化工学报 ›› 2024, Vol. 75 ›› Issue (4): 1519-1532.DOI: 10.11949/0438-1157.20240007
严孝清1( ), 赵瑛1(
), 赵瑛1( ), 张宇哲1, 欧鸿辉1, 黄起中2, 胡华贵2, 杨贵东1(
), 张宇哲1, 欧鸿辉1, 黄起中2, 胡华贵2, 杨贵东1( )
)
收稿日期:2024-01-03
修回日期:2024-03-22
出版日期:2024-04-25
发布日期:2024-06-07
通讯作者:
杨贵东
作者简介:严孝清(1990—),男,博士研究生,助理教授,xq-yan@xjtu.edu.cn基金资助:
Xiaoqing YAN1( ), Ying ZHAO1(
), Ying ZHAO1( ), Yuzhe ZHANG1, Honghui OU1, Qizhong HUANG2, Huagui HU2, Guidong YANG1(
), Yuzhe ZHANG1, Honghui OU1, Qizhong HUANG2, Huagui HU2, Guidong YANG1( )
)
Received:2024-01-03
Revised:2024-03-22
Online:2024-04-25
Published:2024-06-07
Contact:
Guidong YANG
摘要:
合理设计高活性、高选择性、高稳定性、低成本纳米结构催化剂是电催化硝酸根还原制氨的一个重大挑战。采用水热法耦合原位还原法制备了厚度可控的聚吡咯包裹五重孪晶铜纳米线催化剂,实现了低偏压下产氨活性、法拉第效率的提高以及对抗腐蚀能力的大幅提升。偏压为-0.4 V(可逆氢电极)时T-CuNW-10样品合成氨活性达到13.83
中图分类号:
严孝清, 赵瑛, 张宇哲, 欧鸿辉, 黄起中, 胡华贵, 杨贵东. 五重孪晶铜纳米线@聚吡咯制备及其电催化硝酸盐还原制氨[J]. 化工学报, 2024, 75(4): 1519-1532.
Xiaoqing YAN, Ying ZHAO, Yuzhe ZHANG, Honghui OU, Qizhong HUANG, Huagui HU, Guidong YANG. Preparation of five-fold twinned copper nanowires@polypyrrole and their electrocatalytic conversion of nitrate to ammonia[J]. CIESC Journal, 2024, 75(4): 1519-1532.
| 样品编号 | T-CuNW/mg | 吡咯/μl | 过硫酸铵/mg | 碳酸氢钠/mg |
|---|---|---|---|---|
| T-CuNW | 50 | 0 | 0 | 0 |
| T-CuNW-5 | 50 | 5 | 0.0114 | 0.0084 |
| T-CuNW-10 | 50 | 10 | 0.0228 | 0.0168 |
| T-CuNW-20 | 50 | 20 | 0.0456 | 0.0336 |
表1 T-CuNW@ppy催化剂的制备参数
Table 1 Preparation parameters of T-CuNW@ppy
| 样品编号 | T-CuNW/mg | 吡咯/μl | 过硫酸铵/mg | 碳酸氢钠/mg |
|---|---|---|---|---|
| T-CuNW | 50 | 0 | 0 | 0 |
| T-CuNW-5 | 50 | 5 | 0.0114 | 0.0084 |
| T-CuNW-10 | 50 | 10 | 0.0228 | 0.0168 |
| T-CuNW-20 | 50 | 20 | 0.0456 | 0.0336 |
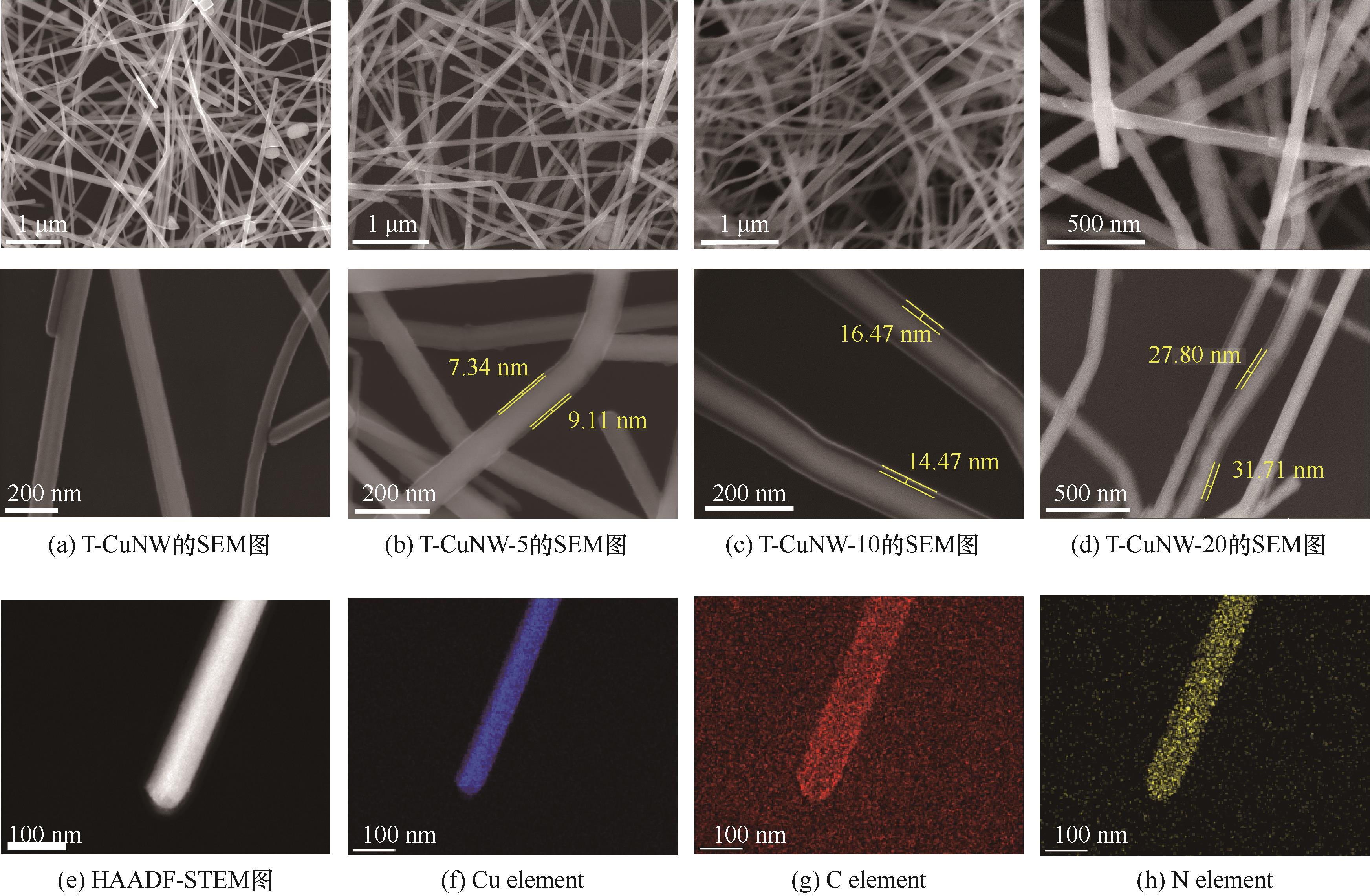
图3 T-CuNW和T-CuNW@ppy系列样品的SEM图[(a)~(d)];T-CuNW-10样品的HAADF-STEM图(e)和EDS图[(f)~(h)]
Fig.3 SEM images of T-CuNW and T-CuNW@ppy samples [(a)—(d)], HAADF-STEM diagram (e) and EDS diagram [(f)—(h)] of T-CuNW-10 sample
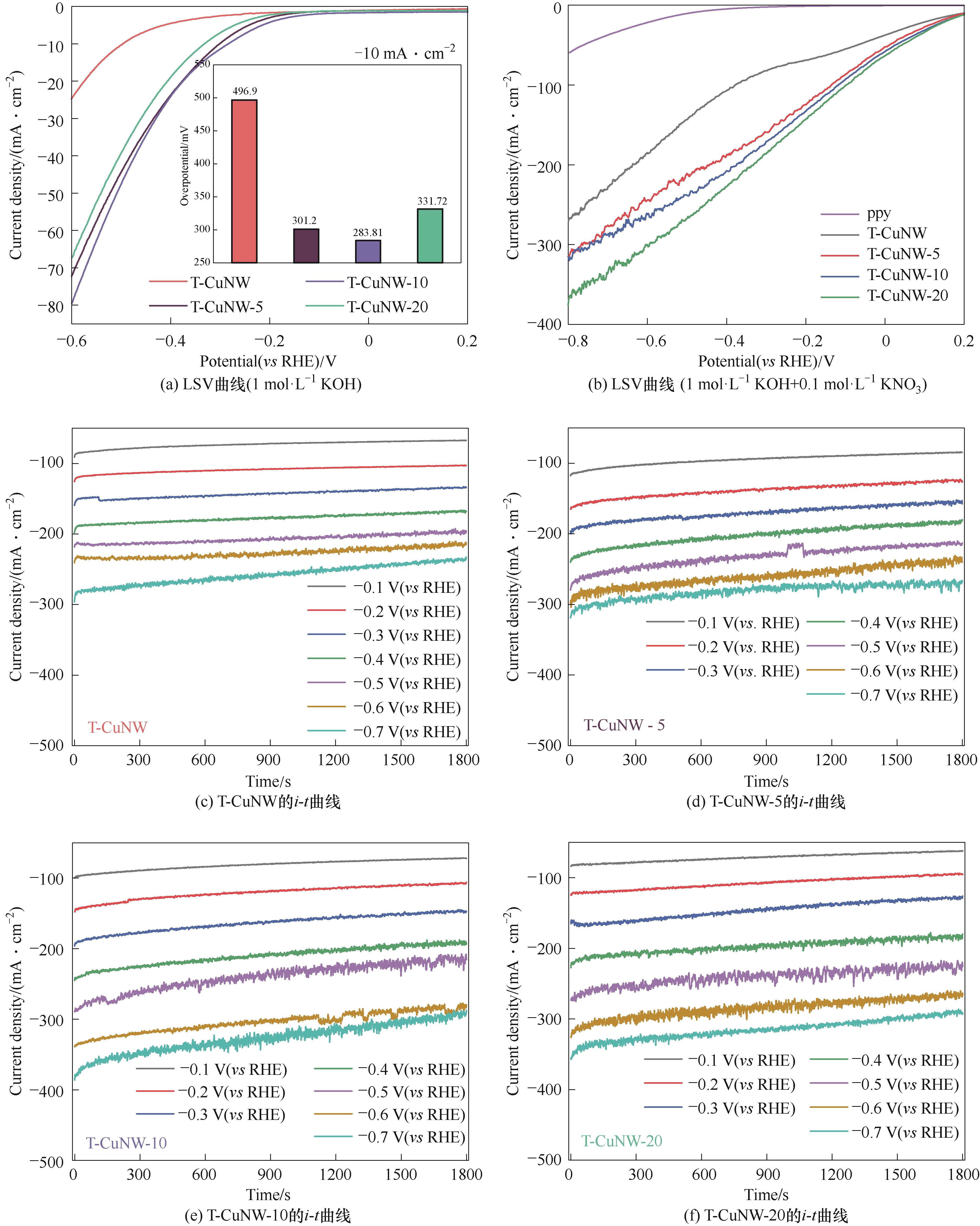
