D. Molecular conjugation using non-covalent click chemistry[J]. Nat. Rev. Chem., 2019, 3: 393-400. |
| 4 | Zhang S G. Fabrication of novel biomaterials through molecular self-assembly[J]. Nat. Biotechnol., 2003, 21: 1171-1178. |
| 5 | Zimmerman J B, Anastas P T, Erythropel H C, et al. Designing for a green chemistry future[J]. Science, 2020, 367(6476): 397-400. |
| 6 | Chang R, Nikoloudakis E, Zou Q L, et al. Supramolecular nanodrugs constructed by self-assembly of peptide nucleic acid-photosensitizer conjugates for photodynamic therapy[J]. ACS Appl. Bio. Mater., 2020, 3(1): 2-9. |
| 7 | Abbas M, Zou Q L, Li S K, et al. Self-assembled peptide- and protein-based nanomaterials for antitumor photodynamic and photothermal therapy[J]. Adv. Mater., 2017, 29(12): 1605021. |
| 8 | Reches M, Gazit E. Casting metal nanowires within discrete self-assembled peptide nanotubes[J]. Science, 2003, 300(5619): 625-627. |
| 9 | Zou Q L, Liu K, Abbas M, et al. Peptide-modulated self-assembly of chromophores toward biomimetic light-harvesting nanoarchitectonics[J]. Adv. Mater., 2016, 28(6): 1031-1043. |
| 10 | Li S K, Xing R R, van Hest J C M, et al. Peptide-based supramolecular assembly drugs toward cancer theranostics[J]. Expert Opin. Drug Deliv., 2022, 19(7): 847-860. |
| 11 | Li S K, Zou Q L, Xing R R, et al. Peptide-modulated self-assembly as a versatile strategy for tumor supramolecular nanotheranostics[J]. Theranostics, 2019, 9(11): 3249-3261. |
| 12 | Han J J, Liu K, Chang R, et al. Photooxidase-mimicking nanovesicles with superior photocatalytic activity and stability based on amphiphilic amino acid and phthalocyanine co-assembly[J]. Angew. Chem. Int. Ed., 2018, 58(7): 2000-2004. |
| 13 | Zhao X B, Pan F, Xu H, et al. Molecular self-assembly and applications of designer peptide amphiphiles[J]. Chem. Soc. Rev., 2010, 39(9): 3480-3498. |
| 14 | Kim S, Kim J H, Lee J S, et al. Beta-sheet-forming, self-assembled peptide nanomaterials towards optical, energy, and healthcare applications[J]. Small, 2015, 11(30): 3623-3640. |
| 15 | Chang R, Yan X H. Supramolecular immunotherapy of cancer based on the self-assembling peptide design[J]. Small Struct., 2020, 1(2): 2000068. |
| 16 | Chen C J, Liu K, Li J B, et al. Functional architectures based on self-assembly of bio-inspired dipeptides: structure modulation and its photoelectronic applications[J]. Adv. Colloid Interface Sci., 2015, 225: 177-193. |
| 17 | Liu Y M, Zhang L, Chang R, et al. Supramolecular cancer photoimmunotherapy based on precise peptide self-assembly design[J]. Chem. Commun., 2022, 58(14): 2247-2258. |
| 18 | Xing R R, Liu Y M, Zou Q L, et al. Self-assembled injectable biomolecular hydrogels towards phototherapy[J]. Nanoscale, 2019, 11(46): 22182-22195. |
| 19 | Liu Y M, Chang R, Xing R R, et al. Bioactive peptide nanodrugs based on supramolecular assembly for boosting immunogenic cell death-induced cancer immunotherapy[J]. Small Methods, 2023, 7(5): 2201708. |
