化工学报 ›› 2025, Vol. 76 ›› Issue (12): 6601-6613.DOI: 10.11949/0438-1157.20250643
张世晨1,3( ), 郭志远1,3, 王艳敏1,3, 郝亚超2, 汪婧1,3, 张盼盼1,3, 纪志永1,3(
), 郭志远1,3, 王艳敏1,3, 郝亚超2, 汪婧1,3, 张盼盼1,3, 纪志永1,3( )
)
收稿日期:2025-06-16
修回日期:2025-08-28
出版日期:2025-12-31
发布日期:2026-01-23
通讯作者:
纪志永
作者简介:张世晨(2000—),男,硕士研究生,zsc15163663375@163.com
基金资助:
Shichen ZHANG1,3( ), Zhiyuan GUO1,3, Yanmin WANG1,3, Yachao HAO2, Jing WANG1,3, Panpan ZHANG1,3, Zhiyong JI1,3(
), Zhiyuan GUO1,3, Yanmin WANG1,3, Yachao HAO2, Jing WANG1,3, Panpan ZHANG1,3, Zhiyong JI1,3( )
)
Received:2025-06-16
Revised:2025-08-28
Online:2025-12-31
Published:2026-01-23
Contact:
Zhiyong JI
摘要:
油气田采出水作为开采石油、天然气及页岩气等产生的伴生废水,亟须进行资源化处置,其所含的锂具有较高的开发潜力,但其组成复杂、锂含量较低、开发难度大。基于此,提出电化学吸附-选择性电渗析纯化浓缩工艺,以最终实现从油气田采出水中获取碳酸锂产品。探究了油气田采出水中特征杂质离子(Ca2+、Sr2+、Ba2+)对电化学吸附提锂性能的影响,并优化了操作条件,在模拟油气田采出水体系下,锂纯度由0.066%提升至44.991%,锂含量由50 mg·L-1富集至124.35 mg·L-1。与选择性电渗析进行耦合,在优化条件下浓缩液中Li+浓度富集至5.68 g·L-1,再经除杂、沉淀、洗涤及干燥可得纯度为99.06%的碳酸锂(Li2CO3)。耦合工艺的能耗和药剂消耗共计3200~3350 CNY·t-1 Li2CO3。研究结果可为油气田采出水中低含量锂的提取/回收提供理论指导与技术参考。
中图分类号:
张世晨, 郭志远, 王艳敏, 郝亚超, 汪婧, 张盼盼, 纪志永. 油气田采出水电化学吸附-选择性电渗析耦合锂提取回收研究[J]. 化工学报, 2025, 76(12): 6601-6613.
Shichen ZHANG, Zhiyuan GUO, Yanmin WANG, Yachao HAO, Jing WANG, Panpan ZHANG, Zhiyong JI. Research on lithium extraction and recovery from oil and gas field produced water coupled with electrochemical adsorption-selective electrodialysis[J]. CIESC Journal, 2025, 76(12): 6601-6613.
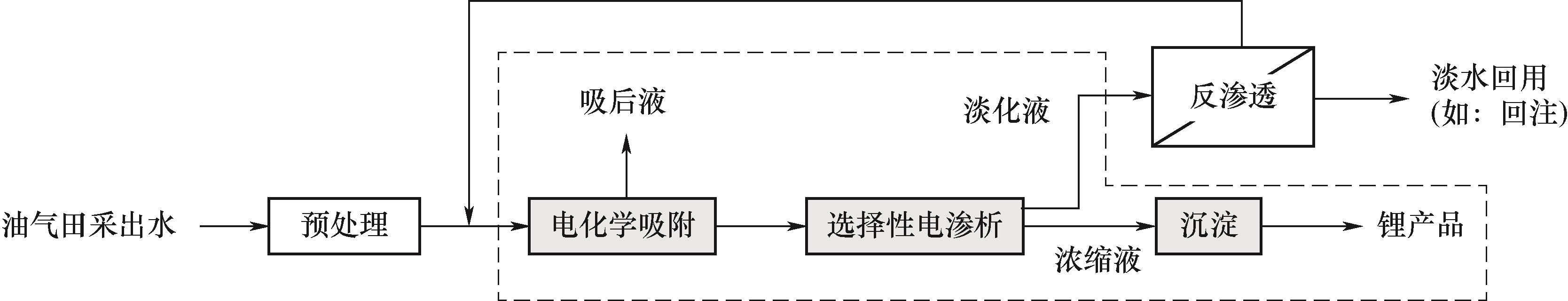
图1 油气田采出水电化学吸附-选择性电渗析耦合锂提取回收工艺示意图
Fig.1 Schematic diagram of electrochemical adsorption-selective electrodialysis coupled with lithium extraction and recovery process for oil and gas field produced water
| 采出水组成 | 离子浓度/(mg/L) |
|---|---|
| Li+ | 50.0 |
| Mg2+ | 121.7 |
| Na+ | 72510.0 |
| Ca2+ | 1273.8 |
| Sr2+ | 928.3 |
| Ba2+ | 1135.3 |