图5 T-CuNW@ppy系列样品在1 mol·L-1 KOH电解液(a)和1 mol·L-1 KOH+0.1 mol·L-1 KNO3电解液(b)中的LSV曲线;不同电压下系列样品在1 mol·L-1 KOH+0.1 mol·L-1 KNO3溶液中的i-t曲线[(c) T-CuNW; (d) T-CuNW-5; (e) T-CuNW-10; (f) T-CuNW-20];不同电压下T-CuNW、ppy和T-CuNW@ppy系列样品在1 mol·L-1 KOH+0.1 mol·L-1 KNO3溶液中的NH3产品活性(g)和NH3的法拉第效率(h);T-CuNW-10在-0.4 V (vs RHE)下1 mol·L-1 KOH(有0.1 mol·L-1 KNO3、无0.1 mol·L-1 KNO3)溶液中和无施加偏压下1 mol·L-1 KOH(有0.1 mol·L-1 KNO3)溶液中的NH3产量(i)
Fig.5 LSV polarization curves of as-synthesized samples in electrolytes using 0.1 mol·L-1 KOH as solvent without (a) and with (b) adding 0.1 mol·L-1NO3-; i-t curves of as-synthesized samples [(c) T-CuNW; (d) T-CuNW-5; (e) T-CuNW-10, (f) T-CuNW-20]; eNITRR performance of as-synthesized samples under different potential in 1 mol·L-1 KOH with 0.1 mol·L-1 KNO3 (g), FE of as-synthesized samples under different potential in 1 mol·L-1 KOH with 0.1 mol·L-1 KNO3 (h); eNITRR performance of T-CuNW-10 in electrolytes using 0.1 mol·L-1 KOH as solvent without and with adding 0.1 mol·L-1NO3- (i)
| 催化剂 | 偏压(vs RHE)/V | NH3产生速率 | 法拉第效率/% | 文献 |
|---|---|---|---|---|
| T-CuNW | -0.4 | 12.04 | 84.1 | 本论文 |
| T-CuNW@ppy | -0.4 | 13.83 | 83.0 | 本论文 |
| Cu49Fe1-NRA | -0.7 | 4.08 mg·cm-2·h-1 | 94.5 | [ |
| Cu SACs | -0.9 | 1.12 mg·cm-2·h-1 | 85.5 | [ |
| Cu1Co1HHTP | -0.6 | 5.09 mg·cm-2·h-1 | 96.4 | [ |
| Cu-Fe2O3 | -0.6 | 179.55 | 约100 | [ |
| Cu Ni NPS/CF | -0.48 | 94.57 mg· cm-2·h-1 | 97.0 | [ |
| T40-CuNCs | -0.6 | 2.62 mg·cm-2·h-1 | 96.8 | [ |
| Cu SCCs | -0.5 | 1.99 mg·cm-2·h-1 | 96.0 | [ |
| Cu-HTBs | -0.7 | 23789.8 | 90.0 | [ |
| Cu5Pd NCs | -0.7 | 32 mg·cm-2·h-1 | 95.5 | [ |
| Ru0.15Cu0.85 | -0.2 | 26.25 | 4.4 | [ |
表2 T-CuNW、T-CuNW@ppy与其他典型材料的硝酸根还原制氨活性比较
Table 2 Comparison of eNITRR performance of T-CuNW, T-CuNW@ppy and other typical materials
| 催化剂 | 偏压(vs RHE)/V | NH3产生速率 | 法拉第效率/% | 文献 |
|---|---|---|---|---|
| T-CuNW | -0.4 | 12.04 | 84.1 | 本论文 |
| T-CuNW@ppy | -0.4 | 13.83 | 83.0 | 本论文 |
| Cu49Fe1-NRA | -0.7 | 4.08 mg·cm-2·h-1 | 94.5 | [ |
| Cu SACs | -0.9 | 1.12 mg·cm-2·h-1 | 85.5 | [ |