| 20 | Wang J, Liu K, Xing R R, et al. Peptide self-assembly: thermodynamics and kinetics[J]. Chem. Soc. Rev., 2016, 45(20): 5589-5604. |
| 21 | Yuan C Q, Ji W, Xing R R, et al. Hierarchically oriented organization in supramolecular peptide crystals[J]. Nat. Rev. Chem., 2019, 3: 567-588. |
| 22 | Yuan C Q, Li Q, Xing R R, et al. Peptide self-assembly through liquid-liquid phase separation[J]. Chem, 2023, 9(9): 2425-2445. |
| 23 | Ulijn R V, Smith A M. Designing peptide based nanomaterials[J]. Chem. Soc. Rev., 2008, 37(4): 664-675. |
| 24 | Xing R R, Yuan C Q, Li S K, et al. Charge-induced secondary structure transformation of amyloid-derived dipeptide assemblies from β-sheet to α-helix[J]. Angew. Chem. Int. Ed., 2018, 57(6): 1537-1542. |
| 25 | Adler-Abramovich L, Gazit E. The physical properties of supramolecular peptide assemblies: from building block association to technological applications[J]. Chem. Soc. Rev., 2014, 43(20): 6881-6893. |
| 26 | Ren P, Li J L, Zhao L Y, et al. Dipeptide self-assembled hydrogels with shear-thinning and instantaneous self-healing properties determined by peptide sequences[J]. ACS Appl. Mater. Interfaces, 2020, 12(19): 21433-21440. |
| 27 | Li S K, Chang R, Zhao L Y, et al. Two-photon nanoprobes based on bioorganic nanoarchitectonics with a photo-oxidation enhanced emission mechanism[J]. Nat. Commun., 2023, 14: 5227. |
| 28 | Chang R, Zou Q L, Zhao L Y, et al. Amino-acid-encoded supramolecular photothermal nanomedicine for enhanced cancer therapy[J]. Adv. Mater., 2022, 34(16): 2200139. |
| 29 | Chang R, Zhao L Y, Xing R R, et al. Functional chromopeptide nanoarchitectonics: molecular design, self-assembly and biological applications[J]. Chem. Soc. Rev., 2023, 52(8): 2688-2712. |
| 30 | Yan X H, Zhu P L, Li J B. Self-assembly and application of diphenylalanine-based nanostructures[J]. Chem. Soc. Rev., 2010, 39(6): 1877-1890. |
| 31 | Wang J, Liu K, Yan L Y, et al. Trace solvent as a predominant factor to tune dipeptide self-assembly[J]. ACS Nano, 2016, 10(2): 2138-2143. |
| 32 | Li Y X, Yan L Y, Liu K, et al. Solvothermally mediated self-assembly of ultralong peptide nanobelts capable of optical waveguiding[J]. Small, 2016, 12(19): 2575-2579. |
| 33 | Wang J, Yuan C Q, Han Y C, et al. Trace water as prominent factor to induce peptide self-assembly: dynamic evolution and governing interactions in ionic liquids[J]. Small, 2017, 13(44): 1702175. |
| 34 | Zhao K L, Xing R R, Yan X H. Cyclic dipeptides: biological activities and self-assembled materials[J]. Pept. Sci., 2021, 113(2): e24202. |
| 35 | Yang M Y, Xing R R, Shen G Z, et al. A versatile cyclic dipeptide hydrogelator: self-assembly and rheology in various physiological conditions[J]. Colloids Surf. A, 2019, 572: 259-265. |
| 36 | Clover T M, O'Neill C L, Appavu R, et al. Self-assembly of block heterochiral peptides into helical tapes[J]. J. Am. Chem. Soc., 2020, 142(47): 19809-19813. |