表1 油气田采出水组成成分
Table 1 Composition of produced water in oil and gas fields
| 采出水组成 | 离子浓度/(mg/L) |
|---|---|
| Li+ | 50.0 |
| Mg2+ | 121.7 |
| Na+ | 72510.0 |
| Ca2+ | 1273.8 |
| Sr2+ | 928.3 |
| Ba2+ | 1135.3 |
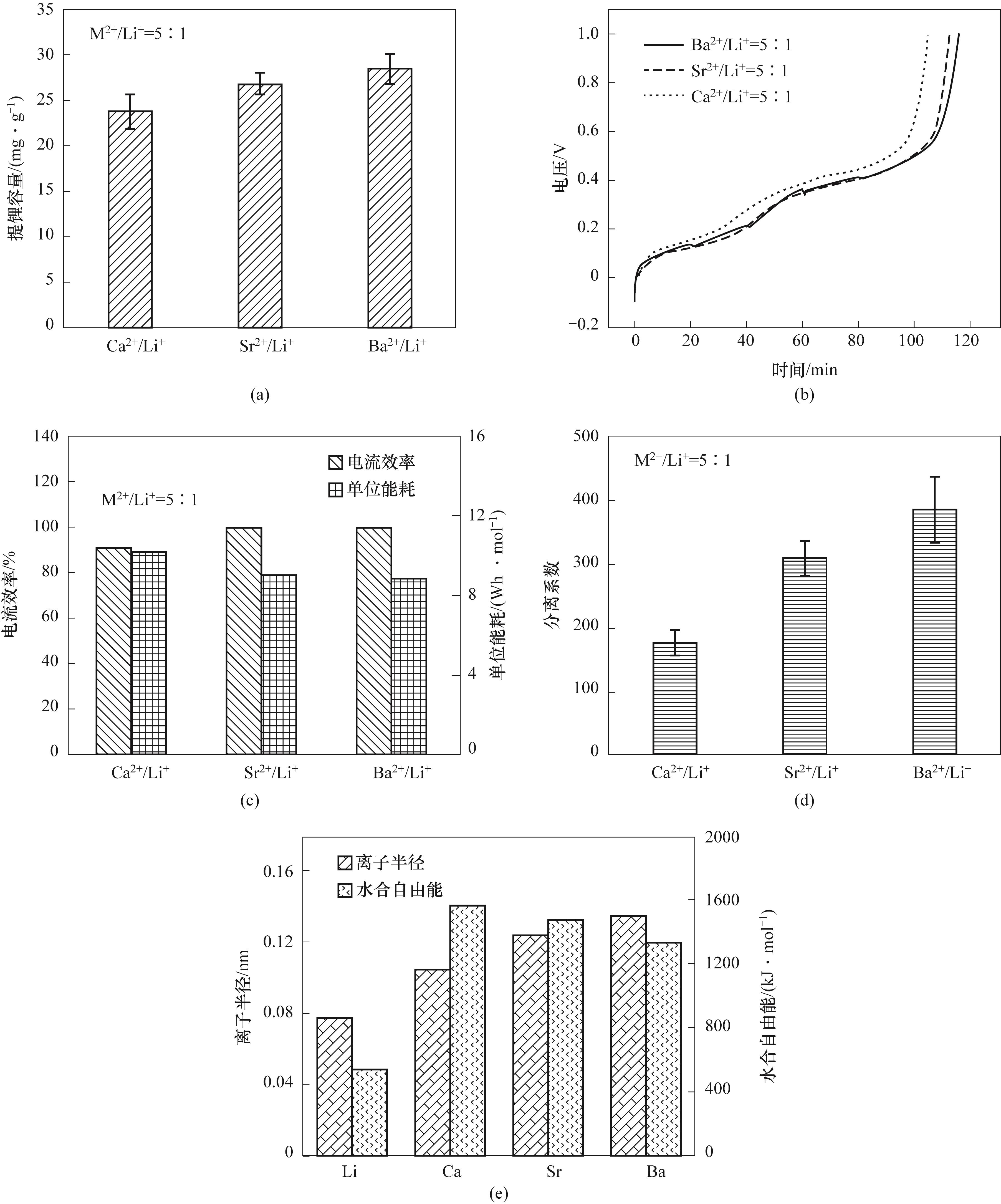
图4 不同杂质离子体系的提锂容量(a)、电压随时间变化趋势(b)、电流效率和单位能耗(c)、分离系数(d)及离子半径与离子水合自由能(e)
Fig.4 Lithium extraction capacity(a), voltage trend with time(b), current efficiency and specific energy consumption(c), separation factor (d) and ionic radius and ionic hydration free energy(e) under different impurity ion systems
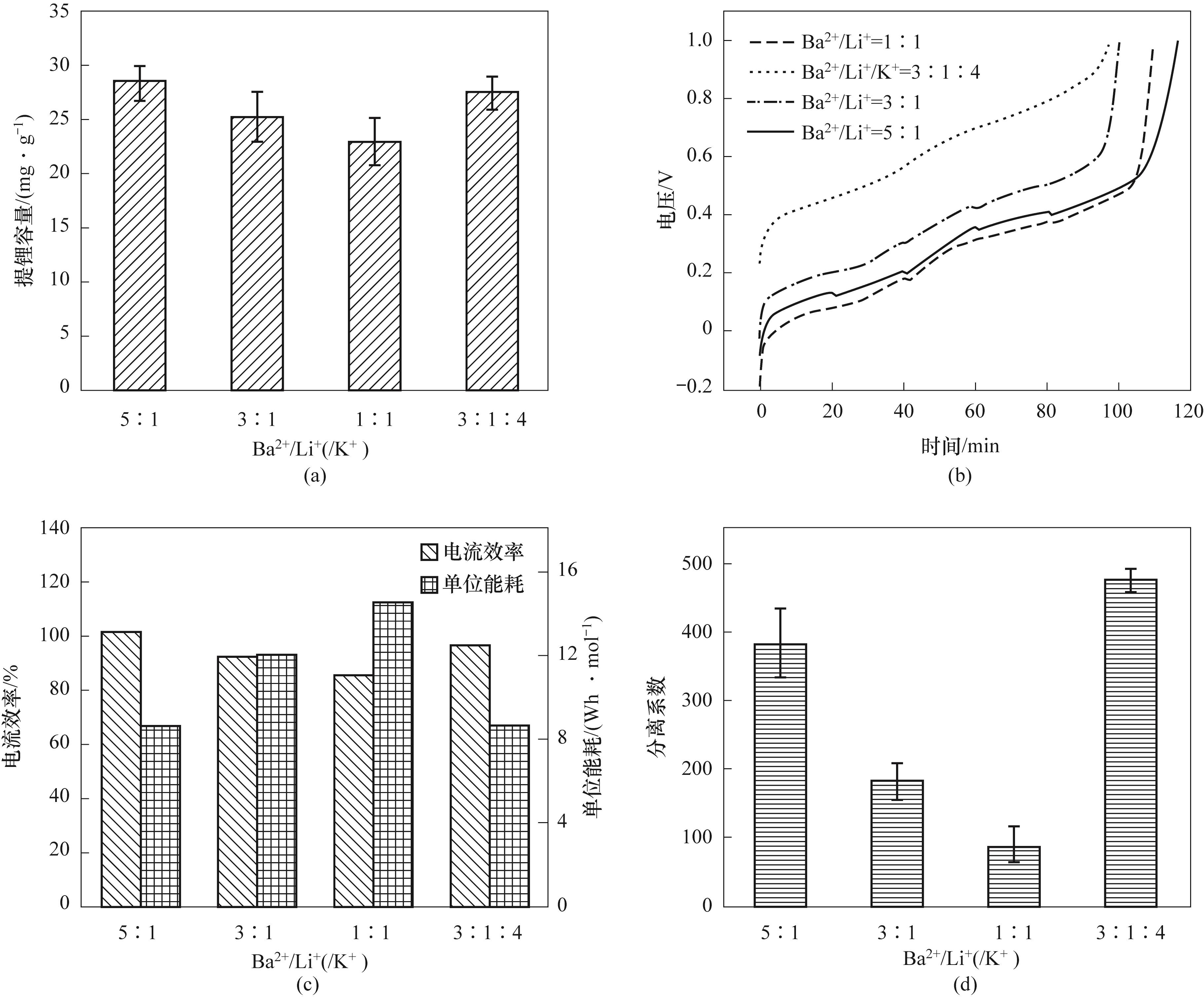
图5 不同Ba2+含量的提锂容量(a)、电压随时间变化趋势(b)、电流效率和单位能耗(c)与分离系数(d)
Fig.5 Lithium extraction capacity (a), voltage trend with time (b), current efficiency and specific energy consumption (c) and separation coefficient (d) with different Ba2+ content
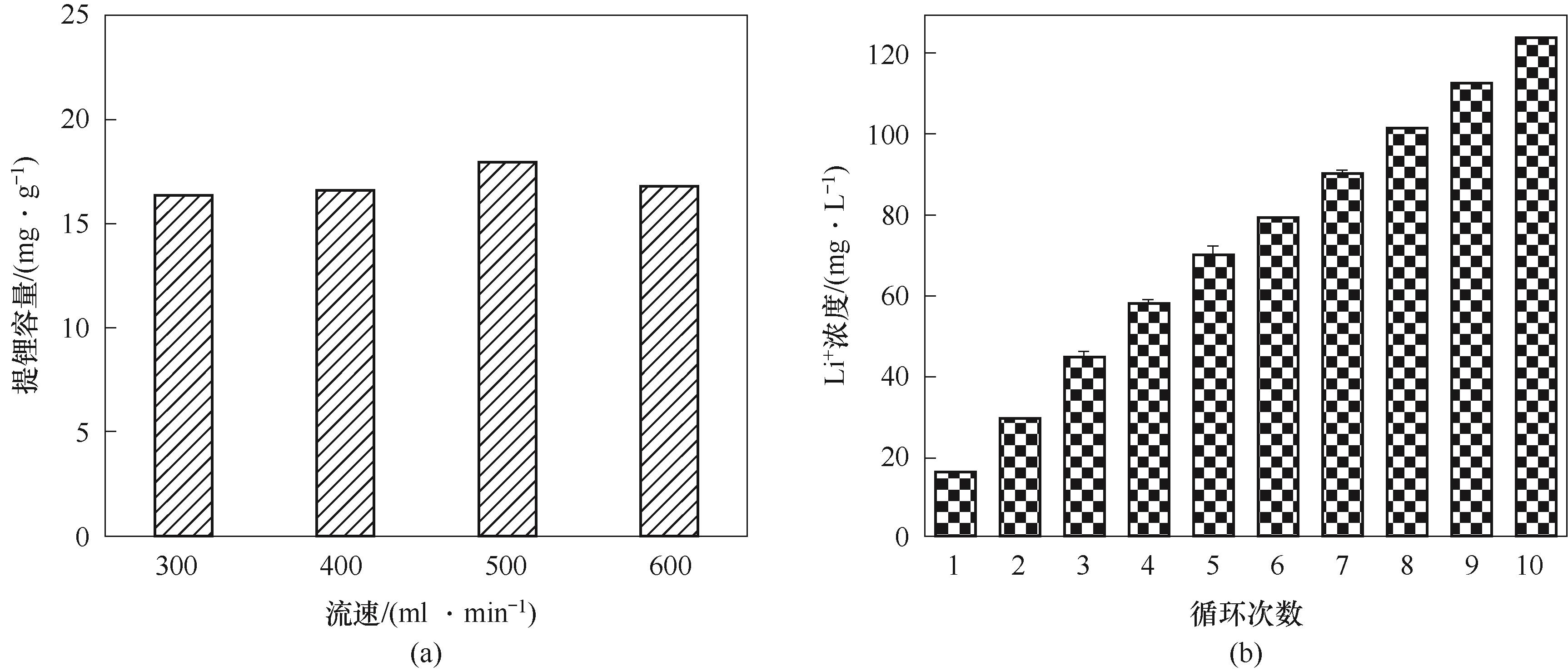
图6 不同流速下5个循环的提锂容量均值(a)和500 ml·min-1条件下10个循环的Li+浓度(b)
Fig.6 Average lithium extraction capacity for 5 cycles at different flow rates (a) and Li+ concentration for 10 cycles at 500 ml·min-1(b)
| 溶液 | 浓度/(mg·L-1) | |||||
|---|---|---|---|---|---|---|
| Li+ | Mg2+ | Na+ | Ca2+ | Sr2+ | Ba2+ | |
| 模拟油气田采出水 | 50.0 | 121.7 | 72510 | 1273.8 | 928.3 | 1135.3 |
| 电化学富锂液 | 124.4 | 1.3 | 145.7 | 2.3 | 1.5 | 1.3 |
| 浓度外推-电化学富锂液 | 2049.3 | 16.9 | 2263.5 | 28 | 18.9 | 14.8 |
| 电渗析浓缩液 | 5680 | 27.2 | 6332 | 48.7 | 31.6 | 23.6 |
表2 各阶段溶液离子组成
Table 2 Ionic composition of the solution at each stage
| 溶液 | 浓度/(mg·L-1) | |||||
|---|---|---|---|---|---|---|
| Li+ | Mg2+ | Na+ | Ca2+ | Sr2+ | Ba2+ | |
| 模拟油气田采出水 | 50.0 | 121.7 | 72510 | 1273.8 | 928.3 | 1135.3 |
| 电化学富锂液 | 124.4 | 1.3 | 145.7 | 2.3 | 1.5 | 1.3 |
| 浓度外推-电化学富锂液 | 2049.3 | 16.9 | 2263.5 | 28 | 18.9 | 14.8 |
| 电渗析浓缩液 | 5680 | 27.2 | 6332 | 48.7 | 31.6 | 23.6 |