| Cu1Co1HHTP | -0.6 | 5.09 mg·cm-2·h-1 | 96.4 | [ |
| Cu-Fe2O3 | -0.6 | 179.55 | 约100 | [ |
| Cu Ni NPS/CF | -0.48 | 94.57 mg· cm-2·h-1 | 97.0 | [ |
| T40-CuNCs | -0.6 | 2.62 mg·cm-2·h-1 | 96.8 | [ |
| Cu SCCs | -0.5 | 1.99 mg·cm-2·h-1 | 96.0 | [ |
| Cu-HTBs | -0.7 | 23789.8 | 90.0 | [ |
| Cu5Pd NCs | -0.7 | 32 mg·cm-2·h-1 | 95.5 | [ |
| Ru0.15Cu0.85 | -0.2 | 26.25 | 4.4 | [ |
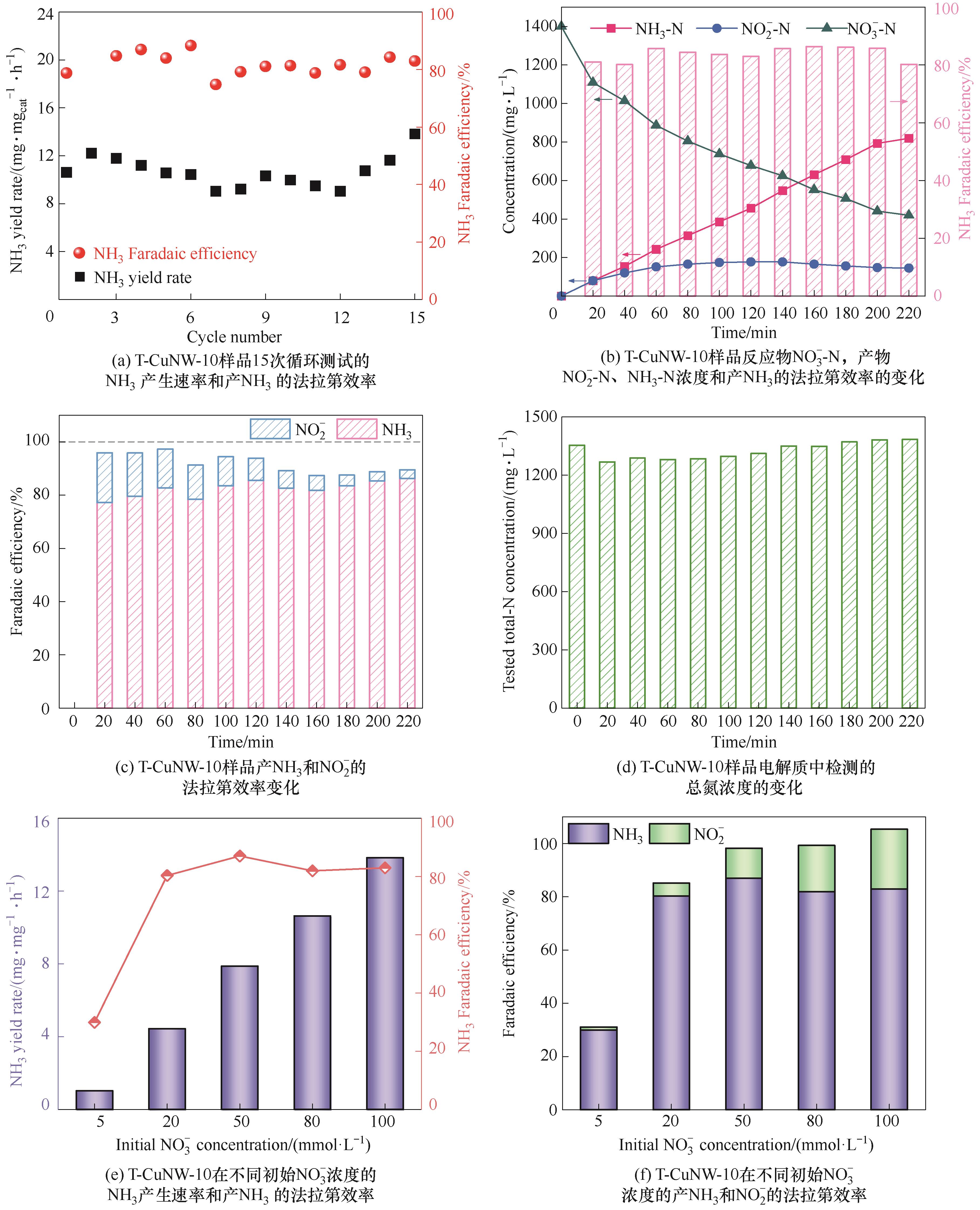
图6 (a)T-CuNW-10在-0.4 V(vs RHE)下含有0.1 mol·L-1NO3-的1 mol·L-1 KOH电解质中15次循环测试的NH3 产品活性和产NH3的法拉第效率; T-CuNW-10在-0.4 V(vs RHE)下1 mol·L-1 KOH+0.1 mol·L-1 KNO3电解质中(b)反应物NO3--N,产物NO2--N、NH3-N浓度和产NH3的法拉第效率随时间的变化规律以及(c)产NH3和NO2-的法拉第效率随时间的变化规律;(d)T-CuNW-10在-0.4 V(vs RHE)下1 mol·L-1 KOH+0.1 mol·L-1 KNO3电解质中检测的总氮浓度随时间的变化规律; T-CuNW在-0.4 V(vs RHE)下含有不同初始浓度NO3-的1 mol·L-1 KOH电解质中的(e)NH3产生速率和产NH3的法拉第效率及(f)产NH3和NO2-的法拉第效率随初始NO3-浓度的变化规律
Fig.6 (a) Cycling tests of T-CuNW-10 for eNITRR tests at -0.4 V(vs RHE); (b) Time dependent concentration change of NO3-, NO2- and NH3 over T-CuNW-10 at -0.4 V(vs RHE); (c) Time dependent concentration change of FE; (d) Time dependent concentration change of total nitrogen at -0.4 V(vs RHE); (e) NH3 yield rate and FE of T-CuNW-10 with different concentrations of nitrate; (f) FE of NH3 and NO2- on T-CuNW-10 with different concentrations of nitrate
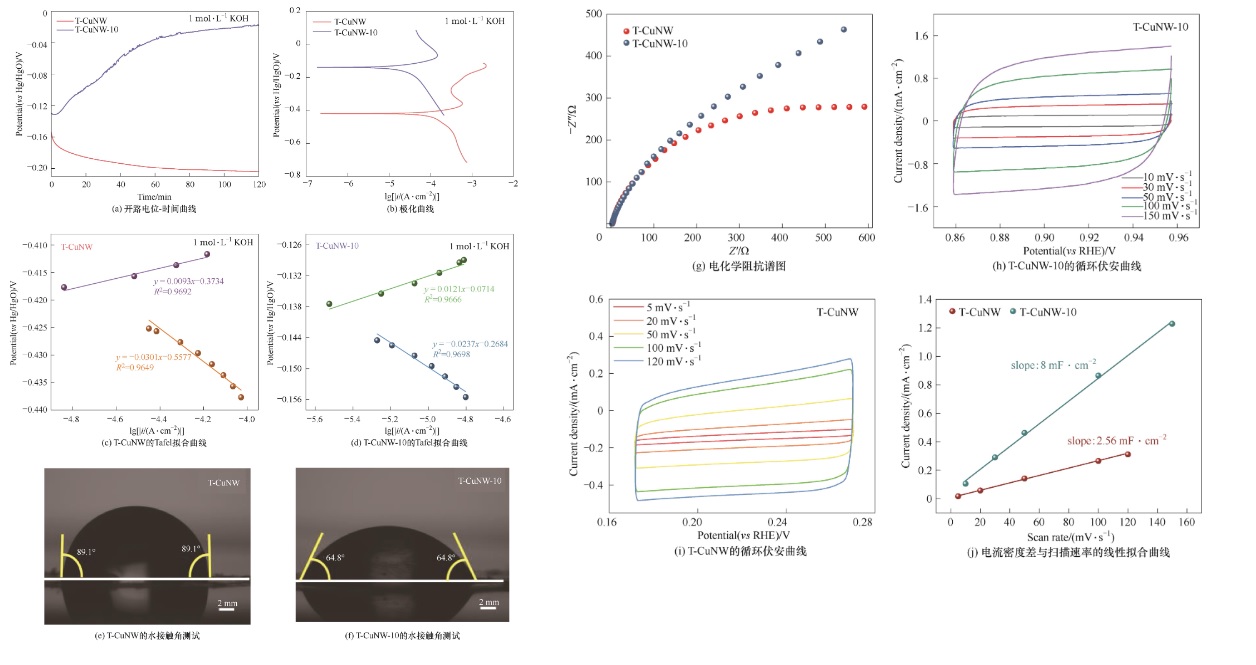
图7 (a)T-CuNW-10和T-CuNW催化剂在1 mol·L-1 KOH溶液中的开路电位-时间曲线;(b)T-CuNW-10和T-CuNW催化剂在1 mol·L-1 KOH溶液中的极化曲线;(c)T-CuNW和(d)T-CuNW-10催化剂在1 mol·L-1 KOH溶液中的Tafel拟合曲线;(e)T-CuNW和(f)T-CuNW-10两种催化剂疏水性测试;(g)T-CuNW-10和T-CuNW催化剂在1 mol·L-1 Na2SO4溶液中的电化学阻抗谱图;(h)T-CuNW-10和(i)T-CuNW催化剂在不同扫描速率下的循环伏安曲线(CV);(j)电流密度差与扫描速率的线性拟合曲线
Fig.7 (a) Open circuit potentials decays with rest time; (b) Polarization curves; (c),(d) Curve of Tafel; (e),(f)Contact angle measurements; (g) Nyquist plots for the as-synthesized samples; (h),(i) Cyclic voltammograms; (j) Linear regression curve of difference in current density and scanning rate
| 样品名称 | Ecorr /mV | icorr/(μA·cm-2) |
