CIESC Journal ›› 2020, Vol. 71 ›› Issue (10): 4733-4749.DOI: 10.11949/0438-1157.20191318
• Surface and interface engineering • Previous Articles Next Articles
Xue LUO1( ),Chuan JING1(
),Chuan JING1( ),Haijun HUANG1,Hongru LI1,Zhiyong WANG1,Zhenqiang WANG1,2,Fang GAO1(
),Haijun HUANG1,Hongru LI1,Zhiyong WANG1,Zhenqiang WANG1,2,Fang GAO1( ),Shengtao ZHANG1
),Shengtao ZHANG1
Received:2019-11-11
Revised:2020-03-28
Online:2020-10-05
Published:2020-10-05
Contact:
Fang GAO
罗雪1( ),荆川1(
),荆川1( ),黄海军1,李红茹1,王治永1,王震强1,2,高放1(
),黄海军1,李红茹1,王治永1,王震强1,2,高放1( ),张胜涛1
),张胜涛1
通讯作者:
高放
作者简介:罗雪(1994—),女,硕士研究生,基金资助:CLC Number:
Xue LUO, Chuan JING, Haijun HUANG, Hongru LI, Zhiyong WANG, Zhenqiang WANG, Fang GAO, Shengtao ZHANG. Study on highly efficient corrosion inhibition of copper by regular self-aggregates of organic molecule[J]. CIESC Journal, 2020, 71(10): 4733-4749.
罗雪, 荆川, 黄海军, 李红茹, 王治永, 王震强, 高放, 张胜涛. 规整有机分子自聚集体对铜的高效缓蚀的研究[J]. 化工学报, 2020, 71(10): 4733-4749.
Add to citation manager EndNote|Ris|BibTeX
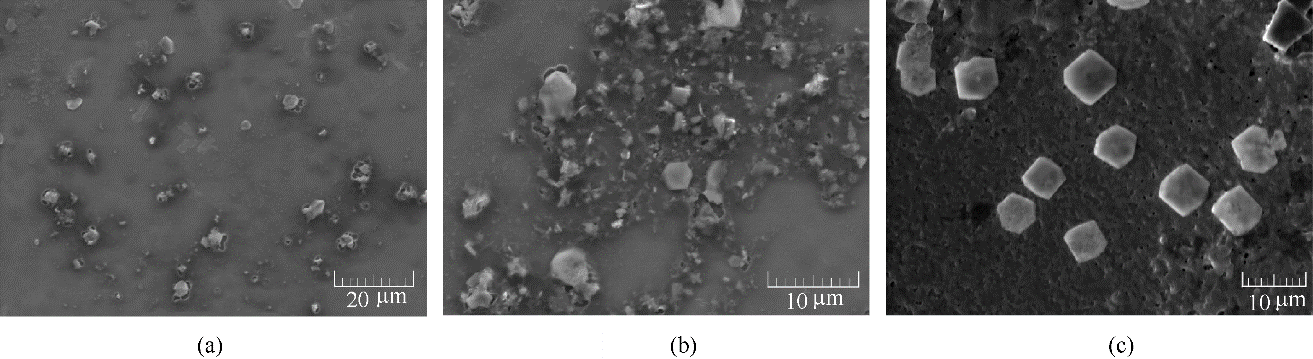
Fig.3 SEM images of BDBD aggregates at 5×10-4 mol/L in the mixed 3.5% NaCl solution/DMSO (40% volume ratio of DMSO) at aggregation time course of 20 min (a), 1 h (b), 2 h (c), respectively
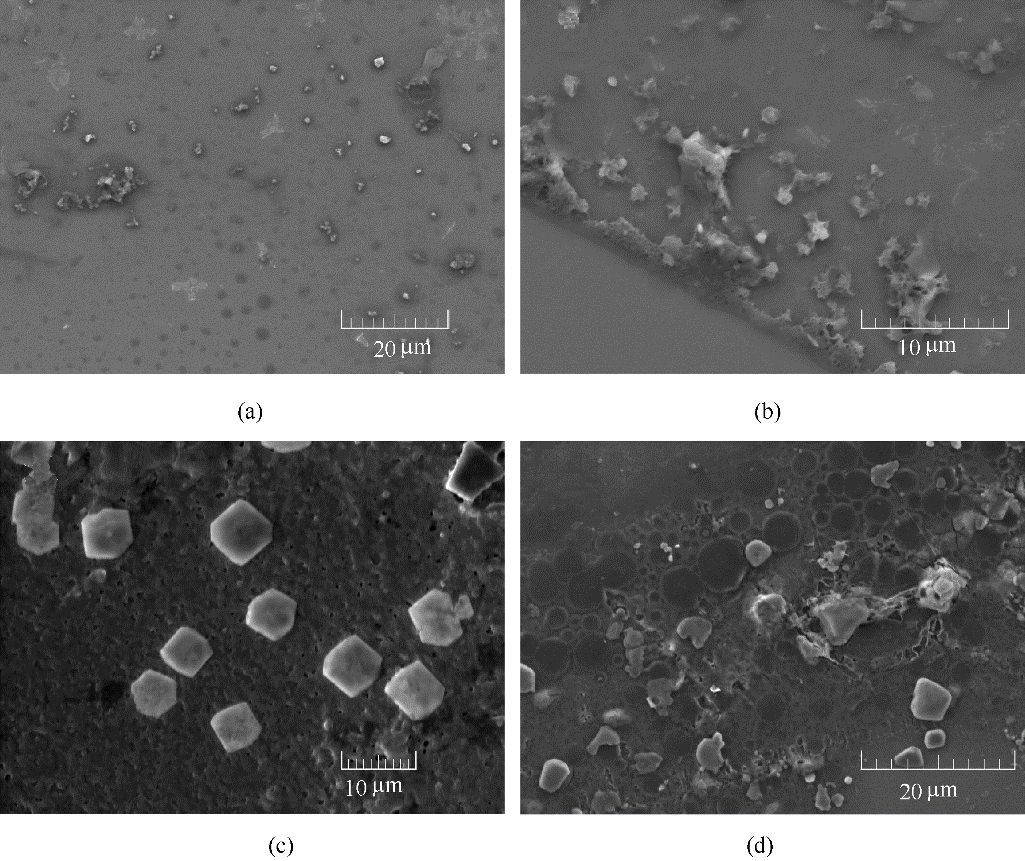
Fig.4 SEM images of the BDBD aggregates in the mixed 3.5% NaCl DMSO aqueous solution (40% DMSO volume ratio) at 2 h evolving time with diflerent BDBD concentration: 1.0×10-4 mol/L (a), 3.0×10-4 mol/L (b), 5.0×10-4 mol/L (c), 7.0×10-4 mol/L (d)

Fig.5 SEM micrographs of the studied Cu specimen surfaces: before the immersion in the 3.5% NaCl/DMSO aqueous solution containing the stable BDBD aggregates (a); after the immersion in the 3.5% NaCl/DMSO aqueous solution with 3.0×10-4 mol/L of the stable BDBD aggregates for 3 h (b); after the immersion in the 3.5% NaCl/DMSO aqueous solution with 5.0×10-4 mol/L of the stable BDBD aggregates for 3 h (c); after the immersion in the 3.5% NaCl/DMSO aqueous solution with 7.0×10-4 mol/L of the stable BDBD aggregates for 3 h (d)