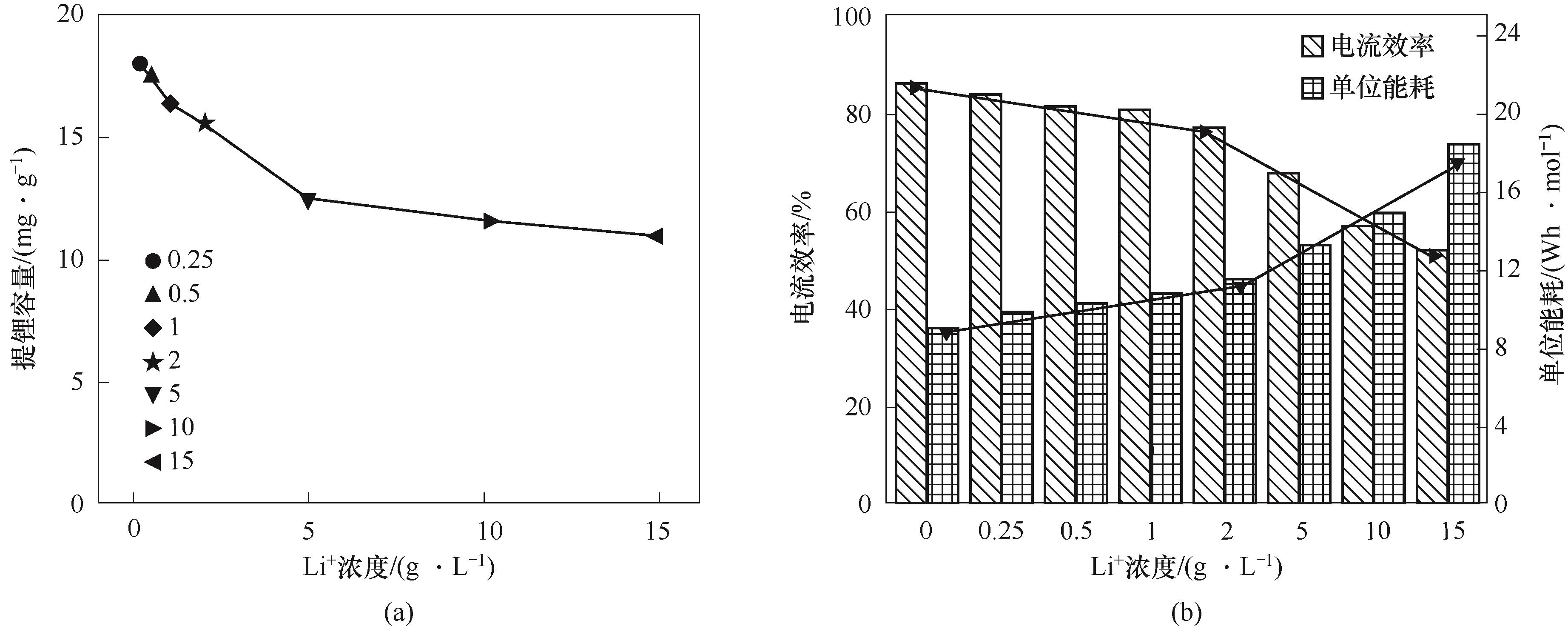
图7 不同浓度梯度下的提锂容量(a)与电流效率和单位能耗(b)
Fig.7 Lithium extraction capacity (a) and current efficiency and specific energy consumption (b) at different concentration gradients
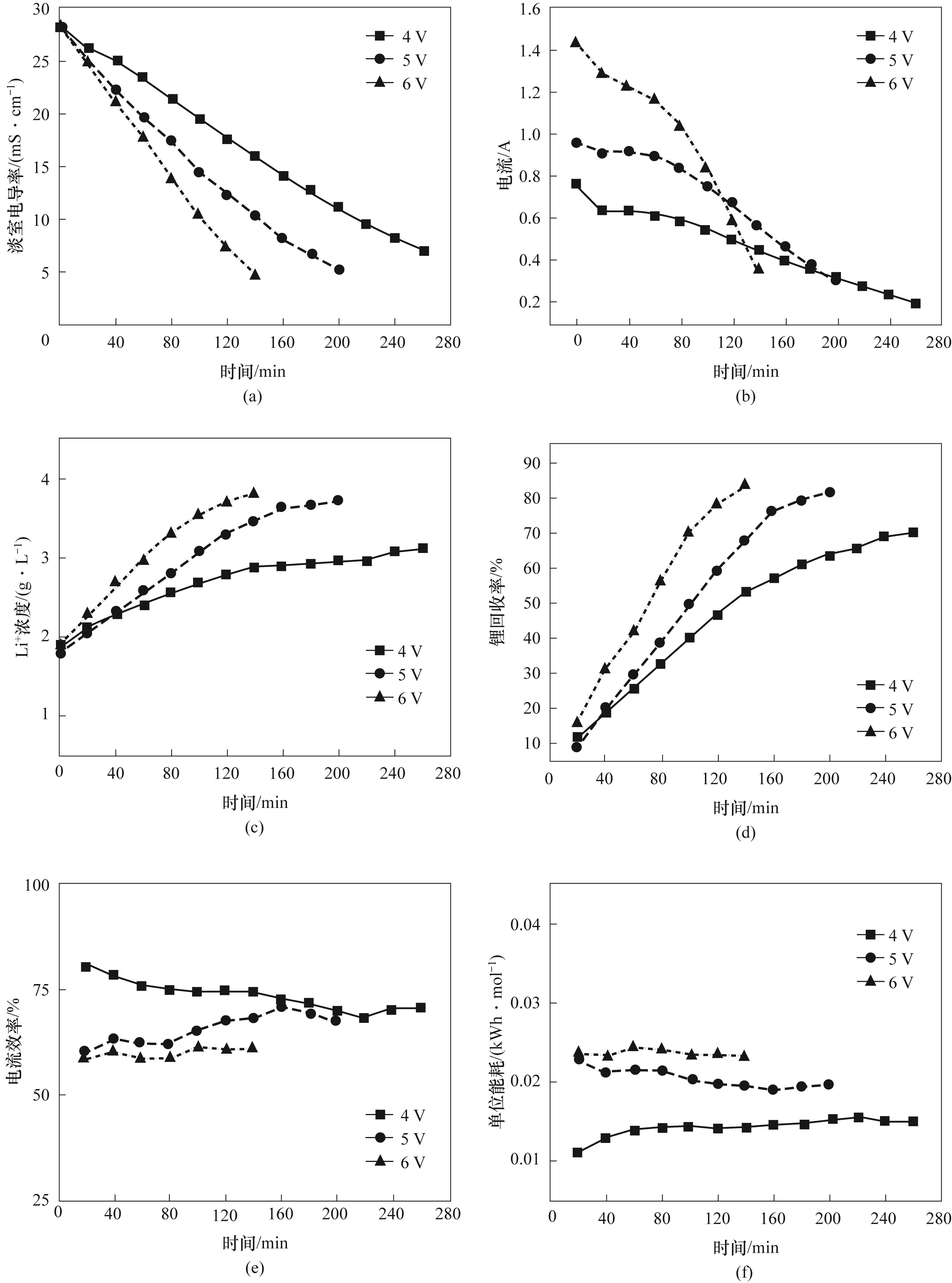
图8 操作电压对淡室电导率(a)、电流(b)、Li+浓度(c)、锂回收率(d)、电流效率(e)和单位能耗(f)的影响
Fig.8 Effect of operating voltage on desalination chamber conductivity (a), current (b), Li+ concentration (c), lithium recovery (d), current efficiency (e) and specific energy consumption (f)
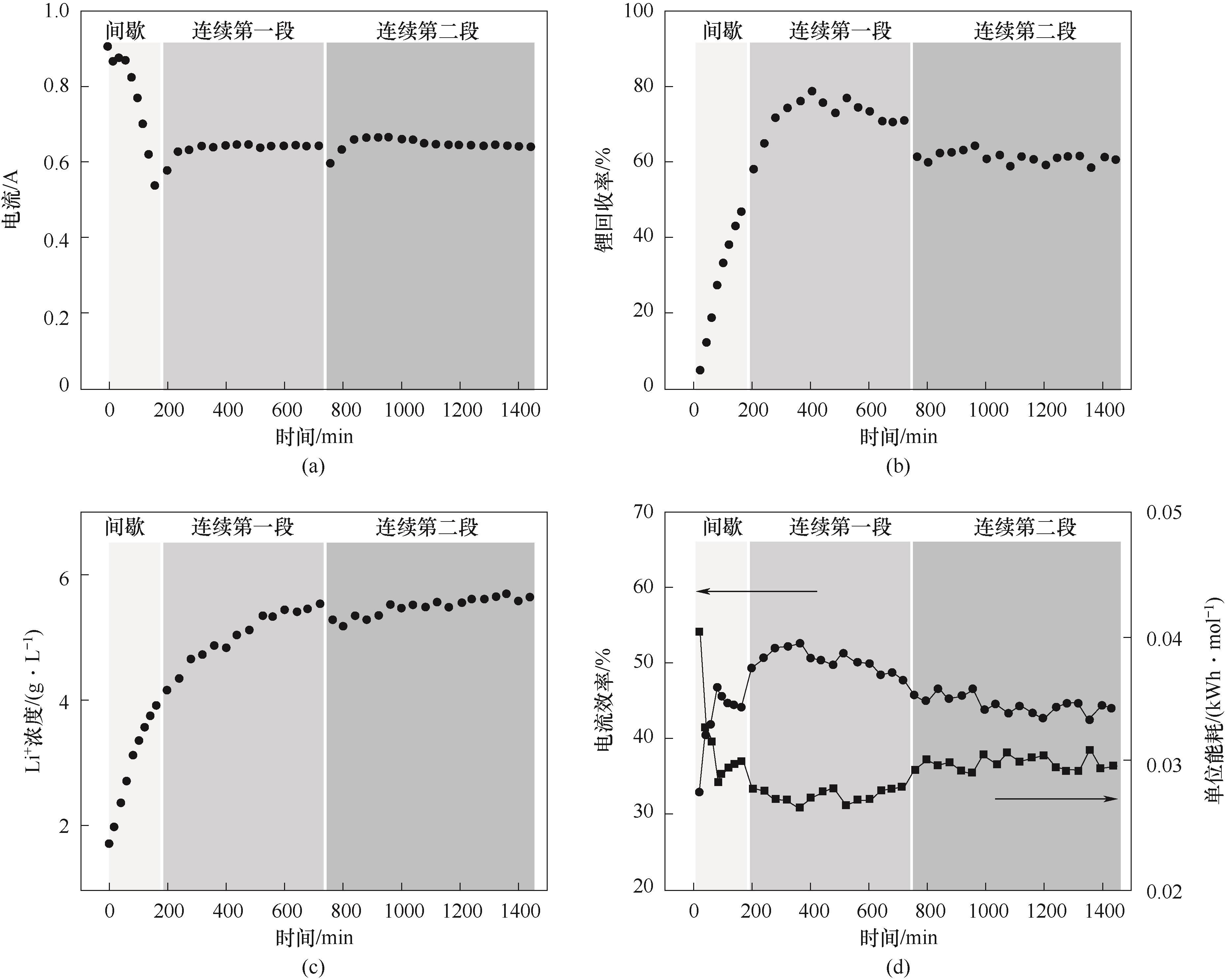
图9 连续电渗析过程中的电流(a)、锂回收率(b)、浓室Li+浓度(c)、电流效率和单位能耗(d)的变化趋势
Fig.9 Trends of current (a), lithium recovery (b), concentration of Li+ in the concentration chamber (c), current efficiency and specific energy consumption (d) during continuous electrodialysis process
| [Li+]/(g·L-1) | [Mg2+]/(mg·L-1) | [Na+]/(g·L-1) | [Ca2+]/(mg·L-1) | [Sr2+]/(mg·L-1) | [Ba2+]/(mg·L-1) |
|---|---|---|---|---|---|
| 3.74 | 0.01 | 7.35 | 6.16 | 2.26 | 1.03 |
表3 除杂后溶液组成
Table 3 Composition of the solution after removal of impurities
| [Li+]/(g·L-1) | [Mg2+]/(mg·L-1) | [Na+]/(g·L-1) | [Ca2+]/(mg·L-1) | [Sr2+]/(mg·L-1) | [Ba2+]/(mg·L-1) |
|---|---|---|---|---|---|
| 3.74 | 0.01 | 7.35 | 6.16 | 2.26 | 1.03 |
| Li+ | Na+ | Mg2+ | Ca2+ | Sr2+ | Ba2+ |
|---|---|---|---|---|---|
| 947 | 1.086 | 0.094 | 4.999 | 1.949 | 0.879 |
表4 产品Li2CO3中各离子浓度(mg·L-1)
Table 4 Concentration of each ion in the product Li2CO3
| Li+ | Na+ | Mg2+ | Ca2+ | Sr2+ | Ba2+ |
|---|---|---|---|---|---|
| 947 | 1.086 | 0.094 | 4.999 | 1.949 | 0.879 |
| 参数 | 数值 |
|---|---|
| 电化学吸附原料液Li+浓度/(mg·L-1) | 50 |
| 电化学吸附处理能力/(ml·min-1) | 500 |
| 电化学吸附单位能耗/(Wh·mol-1 (Li)) | 10~12 |
| 电渗析锂回收率/% | 60 |
| 电渗析处理能力/(L·h-1) | 1.0 |
| 进料浓度/(mg·L-1) | 3971.4 |
| 进料LiCl浓度/(g·L-1) | 2 |
| 浓缩液Li+浓度/(g·L-1) | 5.68 |