|---|---|---|
| T-CuNW | -416.90 | 21.01 |
| T-CuNW-10 | -137.98 | 3.14 |
表3 T-CuNW-10和T-CuNW催化剂在1 mol·L-1 KOH溶液中的Tafel曲线拟合值
Table 3 Tafel curve fitting values of T-CUNW-10 and T-CuNW catalysts in 1 mol·L-1 KOH solution
| 样品名称 | Ecorr /mV | icorr/(μA·cm-2) |
|---|---|---|
| T-CuNW | -416.90 | 21.01 |
| T-CuNW-10 | -137.98 | 3.14 |
| 样品名称 | Cdl/(mF·cm-2) | ECSA/cm2 |
|---|---|---|
| T-CuNW | 2.56 | 64 |
| T-CuNW-10 | 8 | 200 |
表4 T-CuNW-10的ECSA分析结果及与T-CuNW的对比
Table 4 ECSA test of T-CUNW-10 and T-CuNW
| 样品名称 | Cdl/(mF·cm-2) | ECSA/cm2 |
|---|---|---|
| T-CuNW | 2.56 | 64 |
| T-CuNW-10 | 8 | 200 |
| 1 | Wang L, Xia M K, Wang H, et al. Greening ammonia toward the solar ammonia refinery[J]. Joule, 2018, 2(6): 1055-1074. |
| 2 | Guo J P, Chen P. Catalyst: NH3 as an energy carrier[J]. Chem, 2017, 3(5): 709-712. |
| 3 | Sun J, Alam D, Daiyan R, et al. A hybrid plasma electrocatalytic process for sustainable ammonia production[J]. Energy & Environmental Science, 2021, 14(2): 865-872. |
| 4 | 张谭, 刘光, 李晋平, 等. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| Zhang T, Liu G, Li J P, et al. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts[J]. CIESC Journal, 2023, 74(6): 2264-2280. | |
| 5 | Van Langevelde P H, Katsounaros I, Koper M T M. Electrocatalytic nitrate reduction for sustainable ammonia production[J]. Joule, 2021, 5(2): 290-294. |
| 6 | Fan S H, Hu Y N, Zhang T, et al. Highly selective environmental electrocatalytic nitrogen reduction to ammonia on Fe2(MoO4)3/C composite electrocatalyst[J]. International Journal of Hydrogen Energy, 2024, 51: 1198-1206. |
| 7 | Fan S H, Wang Q, Hu Y N, et al. Efficient electrocatalytic conversion of N2 to NH3 using oxygen-rich vacancy lithium niobate cubes[J]. Chinese Journal of Chemical Engineering, 2023, 62: 132-138. |
| 8 | Chen G F, Yuan Y F, Jiang H F, et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper-molecular solid catalyst[J]. Nature Energy, 2020, 5: 605-613. |
