化工学报 ›› 2025, Vol. 76 ›› Issue (7): 3350-3360.DOI: 10.11949/0438-1157.20241421
高凤凤1,2( ), 程慧峰1, 杨博3, 郝晓刚1,2,4(
), 程慧峰1, 杨博3, 郝晓刚1,2,4( )
)
收稿日期:2024-12-09
修回日期:2025-01-23
出版日期:2025-07-25
发布日期:2025-08-13
通讯作者:
郝晓刚
作者简介:高凤凤(1988—),女,博士,副教授,gaofengfeng@tyut.edu.cn
基金资助:
Fengfeng GAO1,2( ), Huifeng CHENG1, Bo YANG3, Xiaogang HAO1,2,4(
), Huifeng CHENG1, Bo YANG3, Xiaogang HAO1,2,4( )
)
Received:2024-12-09
Revised:2025-01-23
Online:2025-07-25
Published:2025-08-13
Contact:
Xiaogang HAO
摘要:
电控离子交换技术(electrochemically switched ion exchange,ESIX)是将电活性离子交换材料(EXIMs)沉积或涂覆在导电基底上,通过电化学控制导电基底上活性材料氧化还原状态实现目标离子置入与释放,从而实现离子的分离。该技术具有痕量提取、无二次污染、速率可控、高选择性等优点。通过共沉淀法制备NiFeMn LDH,并将其与碳纳米管(CNTs)、聚偏二氟乙烯(PVDF)混合涂覆到石墨板上,制得NiFeMn LDH/CNTs/PVDF膜电极。NiFeMn LDH层板上具有丰富的羟基官能团,可与W(Ⅵ)发生羟基配位;层间的阴离子与W(Ⅵ)进行离子交换,可为W(Ⅵ)提供丰富的活性位点。在ESIX系统中,膜电极对W(Ⅵ)的吸附容量可达122.10 mg·g-1,且W(Ⅵ)与Mo(Ⅵ)、Cl-、NO
中图分类号:
高凤凤, 程慧峰, 杨博, 郝晓刚. 电驱动NiFeMn LDH/CNTs/PVDF膜电极选择性提取钨酸根离子[J]. 化工学报, 2025, 76(7): 3350-3360.
Fengfeng GAO, Huifeng CHENG, Bo YANG, Xiaogang HAO. Electrically driven NiFeMn LDH/CNTs/PVDF film electrode for selective extraction of tungstate ions[J]. CIESC Journal, 2025, 76(7): 3350-3360.
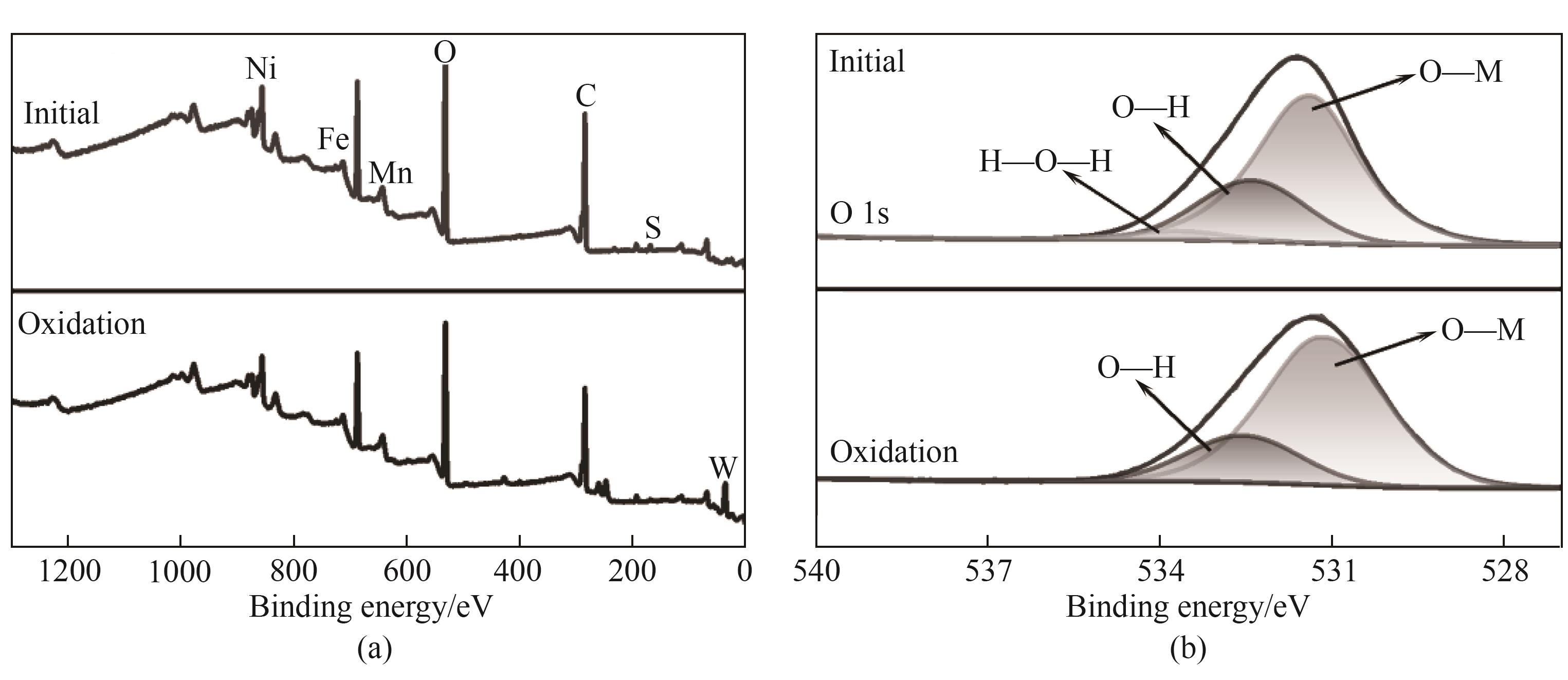
图4 NiFeMn LDH在吸附W(Ⅵ)前后的扫描光谱图(a)和O 1s高分辨光谱(b)
Fig.4 Scanning spectra (a) and O 1s high resolution spectra (b) of NiFeMn LDH before and after adsorption of W(Ⅵ) atoms

图5 (a) NiFeMn LDH/CNTs/PVDF膜电极在不同扫速下的CV曲线;(b)阳极/阴极峰值电流随扫速变化的拟合曲线
Fig.5 (a) CV curves of NiFeMn LDH/CNTs/PVDF at different scan rates; (b) Fitting curves of anode/cathode peak currents with scan rates
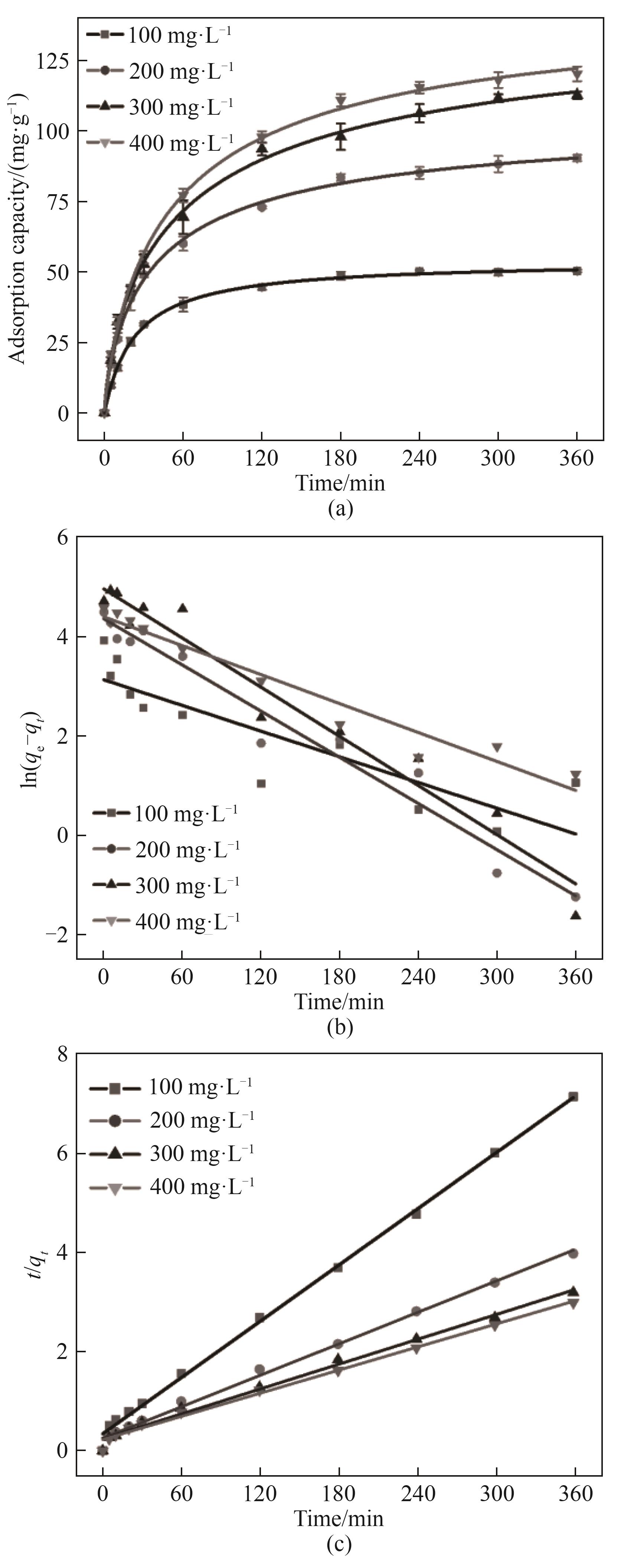
图8 (a) NiFeMn LDH膜在不同初始浓度下的W(Ⅵ)吸附曲线;不同初始W(Ⅵ)浓度的(b)准一级动力学和(c)准二级动力学曲线