| 电压/V | 5 |
| 膜有效面积/m2 | 0.147 |
| 实验时间/h | 24 |
| 电渗析提锂单位能耗/(kWh·mol-1(Li)) | 0.028~0.032 |
| 工业电价/(CNY·kWh-1) | 0.7 |
| 除杂过程药剂消耗/(CNY·t-1 (Li2CO3)) | 14.39 |
| 沉淀过程药剂消耗/(CNY·t-1 (Li2CO3)) | 2363.52 |
表5 实验相关参数
Table 5 Experimental-related parameters
| 参数 | 数值 |
|---|---|
| 电化学吸附原料液Li+浓度/(mg·L-1) | 50 |
| 电化学吸附处理能力/(ml·min-1) | 500 |
| 电化学吸附单位能耗/(Wh·mol-1 (Li)) | 10~12 |
| 电渗析锂回收率/% | 60 |
| 电渗析处理能力/(L·h-1) | 1.0 |
| 进料浓度/(mg·L-1) | 3971.4 |
| 进料LiCl浓度/(g·L-1) | 2 |
| 浓缩液Li+浓度/(g·L-1) | 5.68 |
| 电压/V | 5 |
| 膜有效面积/m2 | 0.147 |
| 实验时间/h | 24 |
| 电渗析提锂单位能耗/(kWh·mol-1(Li)) | 0.028~0.032 |
| 工业电价/(CNY·kWh-1) | 0.7 |
| 除杂过程药剂消耗/(CNY·t-1 (Li2CO3)) | 14.39 |
| 沉淀过程药剂消耗/(CNY·t-1 (Li2CO3)) | 2363.52 |
| [1] | Tarascon J M. Is lithium the new gold?[J]. Nature Chemistry, 2010, 2(6): 510. |
| [2] | Kanagasundaram T, Murphy O, Haji M N, et al. The recovery and separation of lithium by using solvent extraction methods[J]. Coordination Chemistry Reviews, 2024, 509: 215727. |
| [3] | 赵紫伊, 周雪, 王铁夫, 等. 油气田采出水锂资源回收可行性、技术现状及展望[J]. 环境工程技术学报, 2023, 13(4): 1434-1443. |
| Zhao Z Y, Zhou X, Wang T F, et al. Feasibility, technical status and prospects of lithium recovery from produced water in oil and gas fields[J]. Journal of Environmental Engineering Technology, 2023, 13(4): 1434-1443. | |
| [4] | Nikkhah H, Ipekçi D, Xiang W J, et al. Challenges and opportunities of recovering lithium from seawater, produced water, geothermal brines, and salt lakes using conventional and emerging technologies[J]. Chemical Engineering Journal, 2024, 498: 155349. |
| [5] | Zhao Y Z, Xing H F, Rong M, et al. Quantum chemical calculation assisted efficient lithium extraction from unconventional oil and gas field brine by β-diketone synergic system[J]. Desalination, 2023, 565: 116890. |