| 9 | 杨通, 何小波, 银凤翔. M-MOF-74 (M=Ni, Co, Zn) 的制备及其电化学催化合成氨性能[J]. 化工学报, 2020, 71(6): 2857-2870. |
| Yang T, He X B, Yin F X. Preparation of M-MOF-74 (M=Ni, Co, Zn) and its performance in electrocatalytic synthesis of ammonia[J]. CIESC Journal, 2020, 71(6): 2857-2870. | |
| 10 | Gao J N, Jiang B, Ni C C, et al. Enhanced reduction of nitrate by noble metal-free electrocatalysis on P doped three-dimensional Co3O4 cathode: mechanism exploration from both experimental and DFT studies[J]. Chemical Engineering Journal, 2020, 382: 123034. |
| 11 | Pérez-Gallent E, Figueiredo M C, Katsounaros I, et al. Electrocatalytic reduction of nitrate on copper single crystals in acidic and alkaline solutions[J]. Electrochimica Acta, 2017, 227: 77-84. |
| 12 | Min B, Gao Q, Yan Z H, et al. Powering the remediation of the nitrogen cycle: progress and perspectives of electrochemical nitrate reduction[J]. Industrial & Engineering Chemistry Research, 2021, 60(41): 14635-14650. |
| 13 | Yao Q F, Chen J B, Xiao S Z, et al. Selective electrocatalytic reduction of nitrate to ammonia with nickel phosphide[J]. ACS Applied Materials & Interfaces, 2021, 13(26): 30458-30467. |
| 14 | Lv C D, Zhong L X, Liu H J, et al. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide[J]. Nature Sustainability, 2021, 4: 868-876. |
| 15 | Tao Z X, Wu Y S, Wu Z S, et al. Cascade electrocatalytic reduction of carbon dioxide and nitrate to ethylamine[J]. Journal of Energy Chemistry, 2022, 65: 367-370. |
| 16 | Dima G E, de Vooys A C A, Koper M T M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions[J]. Journal of Electroanalytical Chemistry, 2003, 554/555: 15-23. |
| 17 | Garcia-Segura S, Lanzarini-Lopes M, Hristovski K, et al. Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications[J]. Applied Catalysis B: Environmental, 2018, 236: 546-568. |
| 18 | Fu X B, Zhao X G, Hu X B, et al. Alternative route for electrochemical ammonia synthesis by reduction of nitrate on copper nanosheets[J]. Applied Materials Today, 2020, 19: 100620. |
