CIESC Journal ›› 2021, Vol. 72 ›› Issue (2): 748-760.DOI: 10.11949/0438-1157.20201063
• Reviews and monographs • Previous Articles Next Articles
YU Tao1( ),WANG Yundong1(
),WANG Yundong1( ),LIU Zuohua2,MA Jianxiu3,JING Yu3
),LIU Zuohua2,MA Jianxiu3,JING Yu3
Received:2020-07-30
Revised:2020-09-22
Online:2021-02-05
Published:2021-02-05
Contact:
WANG Yundong
通讯作者:
王运东
作者简介:于涛(1997—),男,博士研究生,基金资助:CLC Number:
YU Tao, WANG Yundong, LIU Zuohua, MA Jianxiu, JING Yu. Research progress of hydrogen sulfide deep adsorption materials[J]. CIESC Journal, 2021, 72(2): 748-760.
于涛, 王运东, 刘作华, 马建修, 靖宇. 硫化氢深度吸附材料的研究进展[J]. 化工学报, 2021, 72(2): 748-760.
Add to citation manager EndNote|Ris|BibTeX
| 材料 | 比表面积/(m2/g) | 孔体积/ (cm3/g) | 平均孔径/nm | 气体组成 | 操作 温度/℃ | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|
| WSC | 860 | 0.40 | 0.7 | 50% H2, 15% CO2, 9% CO, 2% N2, 24% H2O, 10-3 H2S 50% H2, 15% CO2, 9% CO, 2% N2, 24% H2O, 10-3 H2S | 150 150 | 71(0.2) | [ |
| WSCU | 945 | 0.43 | 0.8 | 133(0.2) | [ | ||
| S208C | 898 | 0.48 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 27.2(ND) | [ |
| S-A | 905 | 0.48 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 26.4(ND) | [ |
| S-AU | 640 | 0.34 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 34.0(ND) | [ |
| S-AM | 37 | 0.02 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 0.4(-) | [ |
| S-B | 882 | 0.47 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 17.6(ND) | [ |
| S-BU | 808 | 0.43 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 54.1(ND) | [ |
| S-BM | 732 | 0.39 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 71.9(ND) | [ |
| AC-KOH/KI | 1042 | 0.42 | — | 2% O2, 10-4 H2S, 90%相对湿度, N2平衡 | 45 45 | 65.6(0.2) | [ |
| 10-4 H2S, 90%相对湿度, N2平衡 | 34.9(0.2) | [ | |||||
| RB2-NaOH50 | 758 | 0.25 | 1.56 | 2.5×10-4 H2S, N2平衡 | 25 | 43.5(ND) | [ |
| AC-NaOH | — | — | — | 3×10-3 H2S, He平衡 | 30 | 0.4(10) | [ |
| AC-KI | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.56(<10) | [ |
| AC-Na2CO3 | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.58(<10) | [ |
| AC-KOH | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.58(<10) | [ |
| GAC | 1678±41 | 0.75±0.03 | 0.89±0.16 | 80% H2O, 1% H2S, 空气 80% H2O, 1% H2S, 空气 | 25 25 | 53(N) | [ |
| MgO-GAC | 1358±39 | 0.60±0.01 | 0.88±0.23 | 275(N) | [ | ||
| Cu0.5Zn0.5/AC | 570 | 0.76 | — | 10-4 H2S, 2.5×10-3 O2, 50%相对湿度, N2平衡 | 30 | 25.0(ND) | [ |
| NORIT | 978 | 0.30 | — | 5×10-5 H2S, N2平衡 | — | - (<0.5) | [ |
Table 1 Parameters and adsorption ability of active carbon materials for H2S removal
| 材料 | 比表面积/(m2/g) | 孔体积/ (cm3/g) | 平均孔径/nm | 气体组成 | 操作 温度/℃ | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|
| WSC | 860 | 0.40 | 0.7 | 50% H2, 15% CO2, 9% CO, 2% N2, 24% H2O, 10-3 H2S 50% H2, 15% CO2, 9% CO, 2% N2, 24% H2O, 10-3 H2S | 150 150 | 71(0.2) | [ |
| WSCU | 945 | 0.43 | 0.8 | 133(0.2) | [ | ||
| S208C | 898 | 0.48 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 27.2(ND) | [ |
| S-A | 905 | 0.48 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 26.4(ND) | [ |
| S-AU | 640 | 0.34 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 34.0(ND) | [ |
| S-AM | 37 | 0.02 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 0.4(-) | [ |
| S-B | 882 | 0.47 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 17.6(ND) | [ |
| S-BU | 808 | 0.43 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 54.1(ND) | [ |
| S-BM | 732 | 0.39 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 71.9(ND) | [ |
| AC-KOH/KI | 1042 | 0.42 | — | 2% O2, 10-4 H2S, 90%相对湿度, N2平衡 | 45 45 | 65.6(0.2) | [ |
| 10-4 H2S, 90%相对湿度, N2平衡 | 34.9(0.2) | [ | |||||
| RB2-NaOH50 | 758 | 0.25 | 1.56 | 2.5×10-4 H2S, N2平衡 | 25 | 43.5(ND) | [ |
| AC-NaOH | — | — | — | 3×10-3 H2S, He平衡 | 30 | 0.4(10) | [ |
| AC-KI | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.56(<10) | [ |
