CIESC Journal ›› 2020, Vol. 71 ›› Issue (10): 4760-4772.DOI: 10.11949/0438-1157.20200065
• Surface and interface engineering • Previous Articles Next Articles
Xue LUO1( ),Haijun HUANG1(
),Haijun HUANG1( ),Ziping LUO1,Zhiyong WANG1,Xiaojing MU1,Hongru LI1,Xinchao WANG1,2,Shengtao ZHANG1,Fang GAO1(
),Ziping LUO1,Zhiyong WANG1,Xiaojing MU1,Hongru LI1,Xinchao WANG1,2,Shengtao ZHANG1,Fang GAO1( )
)
Received:2020-01-16
Revised:2020-04-13
Online:2020-10-05
Published:2020-10-05
Contact:
Fang GAO
罗雪1( ),黄海军1(
),黄海军1( ),罗自萍1,王治永1,穆小静1,李红茹1,王新潮1,2,张胜涛1,高放1(
),罗自萍1,王治永1,穆小静1,李红茹1,王新潮1,2,张胜涛1,高放1( )
)
通讯作者:
高放
作者简介:罗雪(1994—),女,硕士研究生,基金资助:CLC Number:
Xue LUO, Haijun HUANG, Ziping LUO, Zhiyong WANG, Xiaojing MU, Hongru LI, Xinchao WANG, Shengtao ZHANG, Fang GAO. High efficient corrosion inhibition of steel by nano-micro aggregates of Sapindus mukorossi Gaertn peel extracts[J]. CIESC Journal, 2020, 71(10): 4760-4772.
罗雪, 黄海军, 罗自萍, 王治永, 穆小静, 李红茹, 王新潮, 张胜涛, 高放. 无患子果皮提取物的纳微米聚集体对钢的高效缓蚀[J]. 化工学报, 2020, 71(10): 4760-4772.
Add to citation manager EndNote|Ris|BibTeX
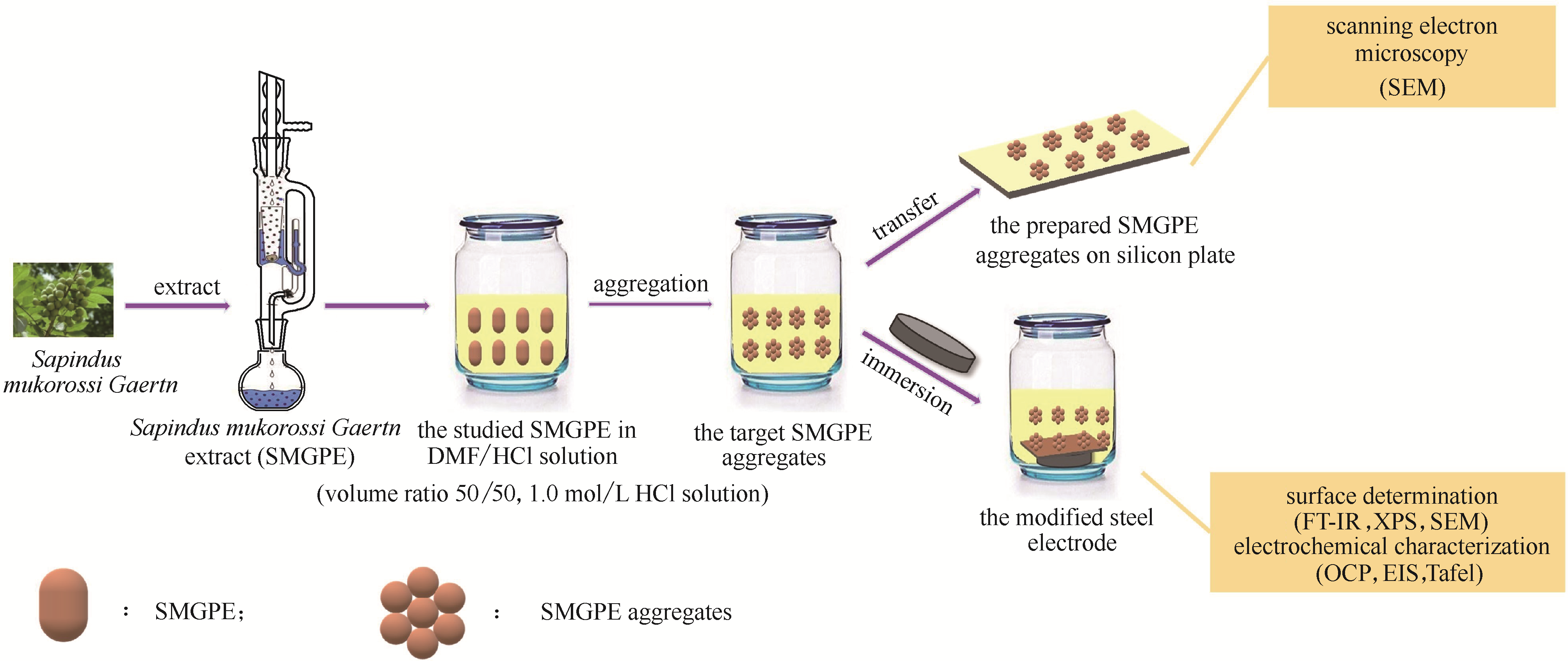
Fig.2 Diagrammatic drawing of the formation of SMGPE aggregates and the preparation of the stable SMGPE aggregates protection film on the studied steel specimen surface
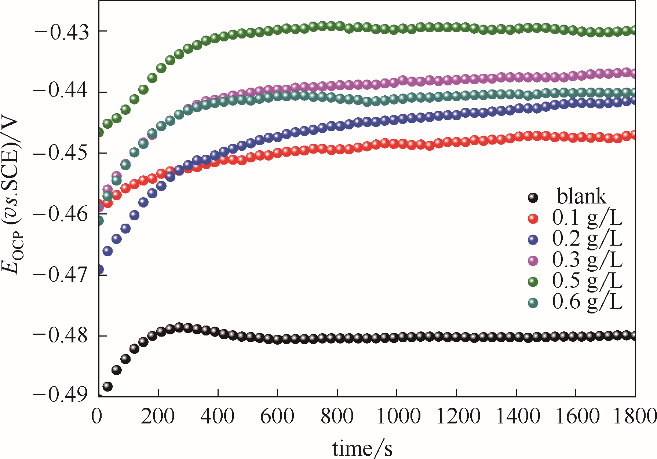
Fig.3 OCP versus time curves in 1.0 mol/L HCl solutions for the investigated unmodified steel electrodes and the stable SMGPE aggregates of different concentrations covered steel electrodes
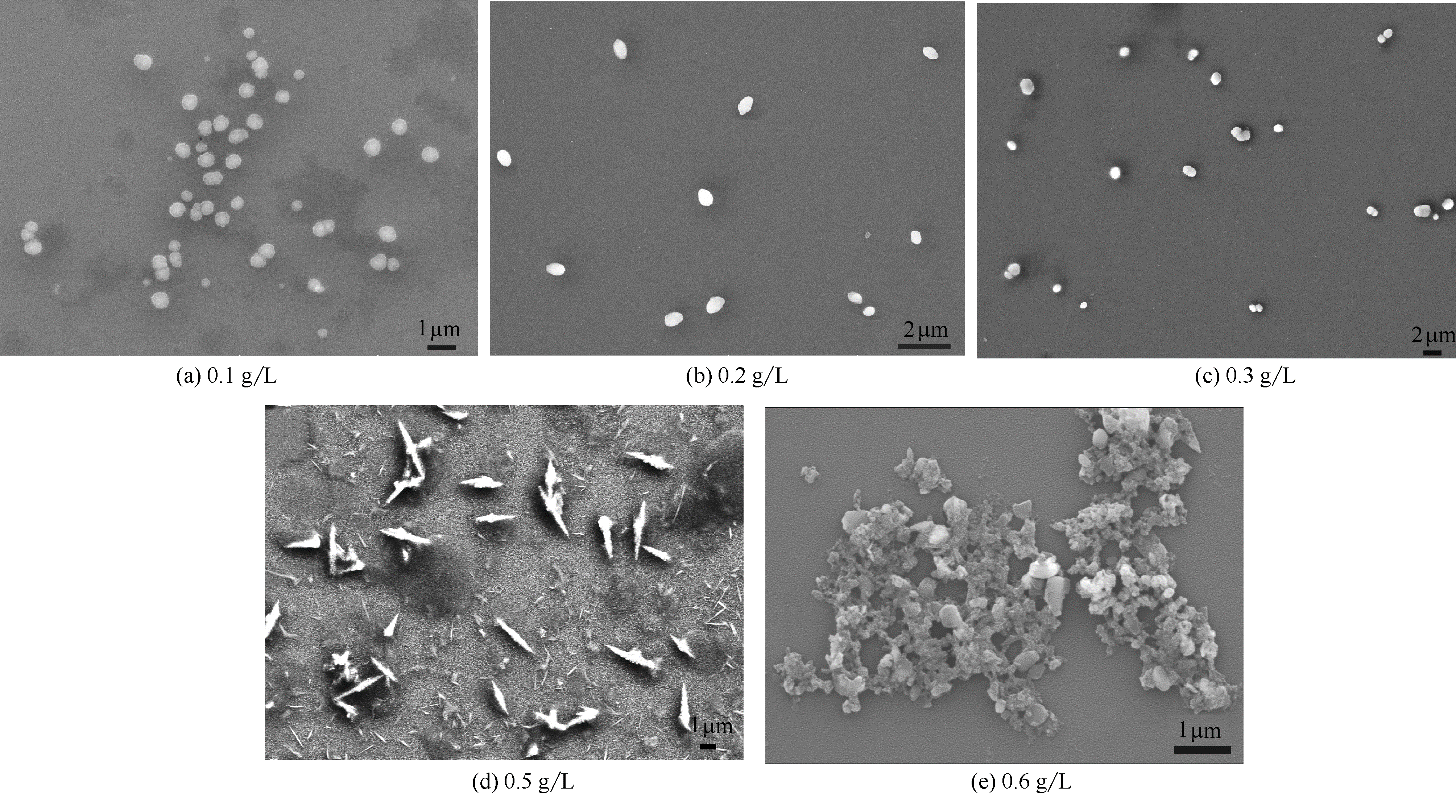
Fig.5 SEM images of SMGPE aggregates in DMF/HCl (vol ratio 50/50,1.0 mol/L HCl solution) mixed solution with different concentrations from 0.1 to 0.6 g/L at 10 h evolution time
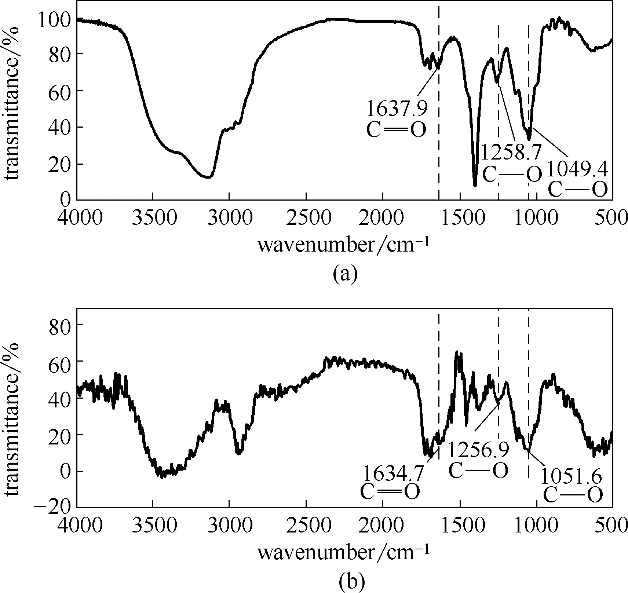
Fig.6 FT-IR spectrum of original SME powder (a); FT-IR spectrum of the stable SMGPE aggregates (aggregation concentration 0.5 g/L, aggregation time 10 h)adsorbed on the studied steel specimen surfaces (b)
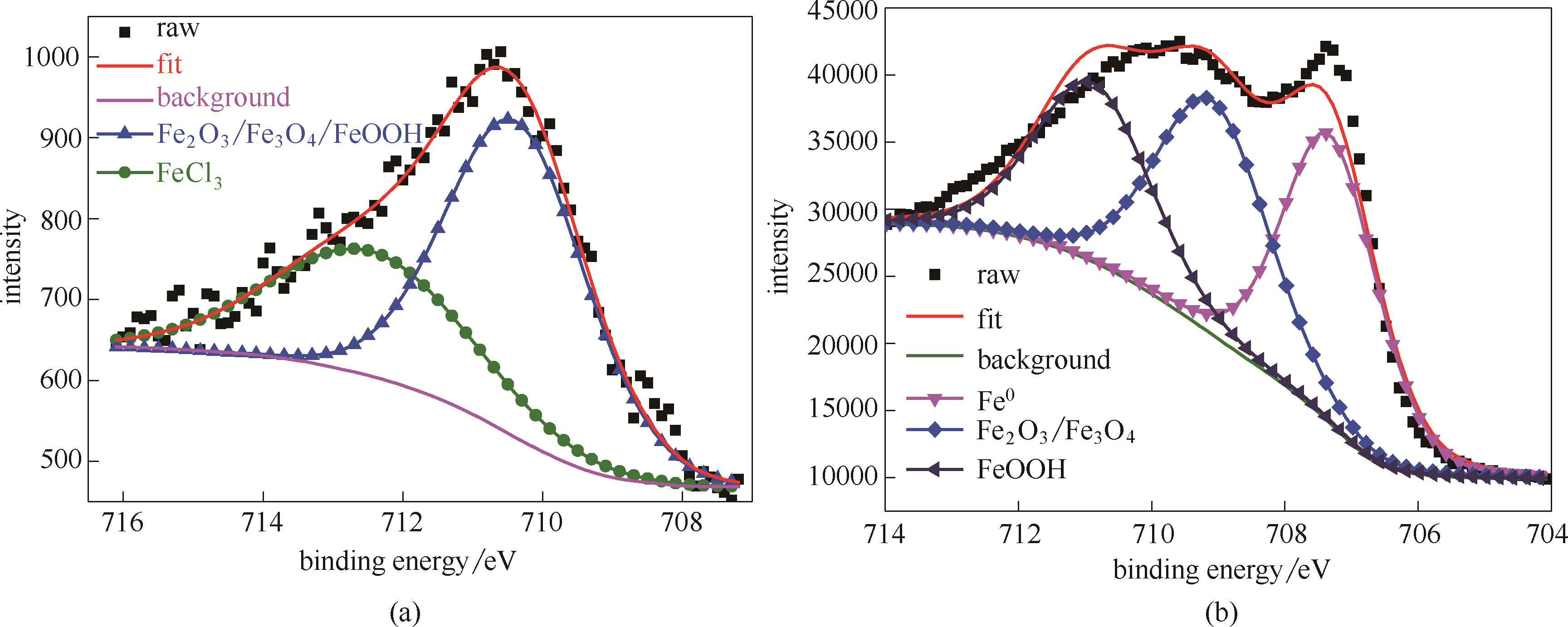
Fig.7 Fe 2p XPS spectra from the surfaces of the investigated bare steel immersed in HCl solution for 1 h (a); Fe 2p XPS spectra from the studied steel specimens treated by the stable SMGPE aggregates (aggregation concentration 0.5 g/L, aggregation time 10 h) immersed in HCl solution for 1 h (b)