| [6] | Wang X M, Numedahl N, Jiang C Q. Direct lithium extraction from Canadian oil and gas produced water using functional ionic liquids—A preliminary study[J]. Applied Geochemistry, 2024, 172: 106126. |
| [7] | Pan Y N, Zhan W Q, Zhang W C. Sustainable lithium extraction from produced water: integrating membrane pretreatment and next-generation adsorbents[J]. Journal of Environmental Management, 2025, 382: 125343. |
| [8] | 王晓丽, 杨文胜. 电化学提锂体系及其电极材料的研究进展[J]. 化工学报, 2021, 72(6): 2957-2971. |
| Wang X L, Yang W S. Research progress of electrochemical lithium extraction systems and electrode materials[J]. CIESC Journal, 2021, 72(6): 2957-2971. | |
| [9] | Meng X R, Jing Y, Li J M, et al. Electrochemical recovery of lithium from brine by highly stable truncated octahedral LiNi0. 05Mn1. 95O4 [J]. Chemical Engineering Science, 2024, 283: 119400. |
| [10] | 朱兴驰, 郭志远, 纪志永, 等. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| Zhu X C, Guo Z Y, Ji Z Y, et al. Simulation and optimization of selective electrodialysis magnesium and lithium separation process[J]. CIESC Journal, 2023, 74(6): 2477-2485. | |
| [11] | Rögener F, Tetampel L. Electrodialysis for the concentration of lithium-containing brines: an investigation on the applicability[J]. Membranes, 2022, 12(11): 1142. |
| [12] | Zhou Y M, Yan H Y, Wang X L, et al. Electrodialytic concentrating lithium salt from primary resource[J]. Desalination, 2018, 425: 30-36. |
| [13] | Gu J, Zhou G L, Chen L L, et al. Particle size control and electrochemical lithium extraction performance of LiMn2O4 [J]. Journal of Electroanalytical Chemistry, 2023, 940: 117487. |
| [14] | Gu J, Chen L L, Fan L J, et al. Multistage regulation of LiMn2O4 electrode for electrochemical lithium extraction from salt-lake[J]. Desalination, 2024, 586: 117828. |