Fig.8 (a) W(Ⅵ) adsorption capacity of NiFeMn LDH film at different initial concentrations; (b) Pseudo-first-order kinetic and (c) pseudo-second-order kinetic curves for different initial W(Ⅵ) concentrations
| Initial concentration/(mg·L-1) | Qexp/(mg·g-1) | Quasi-first-order kinetic | Quasi-second-order kinetic | ||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | Qcal/(mg·g-1) | R2 | k2/(g·mg-1·min-1) | Qcal/(mg·g-1) | R2 | ||
| 100 | 50.22 | 0.00863 | 49.19 | 0.7687 | 0.00115 | 50.46 | 0.9973 |
| 200 | 90.43 | 0.01550 | 90.13 | 0.9683 | 0.00048 | 88.83 | 0.9946 |
| 300 | 112.64 | 0.01651 | 112.26 | 0.9733 | 0.00032 | 110.90 | 0.9917 |
| 400 | 122.10 | 0.00972 | 117.78 | 0.9564 | 0.00030 | 119.20 | 0.9924 |
表1 不同初始浓度下NiFeMn LDH膜吸附W(Ⅵ)的动力学模型模拟相关参数
Table 1 Parameters related to kinetic model simulation of W(Ⅵ) adsorption on NiFeMn LDH films with different initial concentrations
| Initial concentration/(mg·L-1) | Qexp/(mg·g-1) | Quasi-first-order kinetic | Quasi-second-order kinetic | ||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | Qcal/(mg·g-1) | R2 | k2/(g·mg-1·min-1) | Qcal/(mg·g-1) | R2 | ||
| 100 | 50.22 | 0.00863 | 49.19 | 0.7687 | 0.00115 | 50.46 | 0.9973 |
| 200 | 90.43 | 0.01550 | 90.13 | 0.9683 | 0.00048 | 88.83 | 0.9946 |
| 300 | 112.64 | 0.01651 | 112.26 | 0.9733 | 0.00032 | 110.90 | 0.9917 |
| 400 | 122.10 | 0.00972 | 117.78 | 0.9564 | 0.00030 | 119.20 | 0.9924 |
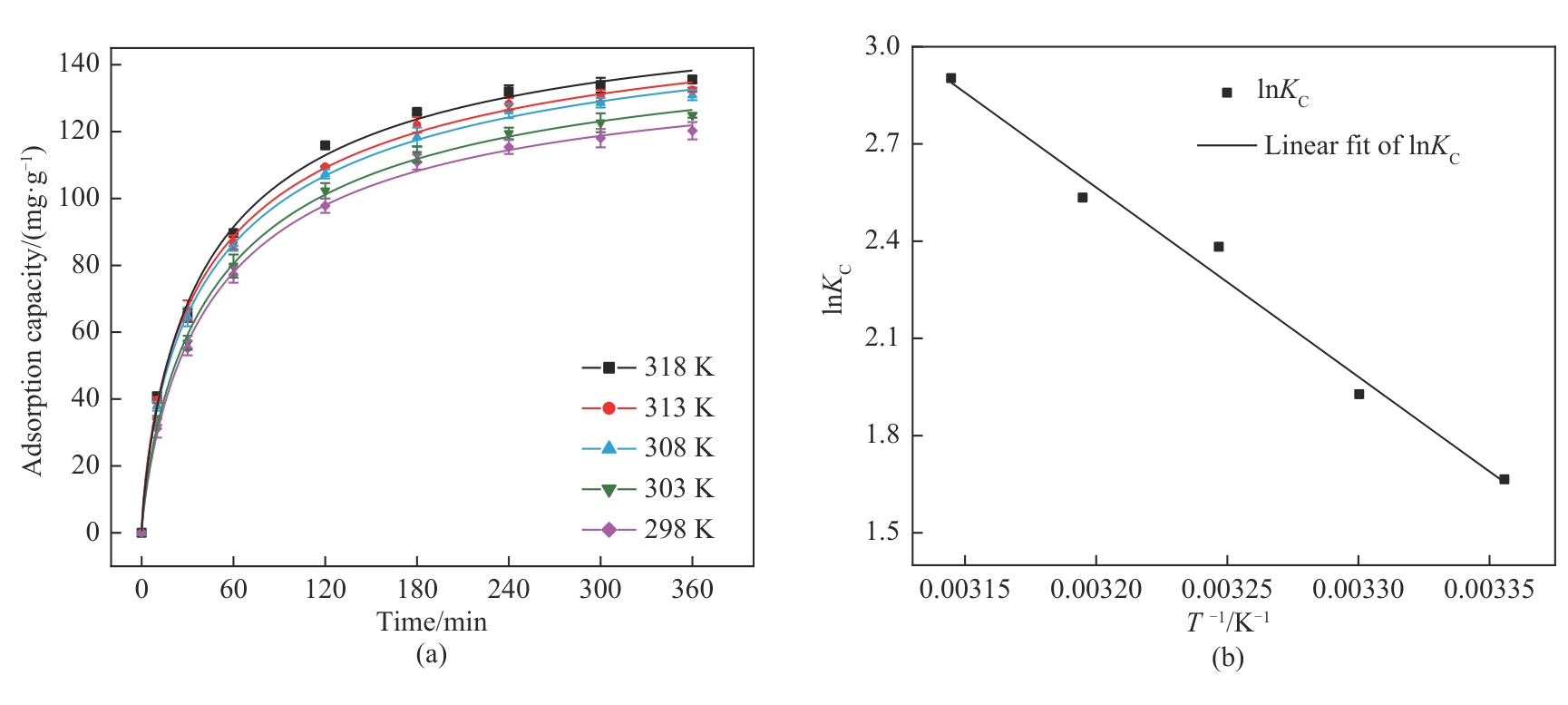
图9 (a)NiFeMn LDH膜在不同温度下的W(Ⅵ)吸附曲线;(b)热力学拟合曲线
Fig.9 (a) W(Ⅵ) adsorption curves of NiFeMn LDH film at different temperatures; (b) Thermodynamic fit curve