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | Fe2O3/Fe3O4/FeOOH | 710.38 | 3.45 |
| FeCl3 | 712.47 | 3.5 | |
| 吸附稳定SMGPE聚集体的钢片 | Fe0 | 707.31 | 1.75 |
| Fe2O3/ Fe3O4 | 709.10 | 2.00 | |
| FeOOH | 710.90 | 2.00 |
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | Fe2O3/Fe3O4/FeOOH | 710.38 | 3.45 |
| FeCl3 | 712.47 | 3.5 | |
| 吸附稳定SMGPE聚集体的钢片 | Fe0 | 707.31 | 1.75 |
| Fe2O3/ Fe3O4 | 709.10 | 2.00 | |
| FeOOH | 710.90 | 2.00 |
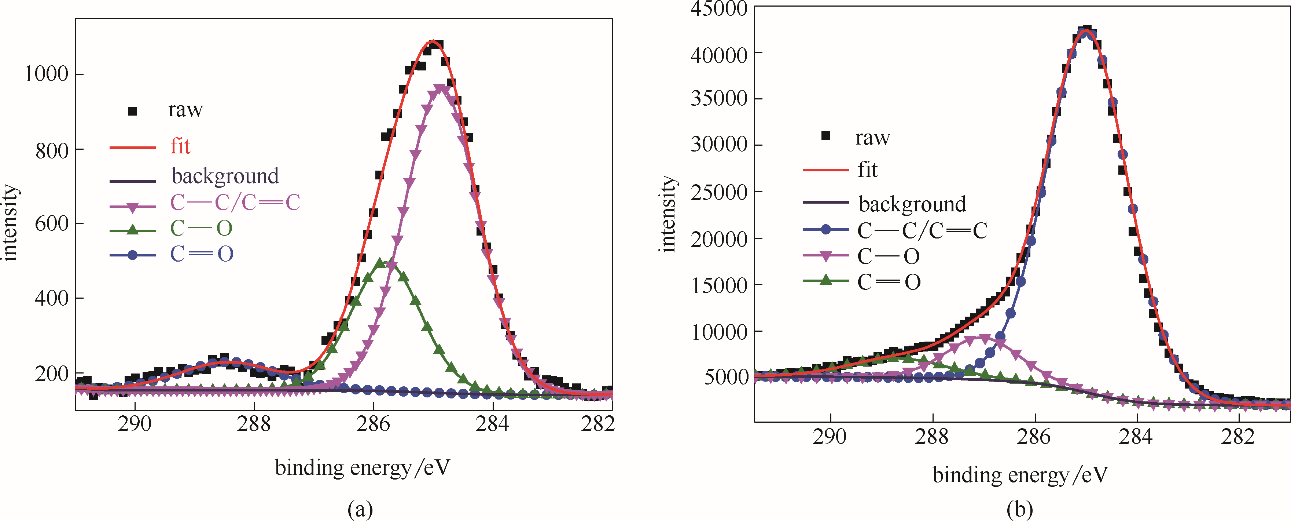
Fig.8 C 1s XPS spectra from surfaces of the investigated bare steel immersed in HCl solution (1.0 mol/L HCl) for 1 h (a); C 1s XPS spectra from surfaces of the studied steel specimens treated by stable SMGPE aggregates (aggregation concentration 0.5 g/L, aggregation time 10 h) immersed in 1.0 mol/L HCl solution for 1 h (b)
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | C—C/C C C | 284.90 | 1.62 |
| C—O | 285.79 | 1.55 | |
C O O | 288.43 | 2.00 | |
| 吸附稳定SMGPE聚集体的钢片 | C—C/C C C | 284.98 | 2.06 |
| C—O | 287.03 | 1.90 | |
C O O | 288.68 | 2.30 |
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | C—C/C C C | 284.90 | 1.62 |
| C—O | 285.79 | 1.55 | |
C O O | 288.43 | 2.00 | |
| 吸附稳定SMGPE聚集体的钢片 | C—C/C C C | 284.98 | 2.06 |
| C—O | 287.03 | 1.90 | |
C O O | 288.68 | 2.30 |
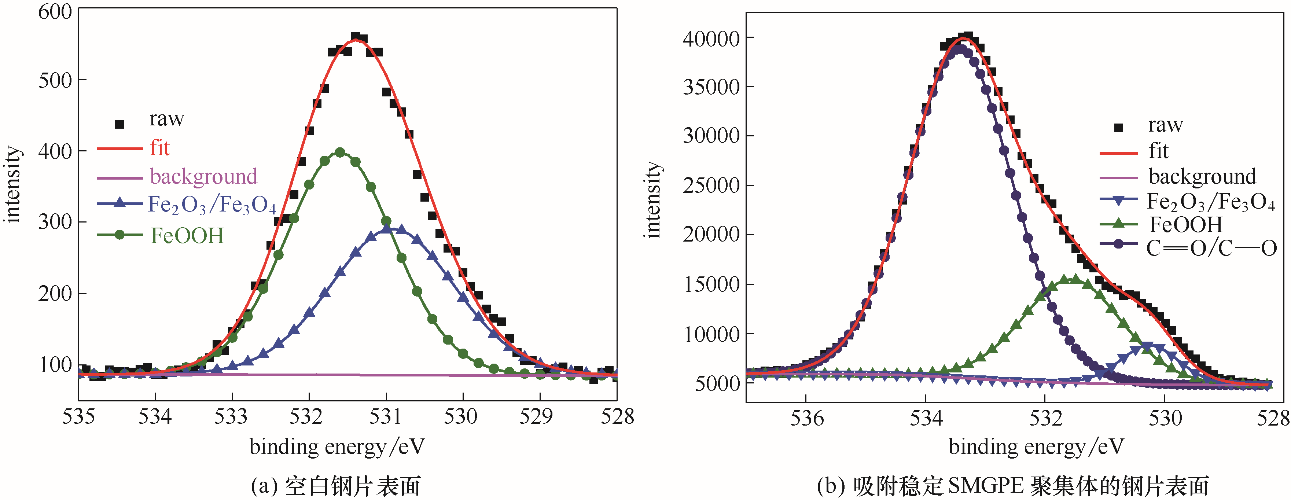
Fig.9 O 1s XPS spectra from surfaces of the investigated bare steel immersed in 1.0 mol/L HCl solution for 3 h; O 1s XPS spectra from surfaces of the studied steel specimens treated by stable SMGPE aggregates (aggregation concentration 0.5 g/L, aggregation time 10 h) immersed in HCl solution for 1 h
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | Fe2O3/ Fe3O4 | 530.92 | 2.00 |
| FeOOH | 531.61 | 1.80 | |
| 吸附稳定SMGPE聚集体的钢片 | Fe2O3/ Fe3O4 | 530.24 | 1.90 |
| FeOOH | 531.55 | 1.75 | |
C O/C—O O/C—O | 533.41 | 1.60 |
Table 3 De-convolution parameters including chemical states, binding energies and FWHMs of O 1s XPS spectra peaks obtained from surfaces of the studied bare steel specimen and the studied steel specimens treated by stable SMGPE aggregates immersed in 1.0 mol/L HCl solution for 1 h (aggregation concentration, 0.5 g/L, aggregation time, 10 h)
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | Fe2O3/ Fe3O4 | 530.92 | 2.00 |