| [15] | Pu Z Y, Zhu Z Q, Lv X J, et al. N, P-doped graphite/LiMn2O4 hybrid electrode for high-performance all-climate dual-ion batteries[J]. Journal of Colloid and Interface Science, 2025, 686: 408-419. |
| [16] | Zhao M Y, Ji Z Y, Zhang Y G, et al. Study on lithium extraction from brines based on LiMn2O4/Li1- x Mn2O4 by electrochemical method[J]. Electrochimica Acta, 2017, 252: 350-361. |
| [17] | Mubita T M, Porada S, Biesheuvel P M, et al. Strategies to increase ion selectivity in electrodialysis[J]. Separation and Purification Technology, 2022, 292: 120944. |
| [18] | 郭志远, 张帆, 纪志永, 等. 选择性电渗析提锂技术的研究进展[J]. 河北工业大学学报, 2022, 51(6): 1-9. |
| Guo Z Y, Zhang F, Ji Z Y, et al. Research and prospect of lithium extraction by selective electrodialysis[J]. Journal of Hebei University of Technology, 2022, 51(6): 1-9. | |
| [19] | Yuan H F, Li M Z, Cui L, et al. Electrochemical extraction technologies of lithium: development and challenges[J]. Desalination, 2025, 598: 118419. |
| [20] | 杨金花, 孙永耀, 赵霞. 基于选择性电渗析技术的盐湖卤水镁锂分离试验研究[J]. 化学与粘合, 2025, 47(2): 192-197. |
| Yang J H, Sun Y Y, Zhao X. Experimental study on magnesium lithium separation in salt lake brine based on selective electrodialysis technology[J]. Chemistry and Adhesion, 2025, 47(2): 192-197. | |
| [21] | Liu Q, Yang P, Tu W W, et al. Lithium recovery from oil and gas produced water: opportunities, challenges, and future outlook[J]. Journal of Water Process Engineering, 2023, 55: 104148. |
| [22] | Karuppasamy K, Mayyas A, Alhseinat E, et al. Exploring lithium extraction technologies in oil and gas field-produced waters: from waste to valuable resource[J]. Chemical Engineering Journal Advances, 2024, 20: 100680. |