| AC-Na2CO3 | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.58(<10) | [ |
| AC-KOH | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.58(<10) | [ |
| GAC | 1678±41 | 0.75±0.03 | 0.89±0.16 | 80% H2O, 1% H2S, 空气 80% H2O, 1% H2S, 空气 | 25 25 | 53(N) | [ |
| MgO-GAC | 1358±39 | 0.60±0.01 | 0.88±0.23 | 275(N) | [ | ||
| Cu0.5Zn0.5/AC | 570 | 0.76 | — | 10-4 H2S, 2.5×10-3 O2, 50%相对湿度, N2平衡 | 30 | 25.0(ND) | [ |
| NORIT | 978 | 0.30 | — | 5×10-5 H2S, N2平衡 | — | - (<0.5) | [ |
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量①/(mg/g) | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| 3DOM-Fe2O3 | 16~44 | — | 20~33 | 5% H2, 3×10-4 H2S, N2平衡 5% H2, 3×10-4 H2S, N2平衡 | 350 350 | — | 37.18(<0.2) | [ |
| 3DOM-Fe2O3/SiO2 | 80~220 | — | 6~17 | — | 38.92(<0.2) | [ | ||
| MFT | — | — | — | 39.8% H2, 32.6% CO2, 2.5×10-3 H2S, N2平衡 | 400 | 216(N) | — | [ |
| Engelhard ZnO | 53.13 | 0.28 | — | 8×10-6 H2S, 34.4% H2, 20% H2O, N2平衡 | 300 | — | 28.1(0.02) | [ |
| ZSCo-0 | 151.3 | 0.42 | 7.40 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 174.6 | 117.8(ND) | [ |
| ZSCo-0.05 | 188.7 | 0.58 | 7.82 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 195.2 | 146.4(ND) | [ |
| ZSCo-0.15 | 206.4 | 0.63 | 9.58 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 208.4 | 169.8(ND) | [ |
| ZSCo-0.3 | 192.1 | 0.55 | 9.24 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 230.5 | 188.4(ND) | [ |
| ZSCo-0.50 | 161.5 | 0.46 | 7.53 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 194.1 | 143.4(ND) | [ |
| Extruded-Fe2O3 | 60.6 | 13.90 | 99.0 | 6×10-4 H2S, 混合气体 | 30 ± 2 | 22.4 | 16.0(N) | [ |
| Fe-Cu-Al-O | 96.7 | — | — | 10-3 H2S, CO2 | 40 | — | 113.9(ND) | [ |
| ZnO | 10.2 | 0.029 | 介-大孔 | 51% H2, 30% He, 10% H2O, 4×10-4 H2S | 400 | 457.3(2) | — | [ |
| Ni-ZnO | 6.8 | 0.025 | 介-大孔 | 51% H2, 30% He, 10% H2O, 4×10-4 H2S | 400 | 730.0(2) | — | [ |
| HZn20SC | 272.59 | 0.17 | — | 33% CO, 39% H2, 5% H2O,3×10-4 H2S, N2平衡 | 450 | — | 44.6(ND) | [ |
| Z20M4C6SC | 207.33 | 0.03 | — | 33% CO, 39% H2, 5×10-4 H2S, N2平衡 | 500 | — | 138.4(ND) | [ |
| ZnO | — | — | — | 54% H2, 21%CO2, 2.5×10-5 H2S, He 平衡 | 150 | — | 131(<0.1) | [ |
| — | — | — | 54% H2, 21%CO2, 2.5×10-5 H2S, He 平衡 | 200 | — | 110(<0.1) | [ | |
| 3DOM-ZnFe2O4/SiO2 | 213.10 | 0.39 | 7.40 | 5% H2, 3% H2O, 0.1% H2S, N2平衡 | 500 | — | 92(<0.5) | [ |
| 3DOM-ZnO/SiO2 | 336 | 0.244 | 1.436 | 3% H2O, 500 mg/m3 H2S, N2平衡 | 室温 | — | 135(<0.72) | [ |
Table 2 Parameters and adsorption ability of porous metal oxide materials for H2S removal
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量①/(mg/g) | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| 3DOM-Fe2O3 | 16~44 | — | 20~33 | 5% H2, 3×10-4 H2S, N2平衡 5% H2, 3×10-4 H2S, N2平衡 | 350 350 | — | 37.18(<0.2) | [ |
| 3DOM-Fe2O3/SiO2 | 80~220 | — | 6~17 | — | 38.92(<0.2) | [ | ||
| MFT | — | — | — | 39.8% H2, 32.6% CO2, 2.5×10-3 H2S, N2平衡 | 400 | 216(N) | — | [ |
| Engelhard ZnO | 53.13 | 0.28 | — | 8×10-6 H2S, 34.4% H2, 20% H2O, N2平衡 | 300 | — | 28.1(0.02) | [ |
| ZSCo-0 | 151.3 | 0.42 | 7.40 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 174.6 | 117.8(ND) | [ |
| ZSCo-0.05 | 188.7 | 0.58 | 7.82 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 195.2 | 146.4(ND) | [ |
| ZSCo-0.15 | 206.4 | 0.63 | 9.58 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 208.4 | 169.8(ND) | [ |
| ZSCo-0.3 | 192.1 | 0.55 | 9.24 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 230.5 | 188.4(ND) | [ |
| ZSCo-0.50 | 161.5 | 0.46 | 7.53 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 194.1 | 143.4(ND) | [ |
| Extruded-Fe2O3 | 60.6 | 13.90 | 99.0 | 6×10-4 H2S, 混合气体 | 30 ± 2 | 22.4 | 16.0(N) | [ |
| Fe-Cu-Al-O | 96.7 | — | — | 10-3 H2S, CO2 | 40 | — | 113.9(ND) | [ |
| ZnO | 10.2 | 0.029 | 介-大孔 | 51% H2, 30% He, 10% H2O, 4×10-4 H2S | 400 | 457.3(2) | — | [ |
| Ni-ZnO | 6.8 | 0.025 | 介-大孔 | 51% H2, 30% He, 10% H2O, 4×10-4 H2S | 400 | 730.0(2) | — | [ |