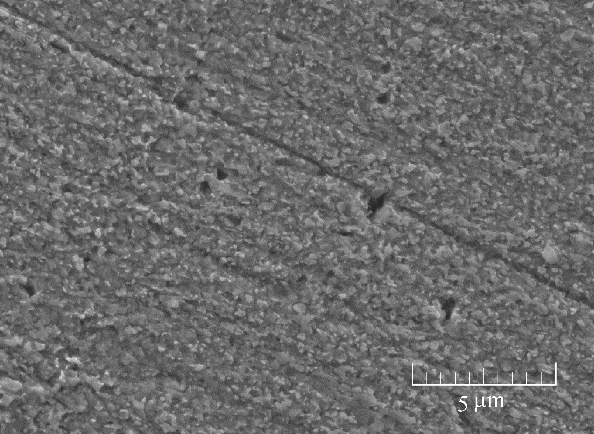
Fig.6 SEM micrographs of the studied Cu specimen surface absorbed with 5.0×10-4 mol/L of the stable BDBD aggregates for 3 h in 3.5% NaCl/ DMSO, which was take out and immersed in the 3.5% NaCl for 14 d

Fig.7 FT-IR spectrum of the BDBD powder (a); FT-IR spectrum of the stable BDBD aggregates adsorbed on the studied copper specimen surfaces (b); Raman spectra of stable BDBD aggregates adsorbed on the studied copper specimen surfaces (c)

Fig.8 Cu 2p (a), O 1s (b), C 1s (c) XPS spectra and the fitted curves measured on the studied copper specimens that were immersion in the mixed 3.5% NaCl DMSO aqueous solution for 3 h (DMSO/H2O: 40/60, volume ratio)
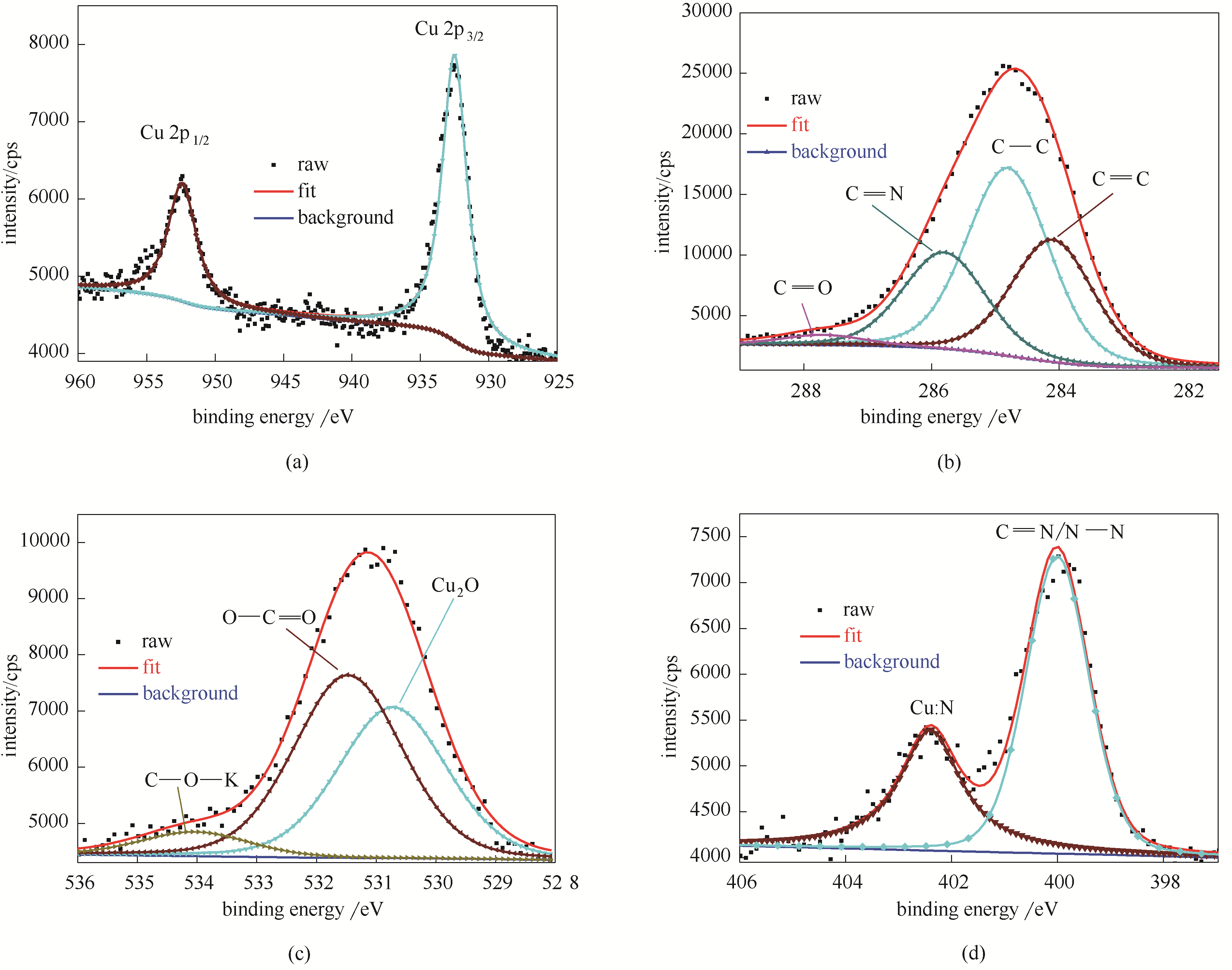
Fig.9 Cu 2p (a), C 1s (b); O 1s (c), N 1s (d) XPS spectra and the fitted curves measured on the studied copper specimens after 3 h of immersion in the mixed 3.5% NaCl DMSO aqueous solution (DMSO/H2O: 40/60, volume ratio) containing the stable BDBD aggregates of 5.0 ×10-4 mol/L
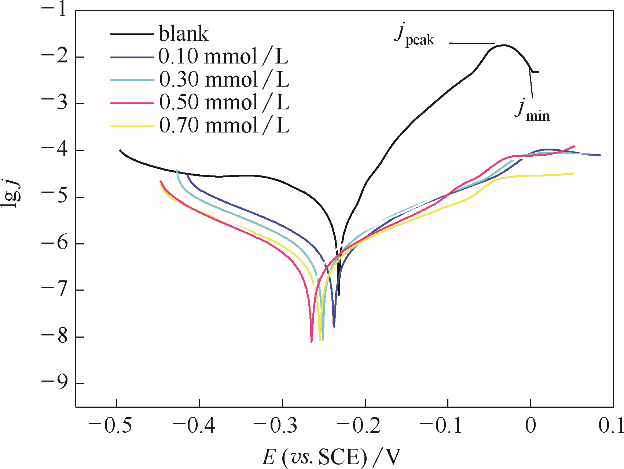
Fig.10 Potentiodynamic polarization curves in 3.5 % NaCl solution for the studied naked copper electrodes, and for the studied stable BDBD-aggregates of different concentrations covered copper electrodes
| 缓蚀剂 | 极化曲线参数 | |||||
|---|---|---|---|---|---|---|
| c (mol/L) | Ecorr(SCE)/ V | jcorr/(A/cm2) | βc/(V/dec) | βa/(V/dec) | ηj /% | |
| 空白 | — | -0.221 | 5.233×10-6 | -0.1667 | 0.04305 | — |
| BDBD自聚集体 | 1.0×10-4 | -0.237 | 1.349×10-6 | -0.1331 | 0.1153 | 74.22 |