| FeOOH | 531.61 | 1.80 | |
| 吸附稳定SMGPE聚集体的钢片 | Fe2O3/ Fe3O4 | 530.24 | 1.90 |
| FeOOH | 531.55 | 1.75 | |
C O/C—O O/C—O | 533.41 | 1.60 |
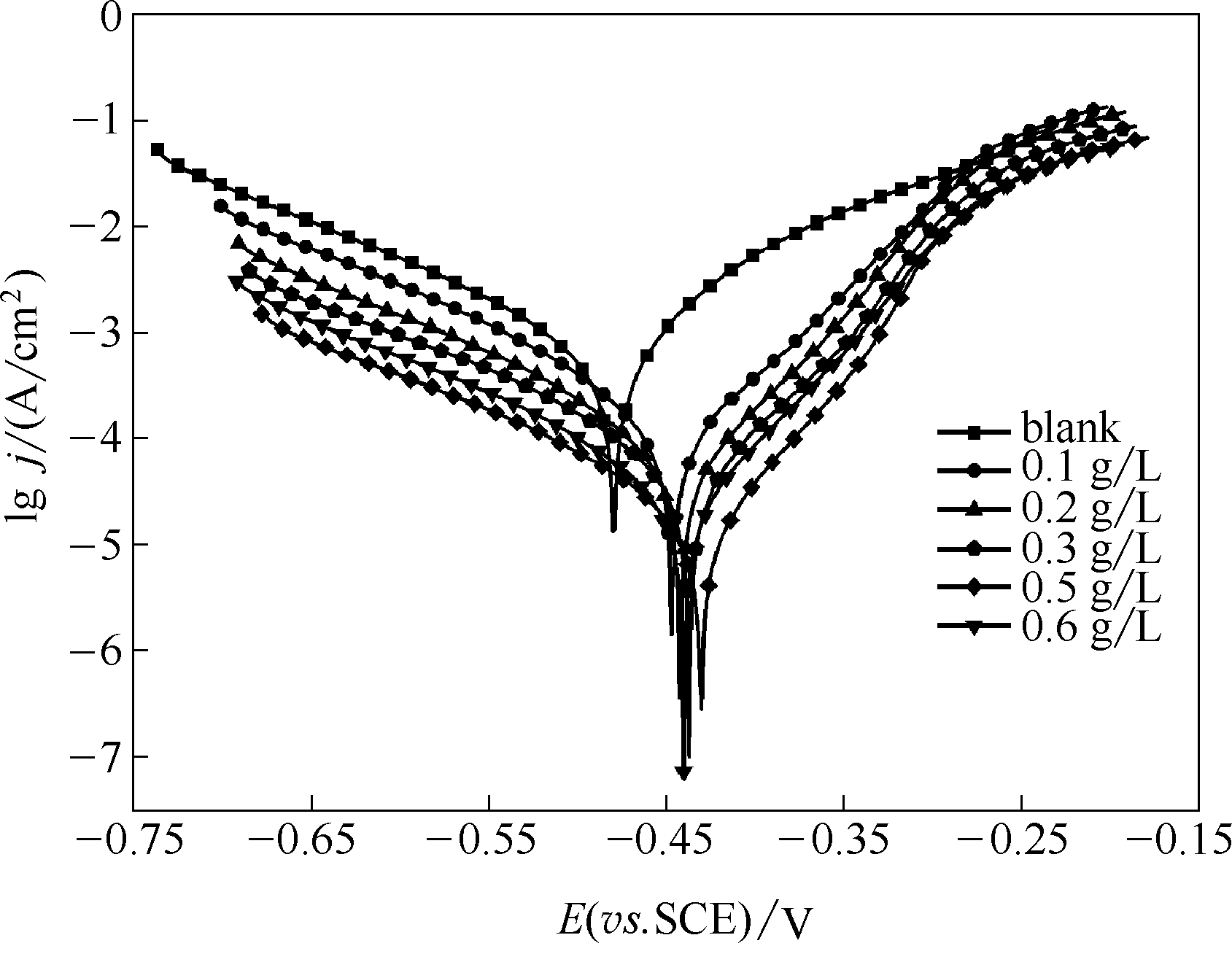
Fig.11 Potentiodynamic polarization curves in 1 mol/L HCl solutions for the investigated unmodified steel electrodes and the stable SMGPE aggregates of different concentrations (aggregation time 10 h) covered steel electrodes
| 电极 | 极化参数 | |||||
|---|---|---|---|---|---|---|
| C/(g/L) | Ecorr (vs. SCE) /V | Icorr/(A/cm2) | βc/(V/dec) | βa/(V/dec) | ηj/% | |
| 空白钢电极 | — | -0.480 | 4.387×10-7 | -0.1278 | 0.1068 | — |
| 稳定SMGPE聚集体吸附的钢电极 | 0.1 | -0.447 | 1.004×10-7 | -0.1146 | 0.0585 | 77.11 |
| 0.2 | -0.442 | 4.957×10-8 | -0.1175 | 0.0508 | 88.7 | |
| 0.3 | -0.437 | 4.549×10-8 | -0.1233 | 0.0515 | 89.63 | |
| 0.5 | -0.430 | 2.820×10-8 | -0.1226 | 0.0424 | 93.57 | |
| 0.6 | -0.440 | 3.767×10-4 | -0.1212 | 0.0524 | 91.41 | |
Table 4 Polarization parameters for the studied unmodified and modified steel specimens by different concentrations of stable SMGPE aggregates (aggregation time 10 h) in 1.0 mol/L HCl solution
| 电极 | 极化参数 | |||||
|---|---|---|---|---|---|---|
| C/(g/L) | Ecorr (vs. SCE) /V | Icorr/(A/cm2) | βc/(V/dec) | βa/(V/dec) | ηj/% | |
| 空白钢电极 | — | -0.480 | 4.387×10-7 | -0.1278 | 0.1068 | — |
| 稳定SMGPE聚集体吸附的钢电极 | 0.1 | -0.447 | 1.004×10-7 | -0.1146 | 0.0585 | 77.11 |
| 0.2 | -0.442 | 4.957×10-8 | -0.1175 | 0.0508 | 88.7 | |
| 0.3 | -0.437 | 4.549×10-8 | -0.1233 | 0.0515 | 89.63 | |
| 0.5 | -0.430 | 2.820×10-8 | -0.1226 | 0.0424 | 93.57 | |
| 0.6 | -0.440 | 3.767×10-4 | -0.1212 | 0.0524 | 91.41 | |
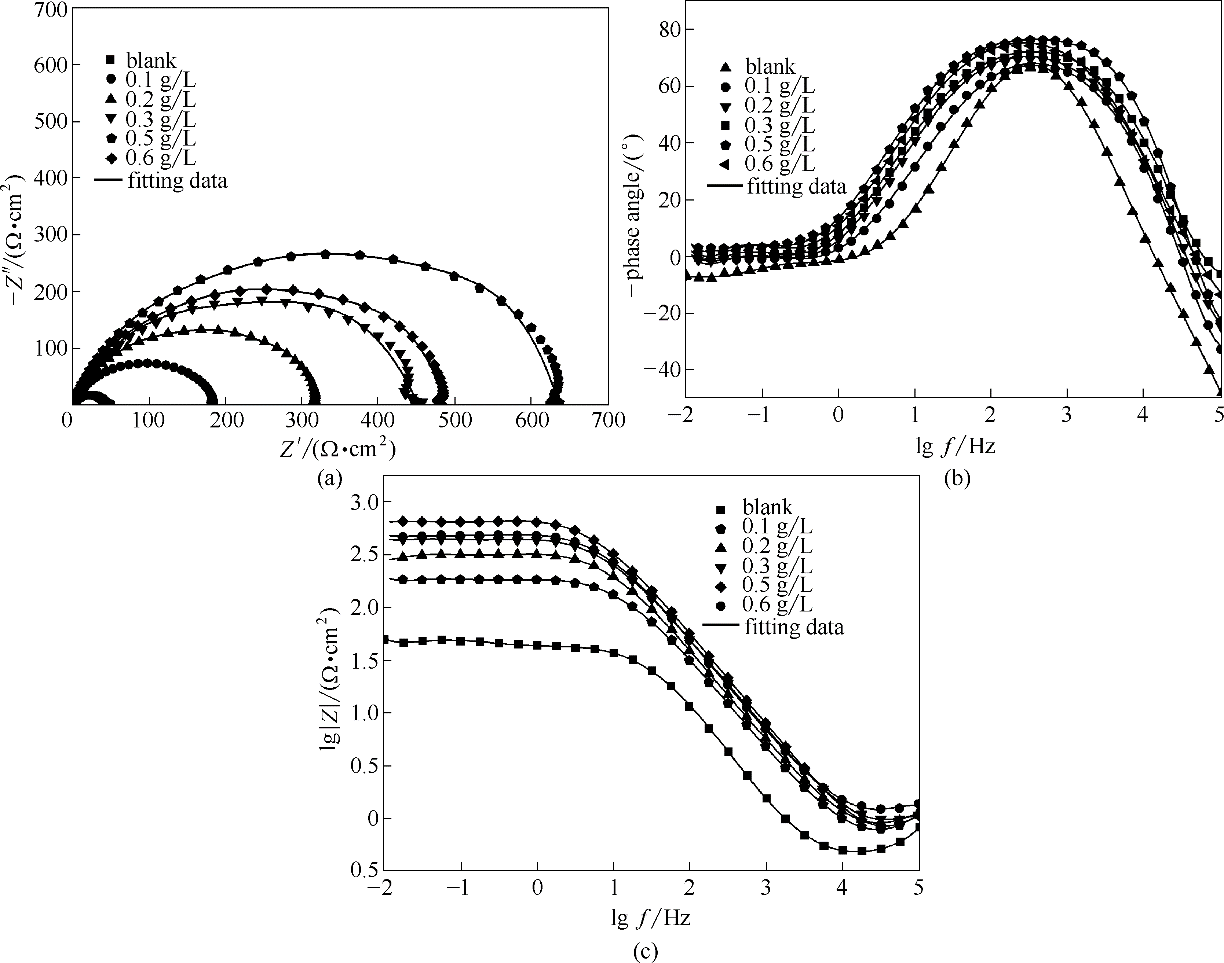
Fig.12 Nyquist curves for the studied naked steel electrodes and SMGPE aggregates (aggregation time 10 h) of various concentrations covered steel electrodes(a); Bode plots for the studied naked steel electrodes (b) and SMGPE aggregates (aggregation time 10 h) of various concentrations covered steel electrodes (c)
| 电极 | C/ (g/L) | 电化学参数 | χ2 | ||||
|---|---|---|---|---|---|---|---|
| Rs/ (Ω·cm2) | Rct / (Ω·cm2) | Cdl / (F/cm2) | n | ηE /% | |||
| 空白钢电极 | — | 0.6096 | 44.36 | 1.408×10-7 | 0.9551 | — | 4.81×103 |
| SMGPE聚集体吸附的钢电极 | 0.1 | 0.8946 | 182.0 | 6.34×10-8 | 0.8832 | 75.63 | 2.41×103 |
| 0.2 | 0.9725 | 315.4 | 5.48×10-8 | 0.8829 | 85.94 | 1.52×103 | |