| HZn20SC | 272.59 | 0.17 | — | 33% CO, 39% H2, 5% H2O,3×10-4 H2S, N2平衡 | 450 | — | 44.6(ND) | [ |
| Z20M4C6SC | 207.33 | 0.03 | — | 33% CO, 39% H2, 5×10-4 H2S, N2平衡 | 500 | — | 138.4(ND) | [ |
| ZnO | — | — | — | 54% H2, 21%CO2, 2.5×10-5 H2S, He 平衡 | 150 | — | 131(<0.1) | [ |
| — | — | — | 54% H2, 21%CO2, 2.5×10-5 H2S, He 平衡 | 200 | — | 110(<0.1) | [ | |
| 3DOM-ZnFe2O4/SiO2 | 213.10 | 0.39 | 7.40 | 5% H2, 3% H2O, 0.1% H2S, N2平衡 | 500 | — | 92(<0.5) | [ |
| 3DOM-ZnO/SiO2 | 336 | 0.244 | 1.436 | 3% H2O, 500 mg/m3 H2S, N2平衡 | 室温 | — | 135(<0.72) | [ |
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量/(mg/g) | 穿透容量①/ (mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| 天然斜发沸石 | 34.2 | 0.12 | 17.9 | 59.95% CH4, 39.95% CO2, 0.1% H2S | 25 | — | 1.4(<3) | [ |
| 4A | 49.5 | — | — | 10-3 H2S, N2平衡 | 50 | — | 8.36(~0) | [ |
| 13X | 567 | 0.34 | — | 2×10-2 H2S, 10-2 SO2, 15% H2O, N2平衡 | 150 | — | 179.7(ND) | [ |
| NaX | 503 | 0.255 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 24.96 | 21.44(ND) | [ |
| ZnX | 538 | 0.307 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 48.96 | 45.44(ND) | [ |
| CoX | 511 | 0.288 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 44.16 | 41.28(ND) | [ |
| AgX | 333 | 0.169 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 51.84 | 48.96(ND) | [ |
| 13X Ex-Cu | 239 | — | — | 2×10-4 H2S, N2平衡 | 120 | — | 40.12±0.6(ND) | [ |
| 13X-Ex-Cu (2.3mmol/g Cu) | 370 | — | — | 8×10-6 H2S, He平衡 | 40 | — | 39.8(0) | [ |
| 5-TiO2/zeolite | 93.42±0.06 | 0.59±0.06 | — | 65% CH4, 35% N2, 0.1% H2S | 25 | — | 4.43(ND) | [ |
| 20Zn/NaA zeolite | 7.99 | — | 2.43 | 2×10-4 H2S, N2平衡 | 28 | — | 15.75(0) | [ |
Table 3 Parameters and adsorption ability of zeolite materials for H2S removal
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量/(mg/g) | 穿透容量①/ (mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| 天然斜发沸石 | 34.2 | 0.12 | 17.9 | 59.95% CH4, 39.95% CO2, 0.1% H2S | 25 | — | 1.4(<3) | [ |
| 4A | 49.5 | — | — | 10-3 H2S, N2平衡 | 50 | — | 8.36(~0) | [ |
| 13X | 567 | 0.34 | — | 2×10-2 H2S, 10-2 SO2, 15% H2O, N2平衡 | 150 | — | 179.7(ND) | [ |
| NaX | 503 | 0.255 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 24.96 | 21.44(ND) | [ |
| ZnX | 538 | 0.307 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 48.96 | 45.44(ND) | [ |
| CoX | 511 | 0.288 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 44.16 | 41.28(ND) | [ |
| AgX | 333 | 0.169 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 51.84 | 48.96(ND) | [ |
| 13X Ex-Cu | 239 | — | — | 2×10-4 H2S, N2平衡 | 120 | — | 40.12±0.6(ND) | [ |
| 13X-Ex-Cu (2.3mmol/g Cu) | 370 | — | — | 8×10-6 H2S, He平衡 | 40 | — | 39.8(0) | [ |
| 5-TiO2/zeolite | 93.42±0.06 | 0.59±0.06 | — | 65% CH4, 35% N2, 0.1% H2S | 25 | — | 4.43(ND) | [ |
| 20Zn/NaA zeolite | 7.99 | — | 2.43 | 2×10-4 H2S, N2平衡 | 28 | — | 15.75(0) | [ |
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量①/(mg/g) | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| MIL-53(Al) | — | — | — | H2S 1.6 MPa | 30 | 376.6(N) | — | [ |
| MIL-53(Cr) | — | — | — | H2S 1.6 MPa | 30 | 419.8(N) | — | [ |
| MIL-53(Fe) | — | — | — | H2S 1.6 MPa | 30 | 273.0(N) | — | [ |
| MIL-47(V) | — | — | — | H2S 1.6 MPa | 30 | 467.2(N) | — | [ |
| MIL-100(Cr) | 1900 | — | — | H2S 2 MPa | 30 | 534.4(N) | — | [ |
| MIL-101(Cr) | 2600 | — | — | H2S 2 MPa | 30 | 1228.8(N) | — | [ |
| MIL-101-NH2(Cr) | 2096 | — | — | 89% CH4, 10% CO2, 1% H2S | 20 | 14.6(ND) | — | [ |
| MIL-125-NH2(Ti) | 1612 | — | — | 89% CH4, 10% CO2, 1% H2S | 20 | 12.3(ND) | — | [ |
| MOF-199 | — | — | — | 400 mg/m3 H2S, 600 mg/m3 CH3CH2SH, 600 mg/m3 CH3SCH3 | 80 | — | 57.2(ND) | [ |
| MOF-199 | 1459 | 0.73 | 0.52 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 53.44(ND) | [ |
| TEA/MOF-199-2 | 187 | 0.15 | 0.43 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 87.68(ND) | [ |