| T/K | ΔG/(kJ·mol-1) | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | R2 |
|---|---|---|---|---|
| 298 | -4.125 | — | — | — |
| 303 | -4.856 | — | — | — |
| 308 | -6.101 | 48.59 | 176.83 | 0.9847 |
| 313 | -6.596 | — | — | — |
| 318 | -7.675 | — | — | — |
表2 NiFeMn LDH膜吸附W(Ⅵ)的热力学计算结果
Table 2 Thermodynamic calculations of W(Ⅵ) adsorption on NiFeMn LDH film
| T/K | ΔG/(kJ·mol-1) | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | R2 |
|---|---|---|---|---|
| 298 | -4.125 | — | — | — |
| 303 | -4.856 | — | — | — |
| 308 | -6.101 | 48.59 | 176.83 | 0.9847 |
| 313 | -6.596 | — | — | — |
| 318 | -7.675 | — | — | — |
| NiFeMn LDH types | Binding energy/eV |
|---|---|
| NiFeMn LDH-WO | -8.55 |
| NiFeMn LDH-SO | -8.53 |
| single layer NiFeMn LDH-WO | -10.06 |
表3 NiFeMn LDH与阴离子的结合能
Table 3 The binding energy of NiFeMn LDH with anions
| NiFeMn LDH types | Binding energy/eV |
|---|---|
| NiFeMn LDH-WO | -8.55 |
| NiFeMn LDH-SO | -8.53 |
| single layer NiFeMn LDH-WO | -10.06 |
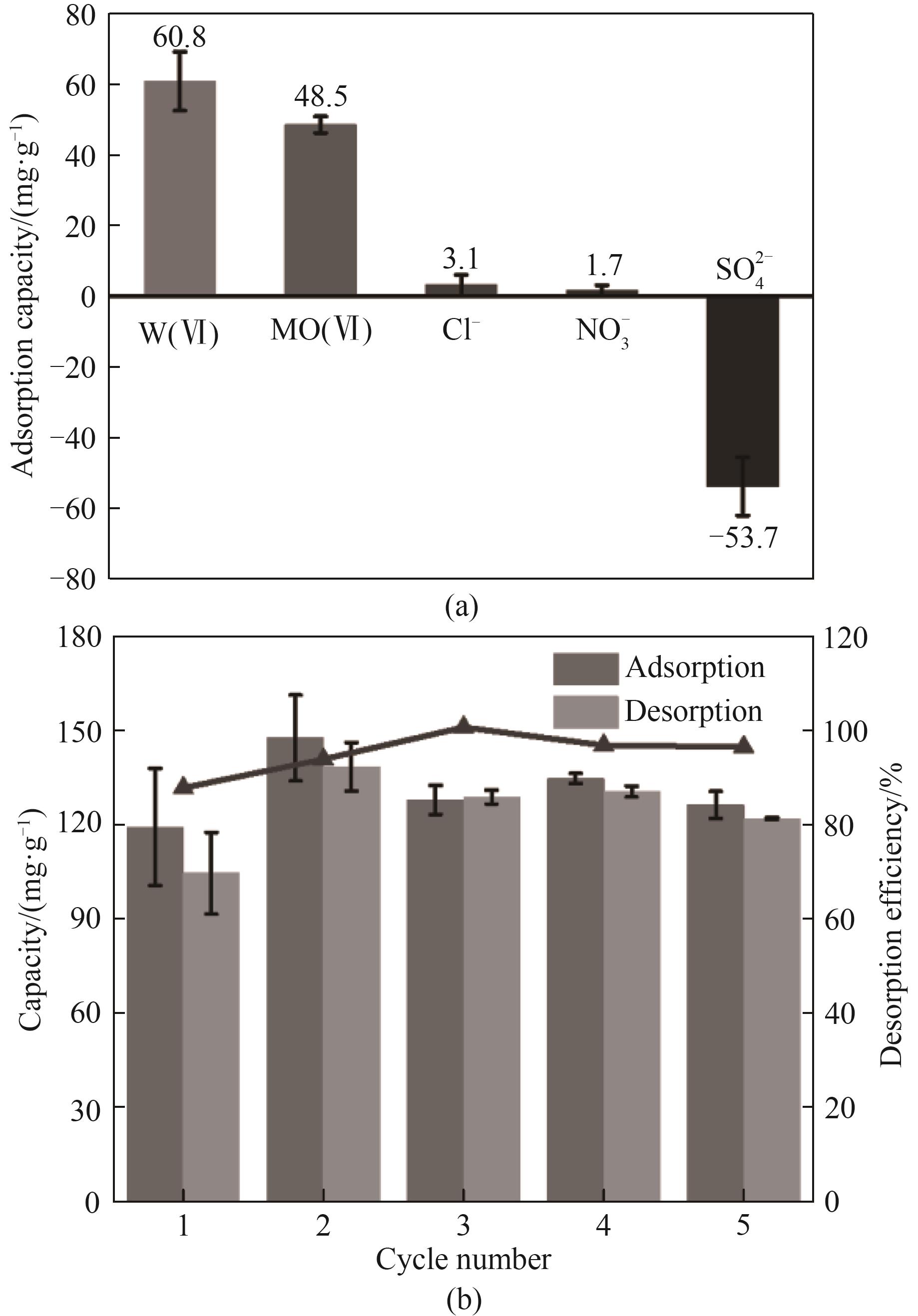
图11 NiFeMn LDH/CNTs/PVDF膜电极的(a)竞争离子存在下的吸附容量和(b)循环稳定性
Fig.11 (a) Adsorption capacity in the presence of competing ions and (b) cycling stability of NiFeMn LDH/CNTs/PVDF film electrode
| Anion types | Separation factor |
|---|---|
| W(Ⅵ) | 1.00 |
| Mo(Ⅵ) | 1.25 |
| Cl- | 19.60 |
| NO | 35.80 |
表4 NiFeMn LDH/CNTs/PVDF复合膜对不同阴离子的分离因子
Table 4 Separation factors of NiFeMn LDH/CNTs/PVDF composite films for different anions
| Anion types | Separation factor |
|---|---|
| W(Ⅵ) | 1.00 |
| Mo(Ⅵ) | 1.25 |
| Cl- | 19.60 |
| NO | 35.80 |
| [1] | 周永敏. 炼油技术开发现状及发展趋势[J]. 石化技术, 2019, 26(12): 120, 115. |