| 0.3 | 0.9667 | 451.2 | 4.49×10-8 | 0.8707 | 90.17 | 4.65×103 | |
| 0.5 | 0.8991 | 635.0 | 3.98×10-8 | 0.8928 | 93.01 | 1.95×103 | |
| 0.6 | 1.2320 | 484.4 | 4.46×10-8 | 0.8867 | 90.92 | 7.48×103 | |
Table 5 Electrochemical impedance parameters for the studied unmodified and different concentrations of stable SMGPE aggregates (aggregation time 10 h) modified steel specimens in 1.0 mol/L HCl solution
| 电极 | C/ (g/L) | 电化学参数 | χ2 | ||||
|---|---|---|---|---|---|---|---|
| Rs/ (Ω·cm2) | Rct / (Ω·cm2) | Cdl / (F/cm2) | n | ηE /% | |||
| 空白钢电极 | — | 0.6096 | 44.36 | 1.408×10-7 | 0.9551 | — | 4.81×103 |
| SMGPE聚集体吸附的钢电极 | 0.1 | 0.8946 | 182.0 | 6.34×10-8 | 0.8832 | 75.63 | 2.41×103 |
| 0.2 | 0.9725 | 315.4 | 5.48×10-8 | 0.8829 | 85.94 | 1.52×103 | |
| 0.3 | 0.9667 | 451.2 | 4.49×10-8 | 0.8707 | 90.17 | 4.65×103 | |
| 0.5 | 0.8991 | 635.0 | 3.98×10-8 | 0.8928 | 93.01 | 1.95×103 | |
| 0.6 | 1.2320 | 484.4 | 4.46×10-8 | 0.8867 | 90.92 | 7.48×103 | |
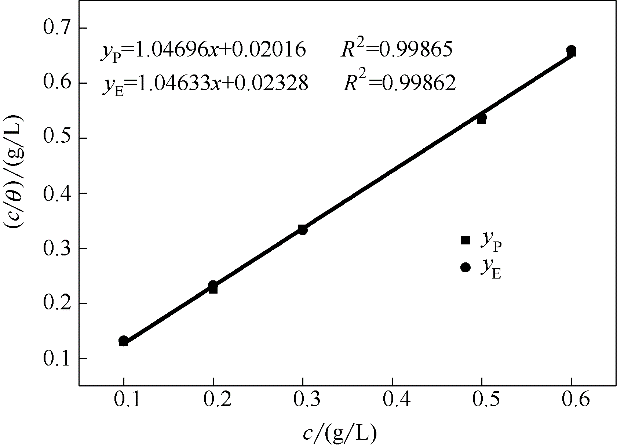
Fig.14 Langmuir adsorption isotherm of stable SMGPE aggregates on the studied steel specimen surfaces in 1.0 mol/L HCl solution at 298 K (yP to Tafel curves, yE to electrochemical impedance spectroscopy)
| 测试方法 | 吸附能 |
|---|---|
| Polarization | -26790 |
| EIS | -26430 |
Table 6 Thermodynamic parameters for the adsorption of SMGPE aggregates at 298 K
| 测试方法 | 吸附能 |
|---|---|
| Polarization | -26790 |
| EIS | -26430 |
| 1 | Wang H, Akid R. Encapsulated cerium nitrate inhibitors to provide high-performance anti-corrosion sol-gel coatings on mild steel[J]. Corrosion Science, 2008, 50: 1142-1148. |
| 2 | Yu J, Zhang Y, Jin X, et al. Fabrication and optical emission spectroscopy of enhanced corrosion-resistant CPEO films on Q235 low carbon steel[J]. Surface and Coatings Technology, 2019, 363: 411-418. |
| 3 | Yi C. Corrosion inhibition effect of 2-hydroxy phosphonoacetic acid and pyrophosfate on Q235 steel, electrochemical noise and EIS analysis[J]. International Journal of Electrochemical Science, 2019, 14: 6759-6772. |
| 4 | Wang C. Synthesis, crystal structure and corrosion inhibition effect of s-benzyl-O,O'-bis(p-tert-butyl phenyl)dithiophosphate for Q235 steel in 1.0 M HCl[J]. International Journal of Electrochemical Science, 2019, 31(6): 3443-3454. |
