化工学报 ›› 2025, Vol. 76 ›› Issue (4): 1788-1799.DOI: 10.11949/0438-1157.20240831
林纬1,2,3( ), 杜建1,2,3, 姚晨1,2,3, 朱家豪1,2,3, 汪威1,2,3(
), 杜建1,2,3, 姚晨1,2,3, 朱家豪1,2,3, 汪威1,2,3( ), 郑小涛1,2,3, 徐建民1,2,3, 喻九阳1,2,3
), 郑小涛1,2,3, 徐建民1,2,3, 喻九阳1,2,3
收稿日期:2024-07-22
修回日期:2024-12-17
出版日期:2025-04-25
发布日期:2025-05-12
通讯作者:
汪威
作者简介:林纬(1987—),男,博士,副教授,linwei@wit.edu.cn
基金资助:
Wei LIN1,2,3( ), Jian DU1,2,3, Chen YAO1,2,3, Jiahao ZHU1,2,3, Wei WANG1,2,3(
), Jian DU1,2,3, Chen YAO1,2,3, Jiahao ZHU1,2,3, Wei WANG1,2,3( ), Xiaotao ZHENG1,2,3, Jianmin XU1,2,3, Jiuyang YU1,2,3
), Xiaotao ZHENG1,2,3, Jianmin XU1,2,3, Jiuyang YU1,2,3
Received:2024-07-22
Revised:2024-12-17
Online:2025-04-25
Published:2025-05-12
Contact:
Wei WANG
摘要:
电化学水软化是一种新型绿色工业循环水阻垢技术,针对平行排列的极板无法有效利用OH-进而导致软化效率低下的问题,提出了基于模拟循环水与氢气泡的相对运动改进的极板垂直布置方式。通过OH-被动扩散形成长期且有效的碱性区域,提升了OH-的利用率,促进了CaCO3晶体在溶液中均匀成核。结果表明,模拟循环水中的Ca2+在到达阴极板之前已经成核,在120 min时CaCO3晶体颗粒数量稳定且尺寸最大可达25 μm,在电流密度为120 A/m2、入水口流量为12 L/h、初始硬度为500 mg/L、阴阳极中心间距为61.5 mm时,软化效率可达108.2 g/(m2·h)。本研究利用气泡运动和水流调控可为电化学循环水软化提供新思路。
中图分类号:
林纬, 杜建, 姚晨, 朱家豪, 汪威, 郑小涛, 徐建民, 喻九阳. 电化学水软化过程中离子输运与成核机理研究[J]. 化工学报, 2025, 76(4): 1788-1799.
Wei LIN, Jian DU, Chen YAO, Jiahao ZHU, Wei WANG, Xiaotao ZHENG, Jianmin XU, Jiuyang YU. Study on ion transport and nucleation mechanism in electrochemical water softening process[J]. CIESC Journal, 2025, 76(4): 1788-1799.
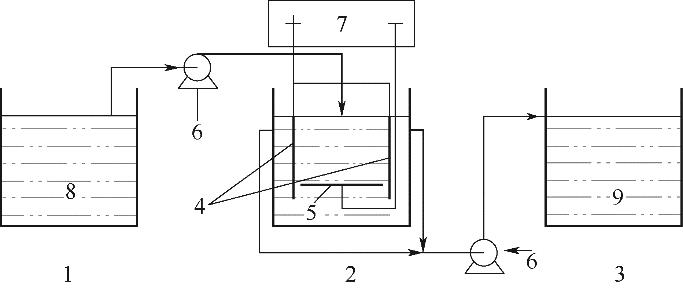
图1 电化学水软化实验装置示意图1—蓄水池;2—反应池;3—收集池;4—阳极;5—阴极;6—蠕动泵;7—电源;8—模拟循环冷却水;9—软化水
Fig.1 Schematic diagram of electrochemical water softening experiment device1—replenishment tank; 2—reactor; 3—reservoir; 4—anode; 5—cathode; 6—peristaltic setup; 7—power; 8—circulating cooling water simulation solution; 9—softened water
| 试剂 | 规格 | 生产厂家 |
|---|---|---|
| NaHCO₃ | 500 g/瓶 | 天津市鼎盛鑫化工有限公司 |
| CaCl2 | 500 g/瓶 | 天津市鼎盛鑫化工有限公司 |
| EDTA-2钠 | 0.01 mol/L | 天津市津北精细化工有限公司 |