| Zhou Y M. Present situation and development trend of refining technology development[J]. Petrochemical Industry Technology, 2019, 26(12): 120, 115. | |
| [2] | 赵中伟, 孙丰龙, 杨金洪, 等. 我国钨资源、技术和产业发展现状与展望[J]. 中国有色金属学报, 2019, 29(9): 1902-1916. |
| Zhao Z W, Sun F L, Yang J H, et al. Status and prospect for tungsten resources, technologies and industrial development in China[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(9): 1902-1916. | |
| [3] | Serdyukov S I, Kniazeva M I, Sizova I A, et al. A new precursor for synthesis of nickel-tungsten sulfide aromatic hydrogenation catalyst[J]. Molecular Catalysis, 2021, 502: 111357. |
| [4] | Haack A, Hillenbrand J, van Gastel M, et al. Spectroscopic and theoretical study on siloxy-based molybdenum and tungsten alkylidyne catalysts for alkyne metathesis[J]. ACS Catalysis, 2021, 11(15): 9086-9101. |
| [5] | Dawood K M, Nomura K. Recent developments in Z-selective olefin metathesis reactions by molybdenum, tungsten, ruthenium, and vanadium catalysts[J]. Advanced Synthesis & Catalysis, 2021, 363(8): 1970-1997. |
| [6] | Bonassi F, Ravelli D, Protti S, et al. Decatungstate photocatalyzed acylations and alkylations in flow via hydrogen atom transfer[J]. Advanced Synthesis & Catalysis, 2015, 357(16/17): 3687-3695. |
| [7] | Yan S Q, Tong T, Li Y, et al. Production of biodiesel through esterification reaction using choline exchanging polytungstoboronic acids as temperature-responsive catalysts[J]. Catalysis Surveys from Asia, 2017, 21(4): 151-159. |
| [8] | Zhao C, Wang C Y, Wang X R, et al. Recovery of tungsten and titanium from spent SCR catalyst by sulfuric acid leaching process[J]. Waste Management, 2023, 155: 338-347. |