| 3.0×10-4 | -0.265 | 9.35×10-7 | -0.1382 | 0.1192 | 82.14 | |
| 5.0×10-4 | -0.269 | 2.22×10-7 | -0.1471 | 0.2025 | 95.76 | |
| 7.0×10-4 | -0.251 | 4.78×10-7 | -0.1425 | 0.1393 | 90.87 | |
Table 1 Polarization parameters for the studied copper specimens covered without and with the stable BDBD aggregates of different concentrations in 3.5% NaCl solution
| 缓蚀剂 | 极化曲线参数 | |||||
|---|---|---|---|---|---|---|
| c (mol/L) | Ecorr(SCE)/ V | jcorr/(A/cm2) | βc/(V/dec) | βa/(V/dec) | ηj /% | |
| 空白 | — | -0.221 | 5.233×10-6 | -0.1667 | 0.04305 | — |
| BDBD自聚集体 | 1.0×10-4 | -0.237 | 1.349×10-6 | -0.1331 | 0.1153 | 74.22 |
| 3.0×10-4 | -0.265 | 9.35×10-7 | -0.1382 | 0.1192 | 82.14 | |
| 5.0×10-4 | -0.269 | 2.22×10-7 | -0.1471 | 0.2025 | 95.76 | |
| 7.0×10-4 | -0.251 | 4.78×10-7 | -0.1425 | 0.1393 | 90.87 | |
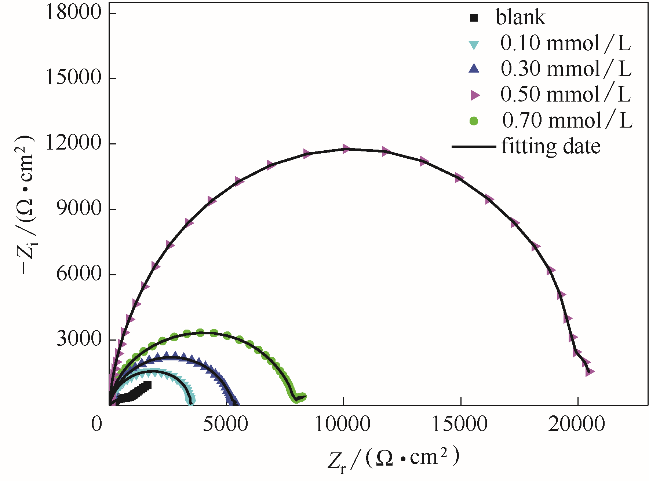
Fig.11 Nyquist plots for the studied naked copper electrodes and the stable BDBD aggregates of different concentrations covered copper electrodes in 3.5% NaCl solution
| 方法 | Kads/ (L/mol) | 吸附能/ (J/mol) |
|---|---|---|
| Polarization | 4.9×104 | -6730 |
| EIS | 4.5×104 | -36510 |
Table 3 Thermodynamic parameters for the adsorption of stable BDBD aggregates in 3.5% NaCl solution at 298 K
| 方法 | Kads/ (L/mol) | 吸附能/ (J/mol) |
|---|---|---|
| Polarization | 4.9×104 | -6730 |
| EIS | 4.5×104 | -36510 |
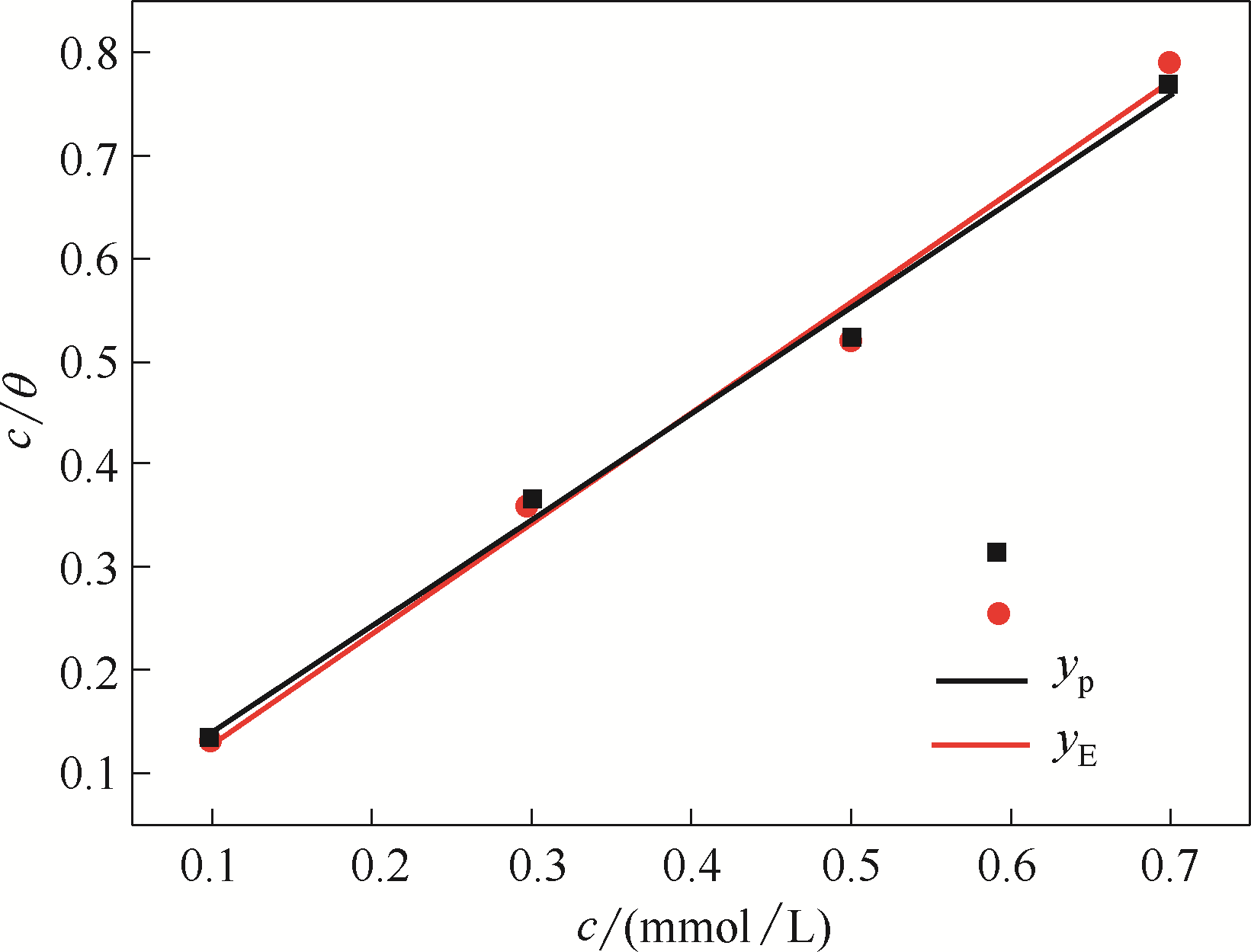
Fig.A4 Langmuir adsorption isotherms of the stable BDBD aggregates covered on the studied copper specimen surfaces in 3.5 % NaCl solution (yp to potentiodynamic polarization and yE to electrochemical impedance spectroscopy
| 1 | Fan H, Li S, Zhao Z, et al. Inhibition of brass corrosion in sodium chloride solutions by self-assembled silane films[J]. Corrosion Science, 2011, 53: 4273-4281. |