| 乙酰丙酮 | 0.975 g/ml | 国药集团化学试剂有限公司 |
| pH 缓冲剂 | pH=10 | 上海市仪电科学仪器有限公司 |
表 1 实验所用试剂
Table 1 Reagents used in the experiment
| 试剂 | 规格 | 生产厂家 |
|---|---|---|
| NaHCO₃ | 500 g/瓶 | 天津市鼎盛鑫化工有限公司 |
| CaCl2 | 500 g/瓶 | 天津市鼎盛鑫化工有限公司 |
| EDTA-2钠 | 0.01 mol/L | 天津市津北精细化工有限公司 |
| 乙酰丙酮 | 0.975 g/ml | 国药集团化学试剂有限公司 |
| pH 缓冲剂 | pH=10 | 上海市仪电科学仪器有限公司 |
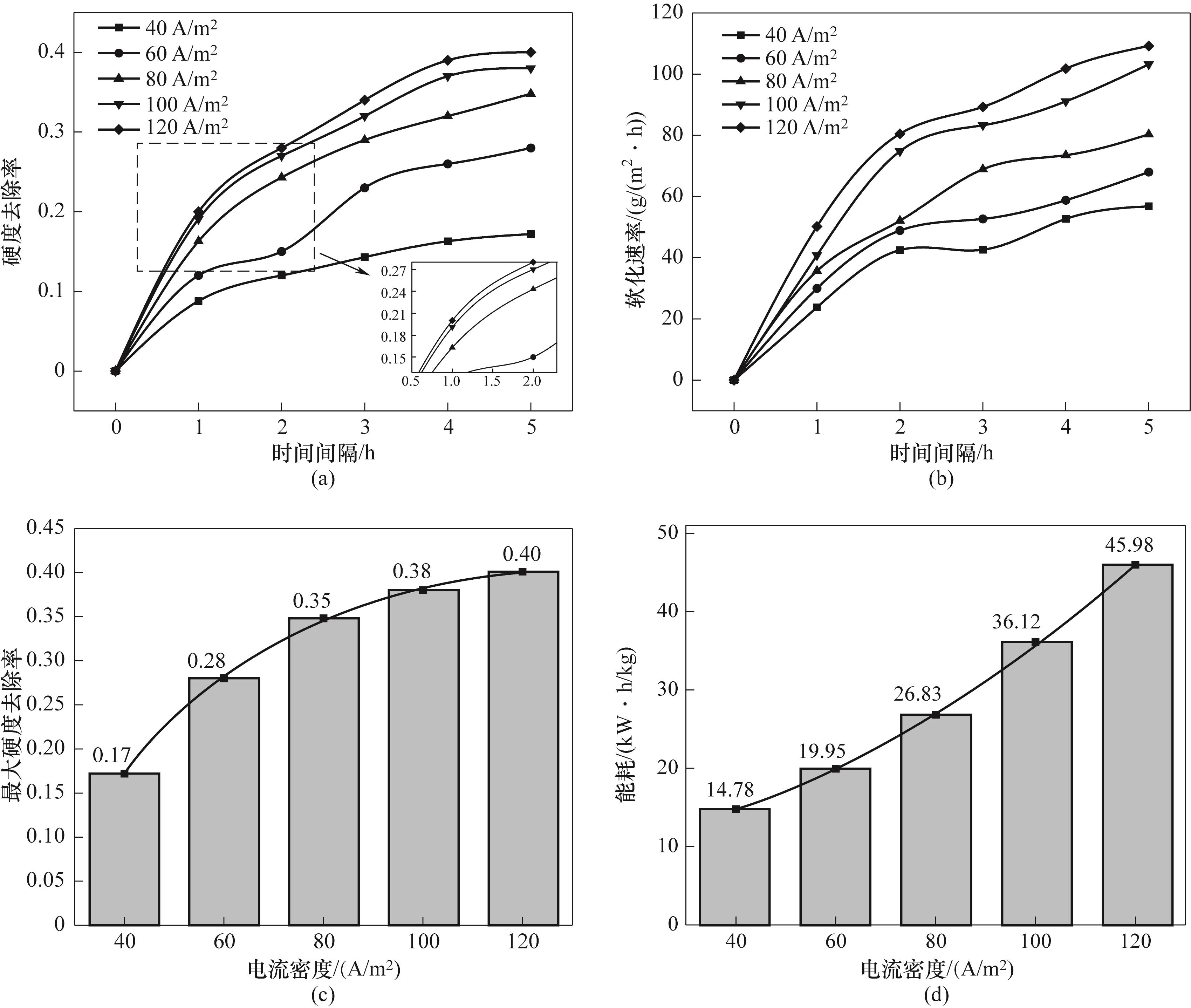
图10 不同电流密度下的硬度去除率(a),软化速率(b),最大硬度去除率(c)和能耗(d)
Fig.10 Hardness removal rate (a), softening rate (b), maximum hardness removal rate (c) and energy consumption (d) at different current densities
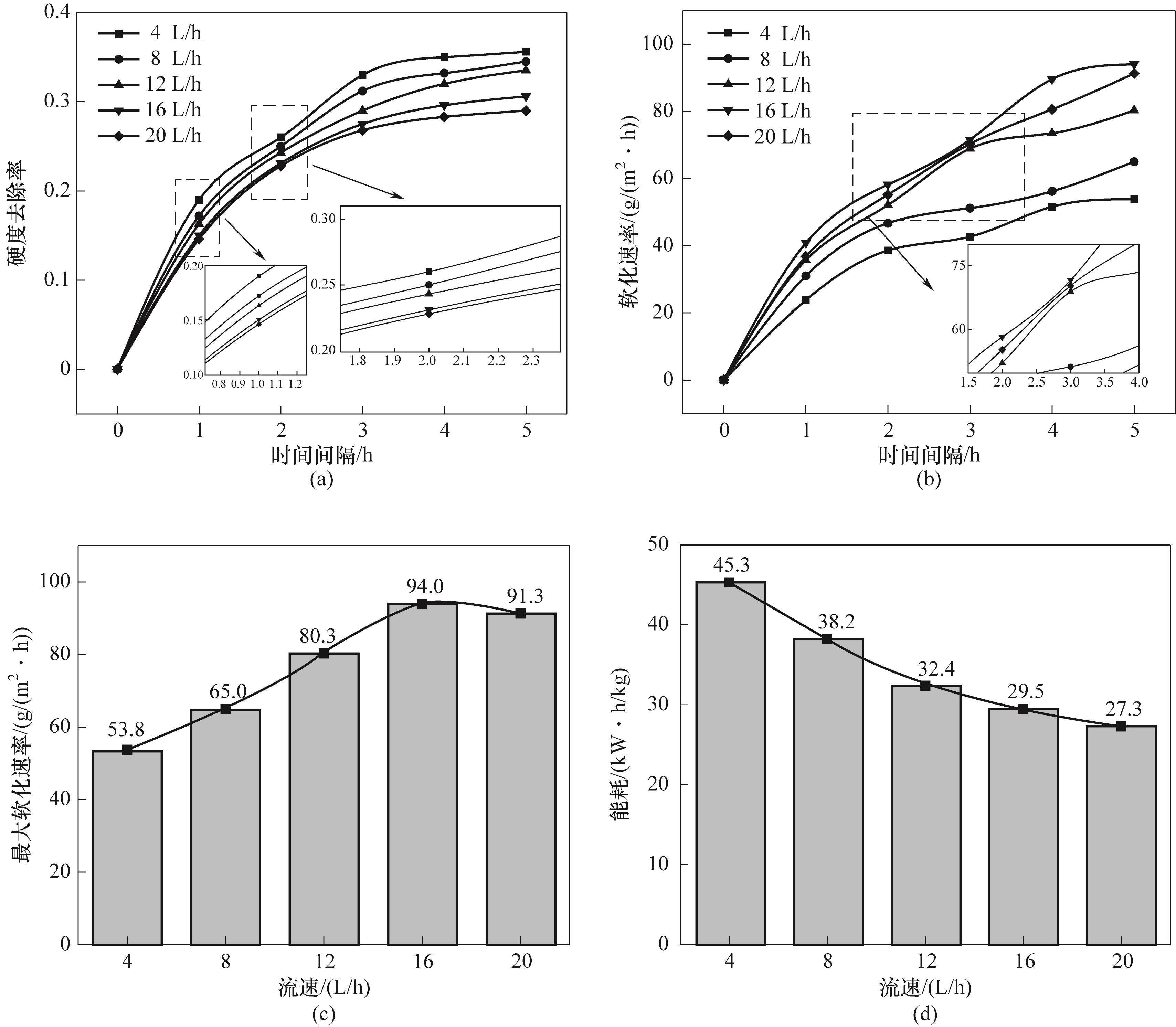
图11 不同入口流速下的硬度去除率(a)、软化速率(b)、最大软化速率(c)和能耗(d)
Fig.11 Hardness removal rate (a), softening rate (b), maximum softening rate (c) and energy consumption (d) at different inlet flow rates

图12 不同初始硬度下的硬度去除率(a),软化速率(b),最大硬度去除率(c)和能耗(d)
Fig.12 Hardness removal rate (a), softening rate (b), maximum hardness removal rate (c) and energy consumption (d) at different initial hardnesses
| 1 | Zhang C H, Tang J W, Zhao G F, et al. Investigation on an electrochemical pilot equipment for water softening with an automatic descaling system: parameter optimization and energy consumption analysis[J]. Journal of Cleaner Production, 2020, 276: 123178. |
| 2 | 林纬, 李吉敏, 汪威, 等. 脉冲电场电化学软化水协同特性分析[J]. 高校化学工程学报, 2023, 37(2): 293-301. |
| Lin W, Li J M, Wang W, et al. Analysis of synergistic characteristics of pulsed electric field and electrochemical on water softening[J]. Journal of Chemical Engineering of Chinese Universities, 2023, 37(2): 293-301. | |
| 3 | Neveux T, Bretaud M, Chhim N, et al. Pilot plant experiments and modeling of CaCO3 growth inhibition by the use of antiscalant polymers in recirculating cooling circuits[J]. Desalination, 2016, 397: 43-52. |