| [9] | Wang B, Yang Q W. Optimization of roasting parameters for recovery of vanadium and tungsten from spent SCR catalyst with composite roasting[J]. Processes, 2021, 9(11): 1923. |
| [10] | Moon G, Kim J H, Lee J Y, et al. Leaching of spent selective catalytic reduction catalyst using alkaline melting for recovery of titanium, tungsten, and vanadium[J]. Hydrometallurgy, 2019, 189: 105132. |
| [11] | 张邦胜, 肖连生, 张启修. 沉淀法分离钨钼的研究进展[J]. 江西有色金属, 2001, 15(2): 26-29. |
| Zhang B S, Xiao L S, Zhang Q X. Progress in W/Mo separation by precipitation[J]. Jiangxi Nonferrous Metals, 2001, 15(2): 26-29. | |
| [12] | 关文娟, 张贵清, 高从堦, 等. 双氧水络合萃取分离钨钼的前驱体料液的制备[J]. 中南大学学报(自然科学版), 2013, 44(5): 1766-1774. |
| Guan W J, Zhang G Q, Gao C J, et al. Preparation of precursor solution for solvent separation of Mo and W by H2O2-complexation[J]. Journal of Central South University (Science and Technology), 2013, 44(5): 1766-1774. | |
| [13] | 袁斌, 邓舜勤. 用离子交换法从钨溶液中分离钼[J]. 湿法冶金, 2003, 22(2): 69-78. |
| Yuan B, Deng S Q. Remove of molybdenum from tungsten solution by ion exchange[J]. Hydrometallurgy of China, 2003, 22(2): 69-78. | |
| [14] | 郭超, 肖连生. 钼酸铵结晶过程中的钨钼分离研究[J]. 稀有金属与硬质合金, 2010, 38(3): 1-4, 15. |
| Guo C, Xiao L S. Study on tungsten and molybdenum separation in the ammonium molybdate crystallization process[J]. Rare Metals and Cemented Carbides, 2010, 38(3): 1-4, 15. | |
| [15] | Zhang W J, Li J T, Zhao Z W, et al. Separation of W and Mo from their peroxoacids solutions by thermal decomposition[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(10): 2731-2737. |
| [16] | 王文强, 赵中伟. 钨提取冶金中钨钼分离研究进展——从“削足适履”到“量体裁衣”[J]. 中国钨业, 2015, 30(1): 49-55. |