| MEA/MOF-199-2 | 18 | 0.12 | 1.28 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 26.65(ND) | [ |
| Cu(NO3)2@UiO-67(bipy) | 549 | — | — | 10-3 H2S,空气, 70% 相对湿度 | 20 | — | 78(N) | [ |
| CuMGO(10%(mass)) | 1002 | 0.53 | — | 10-3 H2S,空气 | 室温 | 120(N) | — | [ |
| MOF-GOPSN | 1722 | 0.68 | — | 10-3 H2S, 空气 | — | — | 109±4(ND) | [ |
| MOF-GOSA | 1419 | 0.58 | — | 10-3 H2S, 空气 | — | — | 133±5(ND) | [ |
| MOF-GOPSN | 1722 | 0.68 | — | 10-3 H2S, 空气, 71% 相对湿度 | — | — | 125±2(ND) | [ |
| MOF-GOSA | 1419 | 0.58 | — | 10-3 H2S, 空气, 71% 相对湿度 | — | — | 241±6(ND) | [ |
| Sep/Cu-BTC | 270.5 | 0.32 | 4.74 | 9.58×10-6 H2S, 50.76% 湿度 | 15 | — | 55.13(<1) | [ |
| MAC-2 | 1415 | 0.66 | — | 500 mg/m3 H2S, 600 mg/m3 CH3SCH3, 湿空气 | — | — | 84.6(ND) | [ |
Table 4 Parameters and adsorption ability of metal organic frames for H2S removal
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量①/(mg/g) | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| MIL-53(Al) | — | — | — | H2S 1.6 MPa | 30 | 376.6(N) | — | [ |
| MIL-53(Cr) | — | — | — | H2S 1.6 MPa | 30 | 419.8(N) | — | [ |
| MIL-53(Fe) | — | — | — | H2S 1.6 MPa | 30 | 273.0(N) | — | [ |
| MIL-47(V) | — | — | — | H2S 1.6 MPa | 30 | 467.2(N) | — | [ |
| MIL-100(Cr) | 1900 | — | — | H2S 2 MPa | 30 | 534.4(N) | — | [ |
| MIL-101(Cr) | 2600 | — | — | H2S 2 MPa | 30 | 1228.8(N) | — | [ |
| MIL-101-NH2(Cr) | 2096 | — | — | 89% CH4, 10% CO2, 1% H2S | 20 | 14.6(ND) | — | [ |
| MIL-125-NH2(Ti) | 1612 | — | — | 89% CH4, 10% CO2, 1% H2S | 20 | 12.3(ND) | — | [ |
| MOF-199 | — | — | — | 400 mg/m3 H2S, 600 mg/m3 CH3CH2SH, 600 mg/m3 CH3SCH3 | 80 | — | 57.2(ND) | [ |
| MOF-199 | 1459 | 0.73 | 0.52 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 53.44(ND) | [ |
| TEA/MOF-199-2 | 187 | 0.15 | 0.43 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 87.68(ND) | [ |
| MEA/MOF-199-2 | 18 | 0.12 | 1.28 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 26.65(ND) | [ |
| Cu(NO3)2@UiO-67(bipy) | 549 | — | — | 10-3 H2S,空气, 70% 相对湿度 | 20 | — | 78(N) | [ |
| CuMGO(10%(mass)) | 1002 | 0.53 | — | 10-3 H2S,空气 | 室温 | 120(N) | — | [ |
| MOF-GOPSN | 1722 | 0.68 | — | 10-3 H2S, 空气 | — | — | 109±4(ND) | [ |
| MOF-GOSA | 1419 | 0.58 | — | 10-3 H2S, 空气 | — | — | 133±5(ND) | [ |
| MOF-GOPSN | 1722 | 0.68 | — | 10-3 H2S, 空气, 71% 相对湿度 | — | — | 125±2(ND) | [ |
| MOF-GOSA | 1419 | 0.58 | — | 10-3 H2S, 空气, 71% 相对湿度 | — | — | 241±6(ND) | [ |
| Sep/Cu-BTC | 270.5 | 0.32 | 4.74 | 9.58×10-6 H2S, 50.76% 湿度 | 15 | — | 55.13(<1) | [ |
| MAC-2 | 1415 | 0.66 | — | 500 mg/m3 H2S, 600 mg/m3 CH3SCH3, 湿空气 | — | — | 84.6(ND) | [ |
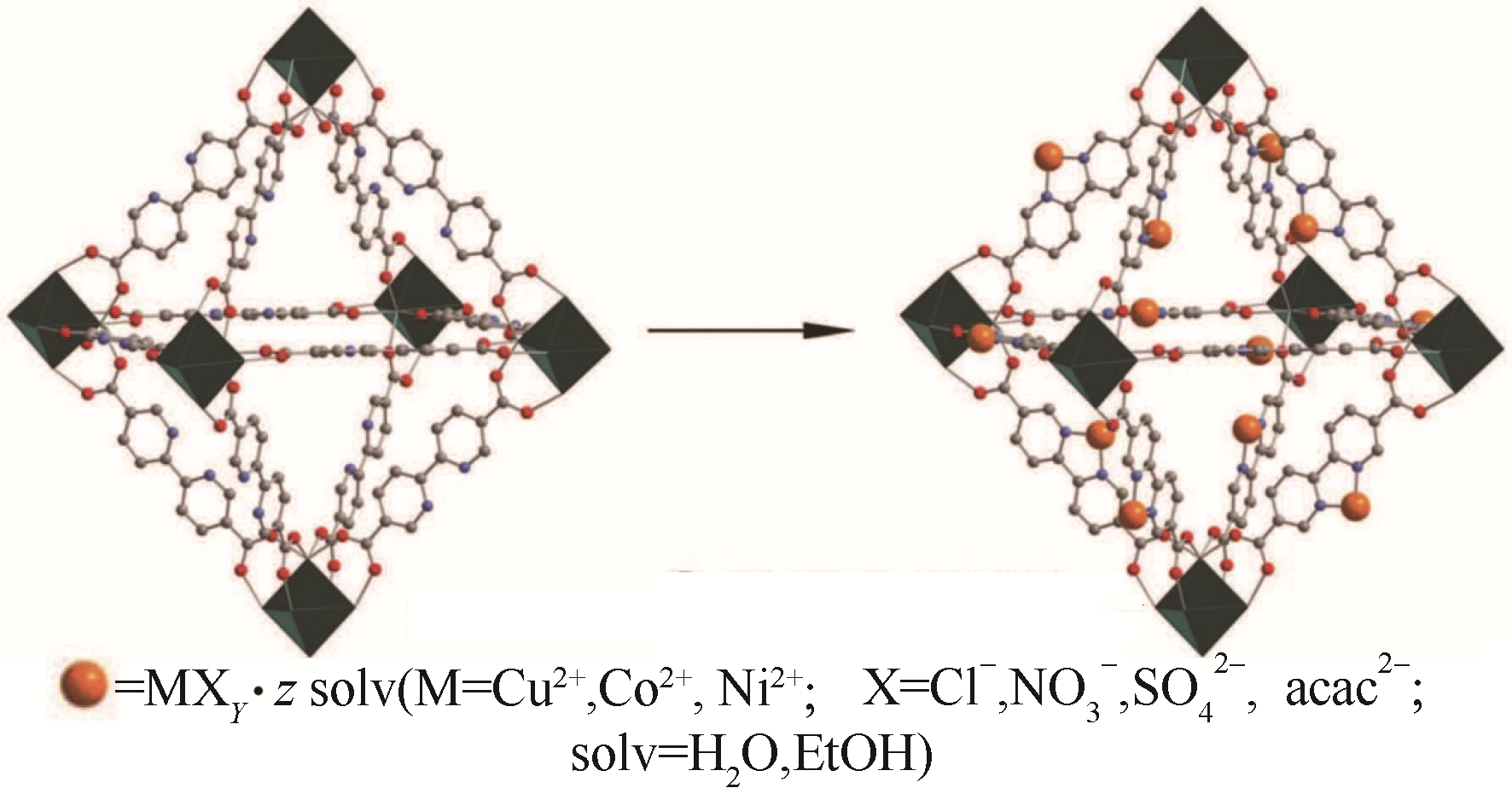
Fig.6 Postsynthetic insertion of metal salts into UiO-67(bipy)[61] (Green octahedra represents the [Zr6O4(OH)4]12+ cluster. Grey, red and blue spheres represent carbon, oxygen and nitrogen atoms, respectively. The orange spheres represent the respective metal complex)
| 65 | Shi R H, Zhang Z R, Fan H L, et al. Cu-based metal-organic framework/activated carbon composites for sulfur compounds removal[J]. Applied Surface Science, 2017, 394: 394-402. |
| 66 | Li Z J, Xiao Y L, Xue W J, et al. Ionic liquid/metal-organic framework composites for H2S removal from natural gas: a computational exploration[J]. The Journal of Physical Chemistry C, 2015, 119(7): 3674-3683. |
| 1 | 刘新鹏. 用于硫化氢脱除与硫资源回收的绿色脱硫新体系性能研究[D]. 济南: 山东大学, 2017. |
| Liu X P. Study on the performance of new green systems for hydrogen sulfide removal and sulfur recovery[D]. Jinan: Shandong University, 2017. | |
| 2 | Bao J E, Krishnan G N, Jayaweera P. Effect of various coal contaminants on the performance of solid oxide fuel cells (Ⅱ): ppm and sub-ppm level testing[J]. Journal of Power Sources, 2009, 193(2): 617-624. |
| 3 | International Standards Organization. Hydrogen fuel—product specification (2): Proton exchange membrane (PEM) fuel cell applications for road vehicles: [S]. 2012. |