| 19 | Gao W S, Xie K F, Xie J, et al. Alloying of Cu with Ru enabling the relay catalysis for reduction of nitrate to ammonia[J]. Advanced Materials, 2023, 35(19): e2202952. |
| 20 | Wang Y T, Zhang P, Lin X Y, et al. Wide-pH-range adaptable ammonia electrosynthesis from nitrate on Cu-Pd interfaces[J]. Science China Chemistry, 2023, 66(3): 913-922. |
| 21 | Song M, Zhou G, Lu N, et al. Oriented attachment induces fivefold twins by forming and decomposing high-energy grain boundaries[J]. Science, 2020, 367(6473): 40-45. |
| 22 | Tang C, Chen Z, Wang Y J, et al. Atomic editing copper twin boundary for precision CO2 reduction[J]. ACS Catalysis, 2022, 12(19): 11838-11844. |
| 23 | Li Y F, Cui F, Ross M B, et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires[J]. Nano Letters, 2017, 17(2): 1312-1317. |
| 24 | Choi C, Kwon S, Cheng T, et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4 [J]. Nature Catalysis, 2020, 3: 804-812. |
| 25 | Cai J, Zhao Q, Hsu W Y, et al. Highly selective electrochemical reduction of CO2 into methane on nanotwinned Cu[J]. Journal of the American Chemical Society, 2023, 145(16): 9136-9143. |
| 26 | Bouzek K, Paidar M, Sadílková A, et al. Electrochemical reduction of nitrate in weakly alkaline solutions[J]. Journal of Applied Electrochemistry, 2001, 31(11): 1185-1193. |
| 27 | Paidar M, Roušar I, Bouzek K. Electrochemical removal of nitrate ions in waste solutions after regeneration of ion exchange columns[J]. Journal of Applied Electrochemistry, 1999, 29(5): 611-617. |
| 28 | Liu Y, Liu Z, Lu N, et al. Facile synthesis of polypyrrole coated copper nanowires: a new concept to engineered core-shell structures[J]. Chemical Communications, 2012, 48(20): 2621-2623. |
| 29 | Wang W, Yan X Q, Geng J F, et al. Engineering a copper@polypyrrole nanowire network in the near field for plasmon-enhanced solar evaporation[J]. ACS Nano, 2021, 15(10): 16376-16394. |