| 4 | Shrestha R, Ban S, Devkota S, et al. Technological trends in heavy metals removal from industrial wastewater: a review[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105688. |
| 5 | Kim J G, Ku J, Jung J, et al. Ion-exchangeable and sorptive reinforced membranes for efficient electrochemical removal of heavy metal ions in wastewater[J]. Journal of Cleaner Production, 2024, 438: 140779. |
| 6 | Lasisi K H, Ajibade T F, Zhang K S. 3,3′-Diaminodiphenyl sulfone engagement in polysulfonamide-based acid-resistant nanofiltration membrane fabrication for efficient separation performance and heavy metal ions removal from wastewater[J]. Journal of Membrane Science, 2022, 661: 120909. |
| 7 | Bera P, Jewrajka S K. Regenerable planting of multifunctional amine: regeneration-enhanced antifouling/antiscaling properties and performance of thin film composite nanofiltration membrane[J]. Journal of Membrane Science, 2024, 692: 122292. |
| 8 | Zhang L, Mishra D, Zhang K L, et al. Electrokinetic study of calcium carbonate and magnesium hydroxide particles in lime softening[J]. Water Research, 2020, 186: 116415. |
| 9 | Shen X L, Li T J, Jiang X P, et al. Desalination of water with high conductivity using membrane-free electrodeionization[J]. Separation and Purification Technology, 2014, 128: 39-44. |
| 10 | 林纬, 王众浩, 汪威, 等. 基于正交实验的电化学法水软化特性分析[J]. 化工学报, 2020, 71(12): 5725-5734. |
| Lin W, Wang Z H, Wang W, et al. Analysis of water softening characteristics of electrochemical method based on orthogonal experiment[J]. CIESC Journal, 2020, 71(12): 5725-5734. | |
| 11 | 栾谨鑫. 复合网状阴极增强电化学除垢性能研究[D]. 大连: 大连理工大学, 2019. |
| Luan J X. Study on multi-meshes coupled cathodes enhanced performance of electrochemical water softening system[D]. Dalian: Dalian University of Technology, 2019. | |