| Wang W Q, Zhao Z W. Research advances of tungsten-molybdenum separation in tungsten extractive metallurgy[J]. China Tungsten Industry, 2015, 30(1): 49-55. | |
| [17] | 高凤凤, 杨言言, 杜晓, 等. 电控离子(交换)膜分离技术——从ESIX到ESIPM[J]. 化学进展, 2020, 32(9): 1344-1351. |
| Gao F F, Yang Y Y, Du X, et al. Electrically switched ion membrane for ion selective separation and recovery: from ESIX to ESIPM[J]. Progress in Chemistry, 2020, 32(9): 1344-1351. | |
| [18] | Hu W T, Sun B C, Zhang X F, et al. New insights into the ion/electron transfer mechanisms of LiMn2O4-based membrane electrodes at different electron fluxes[J]. Small, 2025: 2407656. |
| [19] | Zeng G L, Ye D N, Zhang X F, et al. A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions[J]. Chinese Journal of Chemical Engineering, 2023, 63: 51-62. |
| [20] | Jiang M F, Zhang X F, Du X, et al. An electrochemically induced dual-site adsorption composite film of Ni-MOF derivative/NiCo LDH for selective bromide-ion extraction[J]. Separation and Purification Technology, 2022, 283: 120175. |
| [21] | Hu Y S, Luo Q L, Du X, et al. Film electrode by incorporating polypyrrole/carbon black into cross-linked binders of chitosan/cationic polyacrylamide for selective chloride extraction in wastewater[J]. Separation and Purification Technology, 2024, 330: 125434. |
| [22] | Luo J H, Du X, Gao F F, et al. Electrochemically triggered iodide-vacancy BiOI film for selective extraction of iodide ion from aqueous solutions[J]. Separation and Purification Technology, 2021, 259: 118120. |
| [23] | Song T, Luo Q L, Gao F F, et al. Adsorption and electro-assisted method removal of boron in aqueous solution by nickel hydroxide[J]. Journal of Industrial and Engineering Chemistry, 2023, 118: 372-382. |