| 5 | Guo L, Obot I B, Zheng X W, et al. Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms[J]. Applied Surface Science, 2017, 406: 301-306. |
| 6 | Qiang Y J, Fu S L, Zhang S T, et al. Designing and fabricating of single and double alkyl-chain indazole derivatives self-assembled monolayer for corrosion inhibition of copper[J]. Corrosion Science, 20181, 40: 111-121. |
| 7 | Qiang Y J, Zhang S T, Tan B, et al. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution[J]. Corrosion Science, 2018, 133: 6-16. |
| 8 | Umoren S A. Biomaterials for corrosion protection: evaluation of mustard seed extract as eco-friendly corrosion inhibitor for X60 steel in acid media[J]. Journal of Adhesion Science and Technology, 2016, 30: 1858-1879. |
| 9 | Li L, Xu W, Lei J, et al. Experimental and theoretical investigations of Michelia alba leaves extract as a green highly-effective corrosion inhibitor for different steel materials in acidic solution[J]. RSC Advances, 2015, 5: 93724-93732. |
| 10 | Mobin M, Aslam R, Zehra S, et al. Bio-/environment-friendly cationic gemini surfactant as novel corrosion inhibitor for mild steel in 1 M HCl solution[J]. Journal of Surfactants and Detergents, 2016, 20: 57-74. |
| 11 | El Achouri M, Kertit S, Gouttaya H M, et al. Corrosion inhibition of iron in 1 M HCl by some gemini surfactants in the series of alkanediyl-α, ω-bis-(dimethyl tetradecyl ammonium bromide) [J]. Progress in Organic Coatings, 2001, 43: 267-273. |
| 12 | Huang H C, Liao S C, Chang F R, et al. Molluscicidal saponins from apindus mukorossi, inhibitory agents of golden apple snails, pomacea canaliculata[J]. Journal of Food Agriculture and Environment, 2003, 51: 4916-4919. |
| 13 | 魏敏平. 无患子抑菌成分的分离纯化及其应用研究[D]. 无锡: 江南大学, 2018. |
| Wei M P. Study on the separation and purification of antibacterial components from Sapindus mukurossiand its application[D]. Wuxi: Jiangnan University, 2018. | |
| 14 | 徐圆圆, 贾黎明, 陈仲, 等. 无患子三萜皂苷研究进展[J]. 化学通报, 2018, 12(81): 1078-1088. |
| Xu Y Y, Jia L M, Chen Z, et al. Advances on Triterpenoid Saponin of Sapindus mukorossi[J]. Chemistry, 2018, 12(81): 1078-1088. | |
| 15 | 周礼彬. 川滇无患子总皂苷化学成分及表面活性的应用性能研究[D]. 昆明: 云南中医学院, 2017. |
| Zhou L B. Study on the chemical components and the application of surface active properties of the total saponins from sapindus delavayi[D]. Kunming: Yunnan University of Traditional Chinese Medicine, 2017. | |