| 12 | Jiang B, Ren X Z, Liu Q N, et al. Electrochemical water softening technology: from fundamental research to practical application[J]. Water Research, 2024, 250: 121077. |
| 13 | Gabrielli C, Maurin G, Francy-Chausson H, et al. Electrochemical water softening: principle and application[J]. Desalination, 2006, 201(1/2/3): 150-163. |
| 14 | 於洋. 用于水软化的高性能电沉积反应器研究[D]. 杭州: 浙江大学, 2020. |
| Yu Y. High performance electro-deposition reactor for water softening[D]. Hangzhou: Zhejiang University, 2020. | |
| 15 | Agostinho L C L, Nascimento L, Cavalcanti B F. Water hardness removal for industrial use: application of the electrolysis process[J]. Open Access Scientific Reports, 2012, 1(9): 460-465. |
| 16 | 钱凯凯, 胡将军. 电解参数对循环冷却水处理及倒极除垢效果的影响[J]. 工业水处理, 2020, 40(1): 83-86. |
| Qian K K, Hu J J. Influence of electrolysis parameters on the treatment of circulating cooling water and the descaling effect of reversing electrodes[J]. Industrial Water Treatment, 2020, 40(1): 83-86. | |
| 17 | Jin H C, Yu Y, Zhang L, et al. Polarity reversal electrochemical process for water softening[J]. Separation and Purification Technology, 2019, 210: 943-949. |
| 18 | Yu Y, Jin H C, Meng P J, et al. Electrochemical water softening using air-scoured washing for scale detachment[J]. Separation and Purification Technology, 2018, 191: 216-224. |
| 19 | Yang Q Y, Xu L Q, He Q B, et al. Reduced cathodic scale and enhanced electrochemical precipitation of Ca2+ and Mg2+ by a novel fenced cathode structure: formation of strong alkaline microenvironment and favorable crystallization[J]. Water Research, 2022, 209: 117893. |
| 20 | 夏凡, 仲伟豪, 毛伟, 等. 促进CaCO3在溶液中成核的高效电化学水软化器[J]. 工业水处理, 2024, 44(7): 83-88. |
| Xia F, Zhong W H, Mao W, et al. Construction of an electrochemical reactor for efficient water softening via promoting CaCO3 nucleation in liquid phase[J]. Industrial Water Treatment, 2024, 44(7): 83-88. | |