| 4 | 高齐. 碳材料表面碱性官能团对于低浓度H2S的去除机制研究[D]. 上海: 上海大学, 2018. |
| Gao Q. Removal mechanism of low concentration H2S by surface basic functional groups on carbon materials[D]. Shanghai: Shanghai University, 2018. | |
| 5 | 王睿, 石冈, 魏伟胜, 等. 工业气体中H2S的脱除方法——发展现状与展望[J]. 天然气工业, 1999, 19(3): 84-90. |
| Wang R, Shi G, Wei W S, et al. Methods of removing hydrogen sulfide from industrial gas—present situation and prospects[J]. Natural Gas Industry, 1999, 19(3): 84-90. | |
| 6 | Westmoreland P R, Harrison D P. Evaluation of candidate solids for high-temperature desulfurization of low-Btu gases[J]. Environmental Science & Technology, 1976, 10(7): 659- 661. |
| 7 | Cazorla-Amorós D, Alcañiz-Monge J, Linares-Solano A. Characterization of activated carbon fibers by CO2 adsorption[J]. Langmuir, 1996, 12(11): 2820-2824. |
| 67 | Liu G P, Cadiau A, Liu Y, et al. Enabling fluorinated MOF-based membranes for simultaneous removal of H2S and CO2 from natural gas[J]. Angewandte Chemie-International Edition, 2018, 57(45): 14811-14816. |
| 8 | Peluso A, Gargiulo N, Aprea P, et al. Nanoporous materials as H2S adsorbents for biogas purification: a review[J]. Separation and Purification Reviews, 2019, 48(1): 78-89. |
| 9 | Wu X X, Schwartz V, Overbury S H, et al. Desulfurization of gaseous fuels using activated carbons as catalysts for the selective oxidation of hydrogen sulfide[J]. Energy & Fuels, 2005, 19(5): 1774-1782. |
| 10 | Shen F, Liu J, Zhang Z, et al. Density functional study of hydrogen sulfide adsorption mechanism on activated carbon[J]. Fuel Processing Technology, 2018, 171: 258-264. |
| 11 | Bashkova S, Baker F S, Wu X X, et al. Activated carbon catalyst for selective oxidation of hydrogen sulphide: on the influence of pore structure, surface characteristics, and catalytically-active nitrogen[J]. Carbon, 2007, 45(6): 1354-1363. |
| 12 | Quan W Y, Jiang X, Wang X X, et al. Influence of loading a tertiary amine on activated carbons and effect of CO2 on adsorptive H2S removal from biogas[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(27): 9998-10008. |
| 13 | Seredych M, Bandosz T J. Role of microporosity and nitrogen functionality on the surface of activated carbon in the process of desulfurization of digester gas[J]. The Journal of Physical Chemistry C, 2008, 112(12): 4704-4711. |
| 14 | Barelli L, Bidini G, de Arespacochaga N, et al. Biogas use in high temperature fuel cells: enhancement of KOH-KI activated carbon performance toward H2S removal[J]. International Journal of Hydrogen Energy, 2017, 42(15): 10341-10353. |
| 15 | Tsai J H, Jeng F T, Chiang H L. Removal of H2S from exhaust gas by use of alkaline activated carbon[J]. Adsorption, 2001, 7: 357-366. |
| 16 | Sitthikhankaew R, Predapitakkun S, Kiattikomol R W, et al. Comparative study of hydrogen sulfide adsorption by using alkaline impregnated activated carbons for hot fuel gas purification[J]. Energy Procedia, 2011, 9: 15-24. |
| 17 | Siriwardane I W, Udangawa R, de Silva R M, et al. Synthesis and characterization of nano magnesium oxide impregnated granular activated carbon composite for H2S removal applications[J]. Materials & Design, 2017, 136: 127-136. |
| 18 | Cimino S, Lisi L, Erto A, et al. Role of H2O and O2 during the reactive adsorption of H2S on CuO-ZnO/activated carbon at low temperature[J]. Microporous and Mesoporous Materials, 2020, 295: 109949. |
| 19 | Monteleone G, de Francesco M, Stefano G, et al. Deep H2S removal from biogas for molten carbonate fuel cell (MCFC) systems[J]. Chemical Engineering Journal, 2011, 173(3): 407-414. |
| 20 | Sun M H, Wang X Z, Pan X, et al. Nitrogen-rich hierarchical porous carbon nanofibers for selective oxidation of hydrogen sulfide[J]. Fuel Processing Technology, 2019, 191: 121-128. |