| 2 | Lyon S B, Bingham R, Mills Douglas J. Advances in corrosion protection by organic coatings: what we know and what we would like to know[J]. Progress in Organic Coatings, 2017, 102: 2-7. |
| 3 | Kokalj A, Peljhan S. Density functional theory study of ATA, BTAH, and BTAOH as copper corrosion inhibitors: adsorption onto Cu(111) from gas phase[J]. Langmuir, 2010, 26: 14582-14593. |
| 4 | Finsgar M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part I. Long-term immersion, 3D-profilometry, and electrochemistry[J]. Corrosion Science, 2013, 72: 82-89. |
| 5 | Finsgar M. EQCM and XPS analysis of 1,2,4-triazole and 3-amino-1,2,4-triazole as copper corrosion inhibitors in chloride solution[J]. Corrosion Science, 2013, 77: 350-359. |
| 6 | Wang Z, Gong Y, Jing C, et al. Synthesis of dibenzotriazole derivatives bearing alkylene linkers as corrosion inhibitors for copper in sodium chloride solution: a new thought for the design of organic inhibitors[J]. Corrosion Science, 2016, 113: 64-77. |
| 7 | Sherif M E S. Effects of 2-amino-5-(ethylthio)-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in 3% NaCl solutions[J]. Applied Surface Science, 2006, 252: 8615-8623. |
| 8 | Hong S, Chen W, Zhang Y, et al. Investigation of the inhibition effect of trithiocyanuric acid on corrosion of copper in 3.0wt.% NaCl[J]. Corrosion Science, 2013, 66: 308-314. |
| 9 | Izquierdo J, Santana J J, González S, et al. Uses of scanning electrochemical microscopy for the characterization of thin inhibitor films on reactive metals: The protection of copper surfaces by benzotriazole[J]. Electrochimica Acta, 2010, 55: 8791-8800. |
| 10 | Jafari A H, Hosseini S M A, Jamalizadeh E. Investigation of smart nanocapsules containing inhibitors for corrosion protection of copper[J]. Electrochimica Acta, 2010, 55: 9004-9009. |