| 21 | Zaslavschi I, Shemer H, Hasson D, et al. Electrochemical CaCO3 scale removal with a bipolar membrane system[J]. Journal of Membrane Science, 2013, 445: 88-95. |
| 22 | Hasson D, Sidorenko G, Semiat R. Calcium carbonate hardness removal by a novel electrochemical seeds system[J]. Desalination, 2010, 263(1/2/3): 285-289. |
| 23 | Lin W, Wang Z H, Wang W, et al. Comparative analysis the performance of electrochemical water softening between high frequency electric fields and direct current electric fields based on orthogonal experimental methods[J]. Water Science and Technology, 2021, 83(7): 1677-1690. |
| 24 | 陈琦. 电化学除垢的垂直平面电极间流动特性研究[J]. 化学工程与装备, 2020(6): 5-7. |
| Chen Q. Study on flow characteristics between vertical plane electrodes in electrochemical descaling[J]. Chemical Engineering & Equipment, 2020(6): 5-7. | |
| 25 | Mao W, Gu Y W, Kang W D, et al. Facilitated OH- diffusion via bubble motion and water flow in a novel electrochemical reactor for enhancing homogeneous nucleation of CaCO3 [J]. Water Research, 2023, 242: 120195. |
| 26 | Zhu J, Zhang X H, Lv P Y, et al. An experimental investigation of convective mass transfer characterization in two configurations of electrolysers[J]. International Journal of Hydrogen Energy, 2018, 43(18): 8632-8643. |
| 27 | 胡瑞柱, 黄廷林, 刘泽男. 碳酸钙诱导结晶动力学影响因素研究[J]. 中国环境科学, 2021, 41(8): 3584-3589. |
| Hu R Z, Huang T L, Liu Z N. Influencing factors of induced crystallization kinetics of calcium carbonate[J]. China Environmental Science, 2021, 41(8): 3584-3589. | |
| 28 | 徐浩, 延卫. 阴极电流密度对电化学除垢技术生成水垢的影响[J]. 西安交通大学学报, 2013, 47(7): 47-51. |
| Xu H, Yan W. Effect of cathode current density on scale generated by electrochemical scale removal technique[J]. Journal of Xi'an Jiaotong University, 2013, 47(7): 47-51. | |