| [24] | Song Y F, He L H, Chen X Y, et al. Removal of tungsten from molybdate solution by Fe-Mn binary oxide adsorbent[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(11): 2492-2502. |
| [25] | Srivastava R R, Kim M S, Lee J C. Separation of tungsten from Mo-rich leach liquor by adsorption onto a typical Fe-Mn cake: kinetics, equilibrium, mechanism, and thermodynamics studies[J]. Industrial & Engineering Chemistry Research, 2013, 52(49): 17591-17597. |
| [26] | Chai Q, Yang B, Li X M, et al. A self-driven Ni(OH)2/CB/PVDF film for highly efficient adsorption of tungsten via hydroxyl ligand exchange[J]. Separation and Purification Technology, 2025, 352: 128163. |
| [27] | Zhao Z W, Cao C F, Chen X Y. Separation of macro amounts of tungsten and molybdenum by precipitation with ferrous salt[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(12): 2758-2763. |
| [28] | Zhao Y J, Zhang P J, Liang J R, et al. Unlocking layered double hydroxide as a high-performance cathode material for aqueous zinc-ion batteries[J]. Advanced Materials, 2022, 34(37): 2204320. |
| [29] | Lu Z Y, Qian L, Tian Y, et al. Ternary NiFeMn layered double hydroxides as highly-efficient oxygen evolution catalysts[J]. Chemical Communications, 2016, 52(5): 908-911. |
| [30] | Ye D N, Gao F F, Zeng G L, et al. An electroactive BiOBr/PVDF/CB film electrode for electrochemical extraction of bromine ions from brines[J]. Industrial & Engineering Chemistry Research, 2023, 62(22): 8882-8892. |