| 11 | Khaled K F. Studies of the corrosion inhibition of copper in sodium chloride solutions using chemical and electrochemical measurements[J]. Materials Chemistry and Physics, 2011, 125: 427-433. |
| 12 | Li C C, Guo X. Y, Shen S,et al. Adsorption and corrosion inhibition of phytic acid calcium on the copper surface in 3wt% NaCl solution[J]. Corrosion Science, 2014, 83: 147-154. |
| 13 | Liu Y, Li S, Zhang J, et al. Corrosion inhibition of biomimetic super-hydrophobic electrodeposition coatings on copper substrate[J]. Corrosion Science, 2015, 94: 190-196. |
| 14 | Khiati Z, Othman A A, Sanchez-Moreno M, et al. Corrosion inhibition of copper in neutral chloride media by a novel derivative of 1,2,4-triazole[J]. Corrosion Science, 2011, 53: 3092-3099. |
| 15 | Wang B, Gao F, Ma H. Preparation and XPS studies of macromolecule mixed-valent Cu(I, II) and Fe(II, III) complexes[J]. Journal of Hazardous Materials, 2007, 144: 363-368. |
| 16 | Doong R A, Liao C Y. Enhanced visible-light-responsive photodegradation of bisphenol A by Cu, N-codoped titanate nanotubes prepared by microwave-assisted hydrothermal method[J]. Journal of Hazardous Materials, 2017, 322: 254-262. |
| 17 | Huang H, Fu Y, Wang X, et al. Nano- to micro-self-aggregates of new bisimidazole-based copoly(ionic liquid)s for protecting copper in aqueous sulfuric acid solution[J]. ACS Applied Materials & Interfaces, 2019, 11: 10135-10145. |
| 18 | Zhang D Q, Joo H G, Lee K Y. Investigation of molybdate-benzotriazole surface treatment against copper tarnishing[J]. Surface and Interface Analysis, 2009, 41: 164-169. |
| 19 | Lou W, Cai W, Li J P, et al. Additives-assisted electrodeposition of fine spherical copper powder from sulfuric acid solution[J]. Powder Technology, 2018, 326: 84-88. |