| 29 | Li Y, Wang L, Wang L Y. Facilitating removal efficiency of electrochemical descaling system using confined crystallization membranes[J]. Journal of Water Process Engineering, 2023, 56: 104338. |
| 30 | Zhu T Z, Wang M, Yu D Z, et al. Improving cathode cleaning and current efficiency by regulating loose scale deposition in scale inhibitor-containing water[J]. Separation and Purification Technology, 2023, 323: 124494. |
| [1] | 吴迪, 刘世朋, 王文伟, 姜久春, 杨晓光. 机械压力对锂金属电池性能影响的研究进展[J]. 化工学报, 2025, 76(4): 1422-1431. |
| [2] | 李坤, 黄锐, 丛君, 马海涛, 常龙娇, 罗绍华. NCM622正极材料结构形态和储锂特性的同步演变[J]. 化工学报, 2025, 76(4): 1831-1840. |
| [3] | 马钟琛, 魏子杰, 朱明涛, 叶恒棣, 郭学益, 谭磊. 一步氧化法制备锰酸锂正极材料用电池级四氧化三锰[J]. 化工学报, 2025, 76(3): 1363-1374. |
| [4] | 肖志华, 房浩楠, 郑方植, 孙冬, 陶丽达, 李永峰, 徐春明, 马新龙. NaCl辅助构筑高性能沥青基硬炭负极材料[J]. 化工学报, 2025, 76(2): 846-857. |
| [5] | 纪之骄, 张晓方, 甘汶, 薛云鹏. 载体对单原子电催化剂合成氨性能的影响与调控策略[J]. 化工学报, 2025, 76(1): 18-39. |
| [6] | 郭珊, 田雨, 徐永滨, 王朋, 刘治明. 废旧电池再资源化制备高性能中熵合金催化剂及其性能研究[J]. 化工学报, 2025, 76(1): 231-240. |
| [7] | 徐子易, 席阳, 宋泽文, 周海骏. 碳纳米材料在锌离子电池中的应用研究进展[J]. 化工学报, 2025, 76(1): 40-52. |
| [8] | 吴德威, 汪郑鹏, 周玥, 李晓宁, 陈招, 李卓, 刘成伟, 李学刚, 肖文德. 固定床法制备锂离子电池硅碳负极材料及其储锂性能研究[J]. 化工学报, 2024, 75(S1): 300-308. |
| [9] | 秦思宇, 刘艺佳, 杨佳成, 佟薇, 金立文, 孟祥兆. 受限蒸汽腔内气液两相传热特性研究[J]. 化工学报, 2024, 75(S1): 47-55. |
| [10] | 王舒英, 左涛, 石志伟, 范小明, 张卫新. 阳离子交换树脂基介孔石墨化碳合成与储钠性能[J]. 化工学报, 2024, 75(9): 3338-3347. |
| [11] | 罗欣怡, 徐强, 佘永璐, 聂腾飞, 郭烈锦. 光电分解水制氢气泡动力学特性及其传质机理研究[J]. 化工学报, 2024, 75(9): 3083-3093. |
| [12] | 彭丹, 卢俊杰, 倪文静, 杨媛, 汪靖伦. 高电压钴酸锂电池电解液研究进展[J]. 化工学报, 2024, 75(9): 3028-3040. |
| [13] | 杨明军, 宋维, 张磊, 凌铮, 陈兵兵, 宋永臣. CO2-海水水合物生成强化方法研究[J]. 化工学报, 2024, 75(8): 2939-2948. |
| [14] | 齐琪, 郭利平, 石李明, 郑映, 潘鹏举. 山梨醇类成核剂改性聚丙烯及其共聚物的结晶行为与性能[J]. 化工学报, 2024, 75(7): 2688-2699. |
| [15] | 王天闻, 闫肃, 赵梦园, 杨天让, 刘建国. 固体氧化物电池空气电极铬中毒机理及抗铬性能研究进展[J]. 化工学报, 2024, 75(6): 2091-2108. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号