| [31] | Yang B, Chai Q, Li X M, et al. A potential-driven FeMnO x /CNTs film electrode for efficient extraction of WO 4 2 - via electrochemical coordination and inner-sphere complexation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 699: 134686. |
| [1] | 裴星亮, 叶翠平, 裴赢丽, 李文英. 碱改性MIL-53(Cr)选择性吸附分离二甲苯异构体[J]. 化工学报, 2025, 76(S1): 258-267. |
| [2] | 龚丽芳, 任美慧, 蒋吉春, 郭光召, 胡红云, 黄永达, 姚洪. 垃圾焚烧烟气中芳香烃化合物在线监测和选择性催化还原脱除研究[J]. 化工学报, 2025, 76(6): 3018-3028. |
| [3] | 茅雨洁, 路晓飞, 锁显, 杨立峰, 崔希利, 邢华斌. 工业气体中微量氧深度脱除催化剂研究进展[J]. 化工学报, 2025, 76(5): 1997-2010. |
| [4] | 徐泽海, 刘超, 张国亮. 聚合物基疏水渗透汽化膜及其溶剂回收应用[J]. 化工学报, 2025, 76(5): 2055-2069. |
| [5] | 郭彭涛, 王婷, 薛波, 应允攀, 刘大欢. 用于CH4/N2分离的多吸附位点超微孔MOF[J]. 化工学报, 2025, 76(5): 2304-2312. |
| [6] | 向昕辰, 鲁丹, 赵影, 姚之侃, 寇瑞强, 郑丹军, 周志军, 张林. 聚酰胺纳滤膜表面季铵化提高荷正电性及其锂镁分离性能[J]. 化工学报, 2025, 76(5): 2377-2386. |
| [7] | 张耀辉, 班宇杰, 杨维慎. 以蒸气加工法制备和修饰金属-有机框架膜[J]. 化工学报, 2025, 76(5): 2070-2086. |
| [8] | 尤潇楠, 范小强, 杨遥, 王靖岱, 阳永荣. 超临界乙烯和高压聚乙烯混合物的减压分离过程建模方法[J]. 化工学报, 2025, 76(2): 695-706. |
| [9] | 杨晨, 毛伟, 董兴宗, 田松, 赵锋伟, 吕剑. 选择性加氢脱氯合成烯烃研究进展[J]. 化工学报, 2025, 76(1): 53-70. |
| [10] | 唐宇昊, 张迎迎, 赵智伟, 鲁梦悦, 张飞飞, 王小青, 杨江峰. 弱极性超微孔Sc/In-CPM-66A用于CH4/N2吸附分离性能[J]. 化工学报, 2024, 75(9): 3210-3220. |
| [11] | 吴哲明, 张碧云, 郑仁朝. 腈水解酶立体选择性改造及其合成布瓦西坦[J]. 化工学报, 2024, 75(7): 2633-2643. |
| [12] | 周文轩, 刘珍, 张福建, 张忠强. 高通量-高截留率时间维度膜法水处理机理研究[J]. 化工学报, 2024, 75(7): 2583-2593. |
| [13] | 王涛虹, 王超, 李政, 刘莹, 田歌, 常刚刚, 阳晓宇, 鲍宗必. 固载Cu(Ⅰ)的π络合MOF吸附剂用于乙烷/乙烯的选择性分离[J]. 化工学报, 2024, 75(7): 2565-2573. |
| [14] | 张凯博, 沈佳新, 李玉霞, 谈朋, 刘晓勤, 孙林兵. Y沸石中Cu(Ⅰ)的可控构筑及其乙烯/乙烷吸附分离性能研究[J]. 化工学报, 2024, 75(4): 1607-1615. |
| [15] | 孟园, 倪善, 刘亚锋, 王文杰, 赵越, 朱育丹, 杨良嵘. 功能化多孔氮化碳材料对铀的吸附性能研究[J]. 化工学报, 2024, 75(4): 1616-1629. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号