| 20 | Sherif E S M, Erasmus R M, Comins J D. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions[J]. Electrochimica Acta, 2010, 55: 3657-3663. |
| 21 | Sudheer M A Q. Electrochemical and theoretical investigation of triazole derivatives on corrosion inhibition behavior of copper in hydrochloric acid medium[J]. Corrosion Science, 2013, 70: 161-169. |
| 22 | Mihajlovic M B P, Radovanovic M B, Tasic Z Z, et al. Imidazole based compounds as copper corrosion inhibitors in seawater[J]. Journal of Molecular Liquids, 2017, 225: 127-136. |
| 23 | Qafsaoui W, Kendig M W, Perrot H, et al. Coupling of electrochemical techniques to study copper corrosion inhibition in 0.5 mol·L-1 NaCl by 1-pyrrolidine dithiocarbamate[J]. Electrochimica Acta, 2013, 87: 348-360. |
| 24 | 张景玲. 苯并三氮唑复配体系对铜的协同缓蚀性能的研究[D]. 长沙:湖南大学, 2008. |
| Zhang J L. Investigation of the synergistic effect between BTA and its composite corrosion inhibitiors on copper[D]. Changsha:Hunan University, 2008. | |
| 25 | Zhang D Q, Gao L X, Zhou G D. Inhibition of copper corrosion by bis-(1-benzotriazolymethylene)-(2,5-thiadiazoly)-disulfide in chloride media[J]. Applied Surface Science, 2004, 225: 287-293. |
| 26 | Singh M M, Rastogi R B, Upadhyay B N, et al. Thiosemicarbazide, phenyl isothiocyanate and their condensation product as corrosion inhibitors of copper in aqueous chloride solutions[J]. Materials Chemistry and Physics, 2003, 80: 283-293. |
| 27 | Hu L, Zhang S, Li W, et al. Electrochemical and thermodynamic investigation of diniconazole and triadimefon as corrosion inhibitors for copper in synthetic seawater[J]. Corrosion Science, 2010, 52: 2891-2896. |
| 28 | Qiang Y, Zhang S, Yan S, et al. Three indazole derivatives as corrosion inhibitors of copper in a neutral chloride solution[J]. Corrosion Science, 2017, 126: 295-304. |