| 37 | Li S K, Zhang W J, Xue H D, et al. Tumor microenvironment-oriented adaptive nanodrugs based on peptide self-assembly[J]. Chem. Sci., 2020, 11(33): 8644-8656. |
| 38 | Liu K, Xing R R, Zou Q L, et al. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy[J]. Angew. Chem. Int. Ed., 2016, 55(9): 3036-3039. |
| 39 | Zou Q L, Zhang L, Yan X H, et al. Multifunctional porous microspheres based on peptide-porphyrin hierarchical co-assembly[J]. Angew. Chem. Int. Ed., 2014, 53(9): 2366-2370. |
| 40 | Liu K, Xing R R, Chen C J, et al. Peptide-induced hierarchical long-range order and photocatalytic activity of porphyrin assemblies[J]. Angew. Chem. Int. Ed., 2015, 54(2): 500-505. |
| 41 | Liu K, Zhang R Y, Li Y X, et al. Directed self-assembly: tunable aggregation-induced emission of tetraphenylethylene via short peptide-directed self-assembly[J]. Adv. Mater. Interfaces, 2017, 4(1): 1600183. |
| 42 | Li S K, Zou Q L, Li Y X, et al. Smart peptide-based supramolecular photodynamic metallo-nanodrugs designed by multicomponent coordination self-assembly[J]. J. Am. Chem. Soc., 2018, 140(34): 10794-10802. |
| 43 | Li S K, Zhang W J, Xing R R, et al. Supramolecular nanofibrils formed by coassembly of clinically approved drugs for tumor photothermal immunotherapy[J]. Adv. Mater., 2021, 33(21): 2100595. |
| 44 | Xing R R, Zou Q L, Yuan C Q, et al. Self-assembling endogenous biliverdin as a versatile near-infrared photothermal nanoagent for cancer theranostics[J]. Adv. Mater., 2019, 31(16): 1900822. |
| 45 | Yang M Y, Yuan C Q, Shen G Z, et al. Cyclic dipeptide nanoribbons formed by dye-mediated hydrophobic self-assembly for cancer chemotherapy[J]. J. Colloid Interface Sci., 2019, 557: 458-464. |
| 46 | Zou Q L, Abbas M, Zhao L Y, et al. Biological photothermal nanodots based on self-assembly of peptide-porphyrin conjugates for antitumor therapy[J]. J. Am. Chem. Soc., 2017, 139(5): 1921-1927. |
| 47 | Sun B B, Chang R, Cao S P, et al. Acid-activatable transmorphic peptide-based nanomaterials for photodynamic therapy[J]. Angew. Chem. Int. Ed., 2020, 59(46): 20582-20588. |
| 48 | Song J W, Xing R R, Jiao T F, et al. Crystalline dipeptide nanobelts based on solid-solid phase transformation self-assembly and their polarization imaging of cells[J]. ACS Appl. Mater. Inter., 2018, 10(3): 2368-2376. |
| 49 | Zou Q L, Chang R, Xing R R, et al. Injectable self-assembled bola-dipeptide hydrogels for sustained photodynamic prodrug delivery and enhanced tumor therapy[J]. J. Control. Release, 2020, 319: 344-351. |
| 50 | Li J Y, Kuang Y, Shi J F, et al. The conjugation of nonsteroidal anti-inflammatory drugs (NSAID) to small peptides for generating multifunctional supramolecular nanofibers/hydrogels[J]. Beilstein J. Org. Chem., 2013, 9: 908-917. |
| 51 | Cheetham A G, Zhang P C, Lin Y A, et al. Supramolecular nanostructures formed by anticancer drug assembly[J]. J. Am. Chem. Soc., 2013, 135(8): 2907-2910. |