| 16 | Li X, Deng S, Fu H. Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract[J]. Corrosion Science, 2012, 62: 163-175. |
| 17 | Chidiebere M A, Oguzie E E, Liu L, et al. Ascorbic acid as corrosion inhibitor for Q235 mild steel in acidic environments[J]. Journal of Industrial and Engineering Chemistry, 2015, 26: 182-192. |
| 18 | Mallaiya K, Subramaniam R, Srikandan S S, et al. Electrochemical characterization of the protective film formed by the unsymmetrical Schiff's base on the mild steel surface in acid media[J]. Electrochimica. Acta, 2011, 56: 3857-3863. |
| 19 | Zarrouk A, Hammouti B, Lakhlifi T, et al. New 1 H -pyrrole-2, 5-dione derivatives as efficient organic inhibitors of carbon steel corrosion in hydrochloric acid medium: electrochemical, XPS and DFT studies[J]. Corrosion Science, 2015, 90: 572-584. |
| 20 | Kharbach Y, Qachchachi F Z, Haoudi A, et al. Anticorrosion performance of three newly synthesized isatin derivatives on carbon steel in hydrochloric acid pickling environment: electrochemical, surface and theoretical studies[J]. Journal of Molecular Liquids, 2017, 246: 302-316. |
| 21 | Bouanis M, Tourabi M, Nyassi A, et al. Corrosion inhibition performance of 2, 5-bis(4-dimethylaminophenyl)-1, 3, 4-oxadiazole for carbon steel in HCl solution: gravimetric, electrochemical and XPS studies[J]. Applied Surface Science, 2016, 389: 952-966. |
| 22 | Boumhara K, Tabyaoui M, Jama C, et al. Artemisia Mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1 M HCl solution: electrochemical and XPS investigations[J]. Journal of Industrial and Engineering Chemistry, 2015, 29: 146-155. |
| 23 | Ji G, Anjum S, Sundaram S, et al. Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution[J]. Corrosion Science, 2015, 90: 107-117. |
| 24 | Hussin M H, Jain K M, Razali N N, et al.The effect of Tinospora crispa extracts as a natural mild steel corrosion inhibitor in 1 M HCl solution[J]. Arabian Journal of Chemistry, 2016, 9: 616-624. |
| 25 | Singh A K, Quraishi M A. Investigation of adsorption of isoniazid derivatives at mild steel/hydrochloric acid interface: electrochemical and weight loss methods[J]. Materials Chemistry and Physics, 2010, 123: 666–677. |