| 30 | Niu Z Q, Chen S P, Yu Y, et al. Morphology-controlled transformation of Cu@Au core-shell nanowires into thermally stable Cu3Au intermetallic nanowires[J]. Nano Research, 2020, 13(9): 2564-2569. |
| 31 | Zeng G F, Sun Q, Horta S, et al. A layered Bi2Te3@PPy cathode for aqueous zinc-ion batteries: mechanism and application in printed flexible batteries[J]. Advanced Materials, 2024, 36(1): e2305128. |
| 32 | Wang C H, Liu Z Y, Hu T, et al. Metasequoia-like nanocrystal of iron-doped copper for efficient electrocatalytic nitrate reduction into ammonia in neutral media[J]. ChemSusChem, 2021, 14(8): 1825-1829. |
| 33 | Xu Y T, Xie M Y, Zhong H Q, et al. In situ clustering of single-atom copper precatalysts in a metal-organic framework for efficient electrocatalytic nitrate-to-ammonia reduction[J]. ACS Catalysis, 2022, 12(14): 8698-8706. |
| 34 | Liu H M, Lang X Y, Zhu C, et al. Efficient electrochemical nitrate reduction to ammonia with copper-supported rhodium cluster and single-atom catalysts[J]. Angewandte Chemie International Edition, 2022, 61(23): e202202556. |
| 35 | Fan K, Xie W F, Li J Z, et al. Active hydrogen boosts electrochemical nitrate reduction to ammonia[J]. Nature Communications, 2022, 13: 7958. |
| 36 | Wang Y L, Yin H B, Dong F, et al. N-coordinated Cu-Ni dual-single-atom catalyst for highly selective electrocatalytic reduction of nitrate to ammonia[J]. Small, 2023, 19(20): e2207695. |
| 37 | Li K, Ding L, Xie Z Q, et al. Robust copper-based nanosponge architecture decorated by ruthenium with enhanced electrocatalytic performance for ambient nitrogen reduction to ammonia[J]. ACS Applied Materials & Interfaces, 2023, 15(9): 11703-11712. |
| 38 | Li R, Gao T T, Qiu W X, et al. Unveiling the size effect of nitrogen-doped carbon-supported copper-based catalysts on nitrate-to-ammonia electroreduction[J]. Nano Research, 2023: 1-6. |