| 52 | Matson J B, Stupp S I. Self-assembling peptide scaffolds for regenerative medicine[J]. Chem. Commun., 2012, 48(1): 26-33. |
| 53 | Ke P C, Sani M A, Ding F, et al. Implications of peptide assemblies in amyloid diseases[J]. Chem. Soc. Rev., 2017, 46(21): 6492-6531. |
| 54 | Li Q, Xuan M J, Wang A H, et al. Biogenic sensors based on dipeptide assemblies[J]. Matter, 2022, 5(11): 3643-3658. |
| 55 | Liu K, Xing R R, Li Y X, et al. Mimicking primitive photobacteria: sustainable hydrogen evolution based on peptide-porphyrin co-assemblies with a self-mineralized reaction center[J]. Angew. Chem. Int. Ed., 2016, 55(40): 12503-12507. |
| 56 | Liu K, Yuan C Q, Zou Q L, et al. Self-assembled zinc/cystine-based chloroplast mimics capable of photoenzymatic reactions for sustainable fuel synthesis[J]. Angew. Chem. Int. Ed., 2017, 56(27): 7876-7880. |
| 57 | Kim J H, Lee M, Lee J S, et al. Self-assembled light-harvesting peptide nanotubes for mimicking natural photosynthesis[J]. Angew. Chem. Int. Ed., 2012, 51(2): 517-520. |
| 58 | Meneghin E, Biscaglia F, Volpato A, et al. Biomimetic nanoarchitectures for light harvesting: self-assembly of pyropheophorbide-peptide conjugates[J]. J. Phys. Chem. Lett., 2020, 11(19): 7972-7980. |
| 59 | Tao K, Xue B, Frere S, et al. Multiporous supramolecular microspheres for artificial photosynthesis[J]. Chem. Mater., 2017, 29(10): 4454-4460. |
| 60 | Channon K J, Devlin G L, MacPhee C E. Efficient energy transfer within self-assembling peptide fibers: a route to light-harvesting nanomaterials[J]. J. Am. Chem. Soc., 2009, 131(35): 12520-12521. |
| 61 | Ma K, Xing R R, Jiao T F, et al. Injectable self-assembled dipeptide-based nanocarriers for tumor delivery and effective in vivo photodynamic therapy[J]. ACS Appl. Mater. Inter., 2016, 8(45): 30759-30767. |
| 62 | Zhang W J, Li S K, Liu Y M, et al. Immunosuppressive microenvironment improvement and treatment of aggressive malignancy pancreatic ductal adenocarcinoma based on local administration of injectable hydrogel[J]. Nano Today, 2023, 50: 101832. |
| 63 | Taylor R E, Zahid M. Cell penetrating peptides, novel vectors for gene therapy[J]. Pharmaceutics, 2020, 12(3): 225. |
| 64 | Zhang B Y, Zhang H, Dai S, et al. Cell-penetrating peptide-labelled smart polymers for enhanced gene delivery[J]. Eng. Life Sci., 2017, 17(2): 193-203. |
| 65 | Qu W, Chen W H, Kuang Y, et al. Avidin-biotin interaction mediated peptide assemblies as efficient gene delivery vectors for cancer therapy[J]. Mol. Pharm., 2013, 10(1): 261-269. |
| 66 | Liu Y, Li J F, Shao K, et al. A leptin derived 30-amino-acid peptide modified pegylated poly-L-lysine dendrigraft for brain targeted gene delivery[J]. Biomaterials, 2010, 31(19): 5246-5257. |
| 67 | Fidel J, Kennedy K C, Dernell W S, et al. Preclinical validation of the utility of BLZ-100 in providing fluorescence contrast for imaging spontaneous solid tumors[J]. Cancer Res., 2015, 75(20): 4283-4291. |