| 21 | Qi J, Wei G, Li Y, et al. Porous carbon spheres for simultaneous removal of benzene and H2S[J]. Chemical Engineering Journal, 2018, 339: 499-508. |
| 22 | Zhang Z, Wang J, Li W, et al. Millimeter-sized mesoporous carbon spheres for highly efficient catalytic oxidation of hydrogen sulfide at room temperature[J]. Carbon, 2016, 96: 608-615. |
| 23 | Chen Q J, Wang J T, Liu X J, et al. Alkaline carbon nanotubes as effective catalysts for H2S oxidation[J]. Carbon, 2011, 49(12): 3773-3780. |
| 24 | Khabazipour M, Anbia M. Removal of hydrogen sulfide from gas streams using porous materials: a review[J]. Industrial & Engineering Chemistry Research, 2019, 58(49): 22133-22164. |
| 25 | Fan H L, Sun T, Zhao Y P, et al. Three-dimensionally ordered macroporous iron oxide for removal of H2S at medium temperatures[J]. Environmental Science & Technology, 2013, 47(9): 4859-4865. |
| 26 | Bao W R, Zhang Z Y, Ren X R, et al. Desulfurization behavior of iron-based sorbent with MgO and TiO2 additive in hot coal gas[J]. Energy & Fuels, 2009, 23(7): 3600-3604. |
| 27 | Novochinskii I I, Song C S, Ma X L, et al. Low-temperature H2S removal from steam-containing gas mixtures with ZnO for fuel cell application(1): ZnO particles and extrudates[J]. Energy & Fuels, 2004, 18(2): 576-583. |
| 28 | Yang C, Yang S, Fan H L, et al. A sustainable design of ZnO-based adsorbent for robust H2S uptake and secondary utilization as hydrogenation catalyst[J]. Chemical Engineering Journal, 2020, 382: 122892. |
| 29 | Habeeb O A, Kanthasamy R, Ali G A M, et al. Hydrogen sulfide emission sources, regulations, and removal techniques: a review[J]. Reviews in Chemical Engineering, 2018, 34(6): 837-854. |
| 30 | Pahalagedara L R, Poyraz A S, Song W Q, et al. Low temperature desulfurization of H2S: high sorption capacities by mesoporous cobalt oxide via increased H2S diffusion[J]. Chemistry of Materials, 2014, 26(22): 6613-6621. |
| 31 | Liu X, Meng X, Zhao J. Synthesis of nanocrystalline iron oxides with mesostructure as desulfurizer[J]. Materials Letters, 2013, 92: 255-258. |
| 32 | Long N Q, Loc T X. Experimental and modeling study on room-temperature removal of hydrogen sulfide using a low-cost extruded Fe2O3-based adsorbent[J]. Adsorption, 2016, 22(3): 397-408. |
| 33 | Liu D, Chen S Y, Fei X Y, et al. Regenerable CuO-based adsorbents for low temperature desulfurization application[J]. Industrial & Engineering Chemistry Research, 2015, 54(14): 3556-3562. |
| 34 | Tran D T. Synthesis of porous ZnO based materials using an agarose gel template for H2S desulfurization[J]. RSC Advances, 2016, 6(2): 1339-1345. |
| 35 | Zheng X R, Bao, W R, Jin Q M, et al. Use of high-pressure impregnation in preparing Zn-based sorbents for deep desulfurization of hot coal gas[J]. Energy & Fuels, 2011, 25(7): 2997-3001. |
| 36 | Zheng X R, Bao W R, Chang L P, et al. Interaction among metal components of Zn-Mn-Cu-based sorbents prepared by high-pressure impregnation method and its effect on the removal of H2S from hot coal gas[J]. Energy & Fuels, 2012, 26(6): 3393-3398. |
| 37 | Li L, King D L. H2S removal with ZnO during fuel processing for PEM fuel cell applications[J]. Catalysis Today, 2006, 116(4): 537-541. |
| 38 | Li L, Zhang H B, Zhou P, et al. Three dimensional ordered macroporous zinc ferrite composited silica sorbents with promotional desulfurization and regeneration activity at mid-high temperature[J]. Applied Surface Science, 2019, 470: 177-186. |
| 39 | Wei Y, Liu J, Zhao Z, et al. The catalysts of three-dimensionally ordered macroporous Ce1-xZrxO2-supported gold nanoparticles for soot combustion: the metal-support interaction[J]. Journal of Catalysis, 2012, 287: 13-29. |
| 40 | Wang L J, Fan H L, Shangguan J, et al. Design of a sorbent to enhance reactive adsorption of hydrogen sulfide[J]. ACS Applied Materials & Interfaces, 2014, 6(23): 21167-21177. |