| [23] | Khatoon R, Raksasat R, Ho Y C, et al. Reviewing advanced treatment of hydrocarbon-contaminated oilfield-produced water with recovery of lithium[J]. Sustainability, 2023, 15(22): 16016. |
| [24] | 汤国军, 张宏军, 何化, 等. 油气田采出水提锂技术研究[J]. 天然气与石油, 2023, 41(6): 67-72. |
| Tang G J, Zhang H J, He H, et al. Research on technology for lithium extraction from oil and gas field produced water[J]. Natural Gas and Oil, 2023, 41(6): 67-72. | |
| [25] | Kumar A, Fukuda H, Hatton T A, et al. Lithium recovery from oil and gas produced water: a need for a growing energy industry[J]. ACS Energy Letters, 2019, 4(6): 1471-1474. |
| [26] | Guo Z Y, Ji Z Y, Wang J, et al. Electrochemical lithium extraction based on “rocking-chair” electrode system with high energy-efficient: the driving mode of constant current-constant voltage[J]. Desalination, 2022, 533: 115767. |
| [27] | 李丽, 李宇, 金艳, 等. 高硫高硬气田采出水提锂过程关键技术及应用[J]. 无机盐工业, 2023, 55(1): 74-80. |
| Li L, Li Y, Jin Y, et al. Key technology and application of lithium extraction from produced water in high sulfur and high hardness gas fields[J]. Inorganic Chemicals Industry, 2023, 55(1): 74-80. | |
| [28] | 蒋雨新. 尖晶石锰酸锂电极强化电容去离子脱盐研究[D]. 长沙: 中南大学, 2023. |
| Jiang Y X. A study of capacitive deionization desalination strengthened by spinel LiMn2O4 electrode[D]. Changsha: Central South University, 2023. | |
| [29] | Zhao Y, Mamrol N, Tarpeh W A, et al. Advanced ion transfer materials in electro-driven membrane processes for sustainable ion-resource extraction and recovery[J]. Progress in Materials Science, 2022, 128: 100958. |
| [30] | 陶箴奇, 张志强, 毕秋艳, 等. 氯化锂与碳酸钠反应结晶制备碳酸锂的研究[J]. 无机盐工业, 2016, 48(11): 25-28. |
| Tao Z Q, Zhang Z Q, Bi Q Y, et al. Study on preparation of lithium carbonate via reactive crystallization from lithium chloride and sodium carbonate[J]. Inorganic Chemicals Industry, 2016, 48(11): 25-28. | |