| 39 | Hu Q, Huo Q H, Qi S, et al. Unconventional synthesis of hierarchically twinned copper as efficient electrocatalyst for nitrate-ammonia conversion[J]. Advanced Materials, 2024, 36(11): 2311375. |
| 40 | Song Z M, Qin L, Liu Y, et al. Efficient electroreduction of nitrate to ammonia with CuPd nanoalloy catalysts[J]. ChemSusChem, 2023, 16(22): e202300202. |
| 41 | Luo W J, Wu S L, Jiang Y Y, et al. Efficient electrocatalytic nitrate reduction to ammonia based on DNA-templated copper nanoclusters[J]. ACS Applied Materials & Interfaces, 2023, 15(15): 18928-18939. |
| [1] | 范以薇, 刘威, 李盈盈, 王培霞, 张吉松. 有机液体储氢中全氢化乙基咔唑催化脱氢研究进展[J]. 化工学报, 2024, 75(4): 1198-1208. |
| [2] | 司友明, 郑凌峰, 陈鹏忠, 樊江莉, 彭孝军. 新型锑氧簇光刻胶的性能与机理研究[J]. 化工学报, 2024, 75(4): 1705-1717. |
| [3] | 贾旭东, 杨博龙, 程前, 李雪丽, 向中华. 分步负载金属法制备铁钴双金属位点高效氧还原电催化剂[J]. 化工学报, 2024, 75(4): 1578-1593. |
| [4] | 杨玉维, 李敏, 要智颖, 孙沁林, 刘洋, 葛丹, 孙冰冰. 类器官芯片在纳米药物递送系统研究中的应用及前景[J]. 化工学报, 2024, 75(4): 1209-1221. |
| [5] | 韩宇, 周乐, 张鑫, 罗勇, 孙宝昌, 邹海魁, 陈建峰. 高黏附性Pd/SiO2/NF整体式催化剂的制备及加氢性能研究[J]. 化工学报, 2024, 75(4): 1533-1542. |
| [6] | 李添翼, 武玉泰, 王永胜, 顾佳锐, 宋沂恒, 杨丰铖, 郝广平. 轻同位素分离纯化与催化标记研究进展[J]. 化工学报, 2024, 75(4): 1284-1301. |
| [7] | 孙铭泽, 黄鹤来, 牛志强. 铂基氧还原催化剂:从单晶电极到拓展表面纳米材料[J]. 化工学报, 2024, 75(4): 1256-1269. |
| [8] | 程骁恺, 历伟, 王靖岱, 阳永荣. 镍催化可控/活性自由基聚合反应研究进展[J]. 化工学报, 2024, 75(4): 1105-1117. |
| [9] | 李昂, 赵振宇, 李洪, 高鑫. 微波诱导高分散Pd/FeP催化剂构筑及其电催化性能研究[J]. 化工学报, 2024, 75(4): 1594-1606. |
| [10] | 陈志明, 王泽凤, 马高琪, 王良波, 余承涛, 潘鹏举. 基于亚锡灭活及链端改性提高聚乳酸热稳定性的研究进展[J]. 化工学报, 2024, 75(3): 760-767. |
| [11] | 卫月星, 贺子岳, 燕可洲, 李林玉, 秦育红, 贺冲, 焦路畅. 改性煤气化渣催化降解双酚A的性能研究[J]. 化工学报, 2024, 75(3): 877-889. |
| [12] | 曹宇, 张国辉, 高昂, 杜心宇, 周静, 蔡永茂, 余璇, 于晓明. 二维MXene材料在太阳能电池和金属离子电池中的研究进展[J]. 化工学报, 2024, 75(2): 412-428. |
| [13] | 尹玉华, 方灿, 易清风, 李广. 不同碳导电剂对铁-空气电池性能的影响[J]. 化工学报, 2024, 75(2): 685-694. |
| [14] | 盖星宇, 岳玉学, 杨春华, 张子龙, 蔡天姿, 张海丰, 王柏林, 李小年. 碳负载Cs和Cu基催化剂用于1,1,2-三氯乙烷的气相脱氯化氢[J]. 化工学报, 2024, 75(2): 575-583. |
| [15] | 王雪杰, 崔国庆, 王文涵, 杨扬, 王淙恺, 姜桂元, 徐春明. 电内加热Pt/NPC催化剂高效催化甲基环己烷脱氢反应研究[J]. 化工学报, 2024, 75(1): 292-301. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号