| 41 | 陈曦. 提高Y型和X型分子筛吸附功能深度净化天然气过程基础研究[D]. 上海: 华东理工大学, 2018. |
| Chen X. The fundamental study on enhancing adsorption performance of zeolite Y and X for deep purification of sour natural gas[D]. Shanghai: East China University of Science and Technology, 2018. | |
| 42 | 王洪国. 清洁燃料选择性吸附脱硫过程中硫化物吸附行为的研究[D]. 青岛: 中国石油大学, 2010. |
| Wang H G. Studies of adsorption behavior of sulfur compounds in selective adsorption desulfurization for clean fuel[D]. Qingdao: China University of Petroleum, 2010. | |
| 43 | Kristóf T. Selective removal of hydrogen sulphide from industrial gas mixtures using zeolite NaA[J]. Hungarian Journal of Industrial Chemistry, 2017, 45(1): 9-15. |
| 44 | Alonso-Vicario A, Ochoa-Gómez J R, Gil-Río S, et al. Purification and upgrading of biogas by pressure swing adsorption on synthetic and natural zeolites[J]. Microporous and Mesoporous Materials, 2010, 134(1/2/3): 100-107. |
| 45 | Liu X, Wang R. Effective removal of hydrogen sulfide using 4A molecular sieve zeolite synthesized from attapulgite[J]. Journal of Hazardous Materials, 2017, 326: 157-164. |
| 46 | Yang K, Su B, Shi L, et al. Adsorption mechanism and regeneration performance of 13X for H2S and SO2[J]. Energy & Fuels, 2018, 32(12): 12742-12749. |
| 47 | Chen X, Shen B, Sun H, et al. Ion-exchange modified zeolites X for selective adsorption desulfurization from Claus tail gas: experimental and computational investigations[J]. Microporous and Mesoporous Materials, 2018, 261: 227-236. |
| 48 | Barelli L, Bidini G, Micoli L, et al. 13X Ex-Cu zeolite performance characterization towards H2S removal for biogas use in molten carbonate fuel cells[J]. Energy, 2018, 160: 44-53. |
| 49 | Micoli L, Bagnasco G, Turco M. H2S removal from biogas for fuelling MCFCs: new adsorbing materials[J]. International Journal of Hydrogen Energy, 2014, 39(4): 1783-1787. |
| 50 | Liu C, Zhang R, Wei S, et al. Selective removal of H2S from biogas using a regenerable hybrid TiO2/zeolite composite[J]. Fuel, 2015, 157: 183-190. |
| 51 | Abdullah A H, Mat R, Somderam S, et al. Hydrogen sulfide adsorption by zinc oxide-impregnated zeolite (synthesized from Malaysian kaolin) for biogas desulfurization[J]. Journal of Industrial and Engineering Chemistry, 2018, 65: 334-342. |
| 52 | Fellah M F. Adsorption of hydrogen sulfide as initial step of H2S removal: a DFT study on metal exchanged ZSM-12 clusters[J]. Fuel Processing Technology, 2016, 144: 191-196. |
| 53 | Yaghi O M, Li H L. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels[J]. Journal of the American Chemical Society, 1995, 117: 10401-10402. |
| 54 | Furukawa H, Cordova K E, O'Keeffe M, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341(6149): 1230444. |
| 55 | Jiao L, Seow J Y R, Skinner W S, et al. Metal-organic frameworks: structures and functional applications[J]. Materials Today, 2019, 27: 43-68. |
| 56 | Hamon L, Serre C, Devic T. Comparative study of hydrogen sulfide adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) metal-organic frameworks at room temperature[J]. Journal of the American Chemical Society, 2009, 131: 8775-8777. |
| 57 | Peluso A, Gargiulo N, Aprea P, et al. Modeling hydrogen sulfide adsorption on chromium-based MIL-101 metal organic framework[J]. Science of Advanced Materials, 2014, 6(1): 164-170. |
| 58 | Joshi J N, Zhu G, Lee J J. et al. Probing metal-organic framework design for adsorptive natural gas purification[J]. Langmuir, 2018, 34(29): 8443-8450. |
| 59 | Li Y, Wang L J, Fan H L, et al. Removal of sulfur compounds by a copper-based metal organic framework under ambient conditions[J]. Energy & Fuels, 2015, 29(1): 298-304. |
| 60 | Zhang H Y, Yang C, Geng Q, et al. Adsorption of hydrogen sulfide by amine-functionalized metal organic framework (MOF-199): an experimental and simulation study[J]. Applied Surface Science, 2019, 497: 143815. |