| [31] | Xu Z G, Sun S Y. Preparation of battery-grade lithium carbonate with lithium-containing desorption solution [J]. Metals, 2021, 11(9): 1490. |
| [32] | 郭苏丽, 杨海华. ICP-AES法测定废旧磷酸铁锂回收碳酸锂中锂和金属杂质元素含量[J]. 广东化工, 2020, 47(19): 144-145, 152. |
| Guo S L, Yang H H. Determination of lithium and metal impurity elements in lithium carbonate recovered from waste lithium iron phosphate by ICP-AES method[J]. Guangdong Chemical Industry, 2020, 47(19): 144-145, 152. |
| [1] | 张帅, 徐嘉宇, 华蕾娜, 葛蔚. 气固系统的CG-DPM与MP-PIC耦合模拟方法[J]. 化工学报, 2025, 76(9): 4412-4424. |
| [2] | 袁梦星, 孙琳, 罗雄麟. 多效蒸发海水淡化系统变量相关性分析与全周期操作优化[J]. 化工学报, 2025, 76(6): 2813-2827. |
| [3] | 吴迪, 刘世朋, 王文伟, 姜久春, 杨晓光. 机械压力对锂金属电池性能影响的研究进展[J]. 化工学报, 2025, 76(4): 1422-1431. |
| [4] | 阿如娜, 张浩, 沙帅, 金旭, 刘忠彦, 苏伟, 张家鹏, 邱政. CO2双级压缩热泵喷射特性及容量匹配特性研究[J]. 化工学报, 2025, 76(12): 6658-6668. |
| [5] | 张家豪, 弓志超, 李双喜, 王克俭, 李方俊. 基于流-固-热全耦合数值方法的超高压干气密封的热力变形影响分析及变形协调研究[J]. 化工学报, 2025, 76(11): 5980-5997. |
| [6] | 谭昭轶, 栗晶, 覃羿翔, 柳朝晖. 气固两相湍流大涡模拟中DF模型有效性研究[J]. 化工学报, 2025, 76(11): 5677-5686. |
| [7] | 李焱, 郑利军, 张恩勇, 王云飞. 深水海底管道软管内部流体渗透特性模型与试验研究[J]. 化工学报, 2024, 75(S1): 118-125. |
| [8] | 谢磊, 徐永生, 林梅. 不同截面肋柱-软尾结构单相流动传热比较[J]. 化工学报, 2024, 75(5): 1787-1801. |
| [9] | 朱芝, 许恒杰, 陈维, 毛文元, 邓强国, 孙雪剑. 超临界二氧化碳螺旋槽干气密封热流耦合润滑临界阻塞特性研究[J]. 化工学报, 2024, 75(2): 604-615. |
| [10] | 吕潇峻, 赵长颖, 闫君. 双轴搅拌反应器内Ca(OH)2/CaO热化学储热体系的放热研究[J]. 化工学报, 2024, 75(10): 3718-3729. |
| [11] | 宋嘉豪, 王文. 斯特林发动机与高温热管耦合运行特性研究[J]. 化工学报, 2023, 74(S1): 287-294. |
| [12] | 王阳, 戴永强, 曾炜. 2,5-二羟基苯磺酸增强离子水凝胶材料热电性能的研究[J]. 化工学报, 2023, 74(9): 3946-3955. |
| [13] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [14] | 毕恩哲, 李双喜, 沙廉翔, 刘登宇, 陈凯放. 高温动压涨圈密封结构参数多目标优化分析[J]. 化工学报, 2023, 74(6): 2565-2579. |
| [15] | 朱兴驰, 郭志远, 纪志永, 汪婧, 张盼盼, 刘杰, 赵颖颖, 袁俊生. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号