| 68 | Parrish-Novak J, Byrnes-Blake K, Lalayeva N, et al. Nonclinical profile of BLZ-100, a tumor-targeting fluorescent imaging agent[J]. Int. J. Toxicol., 2017, 36(2): 104-112. |
| 69 | Qi Y Q, Min H, Mujeeb A, et al. Injectable hexapeptide hydrogel for localized chemotherapy prevents breast cancer recurrence[J]. ACS Appl. Mater. Inter., 2018, 10(8): 6972-6981. |
| 70 | Abbas M, Ovais M, Atiq A, et al. Tailoring supramolecular short peptide nanomaterials for antibacterial applications[J]. Coord. Chem. Rev., 2022, 460: 214481. |
| 71 | Liu Y C, Gong H N, Wang Z W, et al. Treatment of superbug infection through a membrane-disruption and immune-regulation cascade effect based on supramolecular peptide hydrogels[J]. Adv. Funct. Mater., 2023, 33(45): 2305726. |
| 72 | Axinte E. Glasses as engineering materials: a review[J]. Mater. Des., 2011, 32(4): 1717-1732. |
| 73 | Shi C J, Zheng K R. A review on the use of waste glasses in the production of cement and concrete[J]. Resour. Conserv. Recycl., 2007, 52(2): 234-247. |
| 74 | Kinchin I M. Water bears and water loss: tardigrades and anhydrobiosis[J]. Biochem, 2008, 30(4): 18-20. |
| 75 | Xing R R, Yuan C Q, Fan W, et al. Biomolecular glass with amino acid and peptide nanoarchitectonics[J]. Sci. Adv., 2023, 9(11): eadd8105. |
| 76 | Yan X H, Yuan C Q, Fan W, et al. Cyclic peptide high-entropy noncovalent glass[J]. Research Square, DOI: 10.21203/rs.21203.rs-3347593/v3347591 . |
| [1] | 刘恺轩, 姜沁源, 汪菲, 李润, 朱平, 王康康, 臧永路, 赵彦龙, 张如范. 高密度超长碳纳米管的可控制备:进展与展望[J]. 化工学报, 2024, 75(4): 1355-1369. |
| [2] | 吴希, 孙博, 刘银东, 齐传磊, 陈凯毅, 王路海, 许崇, 李永峰. 钠离子电池沥青基碳负极材料制备技术研究进展[J]. 化工学报, 2024, 75(4): 1270-1283. |
| [3] | 孟祥军, 花莹曦, 张长金, 张弛, 杨林睿, 杨若昔, 刘鉴漪, 许春建. 6N电子级氘气的制备与纯化技术研究[J]. 化工学报, 2024, 75(1): 377-390. |
| [4] | 李珍宝, 李超, 王虎, 王绍瑞, 黎泉. MPP抑制铝镁合金粉尘爆炸微观机理研究[J]. 化工学报, 2023, 74(8): 3608-3614. |
| [5] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| [6] | 王志龙, 杨烨, 赵真真, 田涛, 赵桐, 崔亚辉. 搅拌时间和混合顺序对锂离子电池正极浆料分散特性的影响[J]. 化工学报, 2023, 74(7): 3127-3138. |
| [7] | 刘晓洋, 喻健良, 侯玉洁, 闫兴清, 张振华, 吕先舒. 螺旋微通道对掺氢甲烷爆轰传播的影响[J]. 化工学报, 2023, 74(7): 3139-3148. |
| [8] | 朱风, 陈凯琳, 黄小凤, 鲍银珠, 李文斌, 刘嘉鑫, 吴玮强, 高王伟. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| [9] | 肖忠良, 尹碧露, 宋刘斌, 匡尹杰, 赵亭亭, 刘成, 袁荣耀. 废旧锂离子电池回收工艺研究进展及其安全风险分析[J]. 化工学报, 2023, 74(4): 1446-1456. |
| [10] | 贠程, 王倩琳, 陈锋, 张鑫, 窦站, 颜廷俊. 基于社团结构的化工过程风险演化路径深度挖掘[J]. 化工学报, 2023, 74(4): 1639-1650. |
| [11] | 张雪婷, 胡激江, 赵晶, 李伯耿. 高分子量聚丙二醇在微通道反应器中的制备[J]. 化工学报, 2023, 74(3): 1343-1351. |
| [12] | 武子超, 汪志雷, 李荣业, 李可昕, 华敏, 潘旭海, 王三明, 蒋军成. 点火方式对欠膨胀氢气射流爆炸超压影响规律研究[J]. 化工学报, 2023, 74(3): 1409-1418. |
| [13] | 靳志远, 单国荣, 潘鹏举. AM/AMPS/SSS三元共聚物的制备及耐温耐盐性能[J]. 化工学报, 2023, 74(2): 916-923. |
| [14] | 杜江龙, 杨雯棋, 黄凯, 练成, 刘洪来. 复合相变材料/空冷复合式锂离子电池模块散热性能[J]. 化工学报, 2023, 74(2): 674-689. |
| [15] | 王燕, 杨帅帅, 张国涛, 徐子晖, 毛文哲, 纪文涛. 改性沸石抑制乙烯爆炸性能及机理研究[J]. 化工学报, 2023, 74(12): 5048-5060. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号