| 61 | Nickerl G, Leistner M, Helten S, et al. Integration of accessible secondary metal sites into MOFs for H2S removal[J]. Inorganic Chemistry Frontiers, 2014, 1(4): 325-330. |
| 62 | Petit C, Bandosz T J. Exploring the coordination chemistry of MOF-graphite oxide composites and their applications as adsorbents[J]. Dalton Transactions, 2012, 41(14): 4027-4035. |
| 63 | Ebrahim A M, Jagiello J, Bandosz T J. Enhanced reactive adsorption of H2S on Cu-BTC/S- and N-doped GO composites[J]. Journal of Materials Chemistry A, 2015, 3(15): 8194-8204. |
| 64 | Kakaei H, Beygzadeh M, Golbabaei F, et al. Preparation of a sepiolite/Cu-BDC nanocomposite and its application as an adsorbent in respirator cartridges for H2S removal[J]. New Journal of Chemistry, 2019, 43(29): 11575-11584. |
| [1] | Yaxin ZHAO, Xueqin ZHANG, Rongzhu WANG, Guo SUN, Shanjing YAO, Dongqiang LIN. Removal of monoclonal antibody aggregates with ion exchange chromatography by flow-through mode [J]. CIESC Journal, 2023, 74(9): 3879-3887. |
| [2] | Xuejin YANG, Jintao YANG, Ping NING, Fang WANG, Xiaoshuang SONG, Lijuan JIA, Jiayu FENG. Research progress in dry purification technology of highly toxic gas PH3 [J]. CIESC Journal, 2023, 74(9): 3742-3755. |
| [3] | Shuang LIU, Linzhou ZHANG, Zhiming XU, Suoqi ZHAO. Study on molecular level composition correlation of viscosity of residual oil and its components [J]. CIESC Journal, 2023, 74(8): 3226-3241. |
| [4] | Yan GAO, Peng WU, Chao SHANG, Zejun HU, Xiaodong CHEN. Preparation of magnetic agarose microspheres based on a two-fluid nozzle and their protein adsorption properties [J]. CIESC Journal, 2023, 74(8): 3457-3471. |
| [5] | Jiayi ZHANG, Jiali HE, Jiangpeng XIE, Jian WANG, Yu ZHAO, Dongqiang ZHANG. Research progress of pervaporation technology for N-methylpyrrolidone recovery in lithium battery production [J]. CIESC Journal, 2023, 74(8): 3203-3215. |
| [6] | Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent [J]. CIESC Journal, 2023, 74(8): 3375-3385. |
| [7] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [8] | Lei XING, Chunyu MIAO, Minghu JIANG, Lixin ZHAO, Xinya LI. Optimal design and performance analysis of downhole micro gas-liquid hydrocyclone [J]. CIESC Journal, 2023, 74(8): 3394-3406. |
| [9] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [10] | Yuanliang ZHANG, Xinqi LUAN, Weige SU, Changhao LI, Zhongxing ZHAO, Liqin ZHOU, Jianmin CHEN, Yan HUANG, Zhenxia ZHAO. Study on selective extraction of nicotine by ionic liquids composite extractant and DFT calculation [J]. CIESC Journal, 2023, 74(7): 2947-2956. |
| [11] | Jinming GAO, Yujiao GUO, Chenglin E, Chunxi LU. Study on the separation characteristics of a downstream gas-liquid vortex separator in a closed hood [J]. CIESC Journal, 2023, 74(7): 2957-2966. |
| [12] | Zhaolun WEN, Peirui LI, Zhonglin ZHANG, Xiao DU, Qiwang HOU, Yegang LIU, Xiaogang HAO, Guoqing GUAN. Design and optimization of cryogenic air separation process with dividing wall column based on self-heat regeneration [J]. CIESC Journal, 2023, 74(7): 2988-2998. |
| [13] | Jie WANG, Xiaolin QIU, Ye ZHAO, Xinyang LIU, Zhongqiang HAN, Yong XU, Wenhan JIANG. Preparation and properties of polyelectrolyte electrostatic deposition modified PHBV antioxidant films [J]. CIESC Journal, 2023, 74(7): 3068-3078. |
| [14] | Kuikui HAN, Xianglong TAN, Jinzhi LI, Ting YANG, Chun ZHANG, Yongfen ZHANG, Hongquan LIU, Zhongwei YU, Xuehong GU. Four-channel hollow fiber MFI zeolite membrane for the separation of xylene isomers [J]. CIESC Journal, 2023, 74(6): 2468-2476. |
| [15] | Xingchi ZHU, Zhiyuan GUO, Zhiyong JI, Jing WANG, Panpan ZHANG, Jie LIU, Yingying ZHAO, Junsheng YUAN. Simulation and optimization of selective electrodialysis magnesium and lithium separation process [J]. CIESC Journal, 2023, 74(6): 2477-2485. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||