| 26 | Sudheer, Quraishi M A. Electrochemical and theoretical investigation of triazole derivatives on corrosion inhibition behavior of copper in hydrochloric acid medium[J]. Corrosion Science, 2013, 70: 161-169. |
| 27 | Huang H J, Fu Y, Li F, et al.Orderly self-assembly of new ionic copolymers for efficiently protecting copper in aggressive sulfuric acid solution[J]. Chemical Engineering Journal, 2020, 384: 123293. |
| 28 | Wang Z Q, Gong Y L, Zhang L, et al. Self-assembly of new dendrimers basing on strong π-π intermolecular interaction for application to protect copper[J]. Chemical Engineering Journal, 2018, 342: 238-250. |
| 29 | Ostovari A, Hoseinieh S M, Peikari M, et al. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid) [J]. Corrosion Science, 2009, 51: 1935-1949. |
| 30 | Sığırcık G, Yildirim D, Tüken T. Synthesis and inhibitory effect of N, N'-bis(1-phenylethanol)ethylenediamine against steel corrosion in HCl media[J]. Corrosion Science, 2017, 120: 184-193. |
| 31 | Li L, Zhang X, Lei J, et al. Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel[J]. Corrosion Science, 2012, 63: 82-90. |
| 32 | Biswas A, Pal S, Udayabhanu G. Experimental and theoretical studies of Xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl[J]. Applied Surface Science, 2015, 353: 173-183. |
| 33 | Chauhan D S, Ansari K R, Sorour A A, et al. Thiosemicarbazide and thiocarbohydrazide functionalized chitosan as ecofriendly corrosion inhibitors for carbon steel in hydrochloric acid solution[J]. International Journal of Biological Macromolecules, 2018, 107: 1747-1757. |
| 34 | Hu Z, Meng Y, Ma X, et al. Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M HCl[J]. Corrosion Science, 2016, 112: 563-575. |
| 35 | Obot I B, Obi-Egbedi N O. Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation[J]. Corrosion Science, 2010, 52: 198-204. |
| [1] | Fei KANG, Weiguang LYU, Feng JU, Zhi SUN. Research on discharge path and evaluation of spent lithium-ion batteries [J]. CIESC Journal, 2023, 74(9): 3903-3911. |
| [2] | Jiaqi CHEN, Wanyu ZHAO, Ruichong YAO, Daolin HOU, Sheying DONG. Synthesis of pistachio shell-based carbon dots and their corrosion inhibition behavior on Q235 carbon steel [J]. CIESC Journal, 2023, 74(8): 3446-3456. |