| 29 | Solomon M M, Umoren S A. In-situ preparation, characterization and anticorrosion property of polypropylene glycol/silver nanoparticles composite for mild steel corrosion in acid solution[J]. Journal of Colloid and Interface Science, 2016, 462: 29-41. |
| 30 | Scendo M. Inhibition of copper corrosion in sodium nitrate solutions with nontoxic inhibitors[J]. Corrosion Science, 2008, 50: 1584-1592. |
| 31 | Scendo M. The effect of purine on the corrosion of copper in chloride solutions[J]. Corrosion Science, 2007, 49: 373-390. |
| 32 | Mendonça G L F, Costa S N, Freire V N, et al. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods[J]. Corrosion Science, 2017, 115: 41-55. |
| 33 | Zhang J, Liu Z, Han G C, et al. Inhibition of copper corrosion by the formation of Schiff base self-assembled monolayers[J]. Applied Surface Science, 2016, 389: 601-608. |
| [1] | Fei KANG, Weiguang LYU, Feng JU, Zhi SUN. Research on discharge path and evaluation of spent lithium-ion batteries [J]. CIESC Journal, 2023, 74(9): 3903-3911. |
| [2] | Yan GAO, Peng WU, Chao SHANG, Zejun HU, Xiaodong CHEN. Preparation of magnetic agarose microspheres based on a two-fluid nozzle and their protein adsorption properties [J]. CIESC Journal, 2023, 74(8): 3457-3471. |
| [3] | Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent [J]. CIESC Journal, 2023, 74(8): 3375-3385. |
| [4] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [5] | Jiaqi CHEN, Wanyu ZHAO, Ruichong YAO, Daolin HOU, Sheying DONG. Synthesis of pistachio shell-based carbon dots and their corrosion inhibition behavior on Q235 carbon steel [J]. CIESC Journal, 2023, 74(8): 3446-3456. |
| [6] | Jie WANG, Xiaolin QIU, Ye ZHAO, Xinyang LIU, Zhongqiang HAN, Yong XU, Wenhan JIANG. Preparation and properties of polyelectrolyte electrostatic deposition modified PHBV antioxidant films [J]. CIESC Journal, 2023, 74(7): 3068-3078. |