| [3] | Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent [J]. CIESC Journal, 2023, 74(8): 3375-3385. |
| [4] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [5] | Yan GAO, Peng WU, Chao SHANG, Zejun HU, Xiaodong CHEN. Preparation of magnetic agarose microspheres based on a two-fluid nozzle and their protein adsorption properties [J]. CIESC Journal, 2023, 74(8): 3457-3471. |
| [6] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [7] | Jie WANG, Xiaolin QIU, Ye ZHAO, Xinyang LIU, Zhongqiang HAN, Yong XU, Wenhan JIANG. Preparation and properties of polyelectrolyte electrostatic deposition modified PHBV antioxidant films [J]. CIESC Journal, 2023, 74(7): 3068-3078. |
| [8] | Jing ZHAO, Chengwen GU, Xigao JIAN, Zhihuan WENG. Preparation and performance evaluation of magnolol-based epoxy resin anti-corrosion coatings [J]. CIESC Journal, 2023, 74(7): 3010-3017. |
| [9] | Yanhui LI, Shaoming DING, Zhouyang BAI, Yinan ZHANG, Zhihong YU, Limei XING, Pengfei GAO, Yongzhen WANG. Corrosion micro-nano scale kinetics model development and application in non-conventional supercritical boilers [J]. CIESC Journal, 2023, 74(6): 2436-2446. |
| [10] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [11] | Chenxin LI, Yanqiu PAN, Liu HE, Yabin NIU, Lu YU. Carbon membrane model based on carbon microcrystal structure and its gas separation simulation [J]. CIESC Journal, 2023, 74(5): 2057-2066. |
| [12] | Shaoyun CHEN, Dong XU, Long CHEN, Yu ZHANG, Yuanfang ZHANG, Qingliang YOU, Chenglong HU, Jian CHEN. Preparation and adsorption properties of monolayer polyaniline microsphere arrays [J]. CIESC Journal, 2023, 74(5): 2228-2238. |
| [13] | Yu PAN, Zihang WANG, Jiayun WANG, Ruzhu WANG, Hua ZHANG. Heat and moisture performance study of Cur-LiCl coated heat exchanger [J]. CIESC Journal, 2023, 74(3): 1352-1359. |
| [14] | Xuanjun WU, Chao WANG, Zijian CAO, Weiquan CAI. Deep learning model of fixed bed adsorption breakthrough curve hybrid-driven by data and physical information [J]. CIESC Journal, 2023, 74(3): 1145-1160. |
| [15] | Xiaowan PENG, Xiaonan GUO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Modeling and simulation of CH4/N2 separation process with two absorption-adsorption columns using ZIF-8 slurry [J]. CIESC Journal, 2023, 74(2): 784-795. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||