| [7] | Jing ZHAO, Chengwen GU, Xigao JIAN, Zhihuan WENG. Preparation and performance evaluation of magnolol-based epoxy resin anti-corrosion coatings [J]. CIESC Journal, 2023, 74(7): 3010-3017. |
| [8] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [9] | Meibo XING, Zhongtian ZHANG, Dongliang JING, Hongfa ZHANG. Enhanced phase change energy storage/release properties by combining porous materials and water-based carbon nanotube under magnetic regulation [J]. CIESC Journal, 2023, 74(7): 3093-3102. |
| [10] | Yanhui LI, Shaoming DING, Zhouyang BAI, Yinan ZHANG, Zhihong YU, Limei XING, Pengfei GAO, Yongzhen WANG. Corrosion micro-nano scale kinetics model development and application in non-conventional supercritical boilers [J]. CIESC Journal, 2023, 74(6): 2436-2446. |
| [11] | Yanmei ZHANG, Tao YUAN, Jiang LI, Yajie LIU, Zhanxue SUN. Study on the construction of high-efficient SRB mixed microflora and its performance under acid stress [J]. CIESC Journal, 2023, 74(6): 2599-2610. |
| [12] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [13] | Chenxin LI, Yanqiu PAN, Liu HE, Yabin NIU, Lu YU. Carbon membrane model based on carbon microcrystal structure and its gas separation simulation [J]. CIESC Journal, 2023, 74(5): 2057-2066. |
| [14] | Shaoyun CHEN, Dong XU, Long CHEN, Yu ZHANG, Yuanfang ZHANG, Qingliang YOU, Chenglong HU, Jian CHEN. Preparation and adsorption properties of monolayer polyaniline microsphere arrays [J]. CIESC Journal, 2023, 74(5): 2228-2238. |
| [15] | Yu PAN, Zihang WANG, Jiayun WANG, Ruzhu WANG, Hua ZHANG. Heat and moisture performance study of Cur-LiCl coated heat exchanger [J]. CIESC Journal, 2023, 74(3): 1352-1359. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||