CIESC Journal ›› 2021, Vol. 72 ›› Issue (4): 2123-2131.DOI: 10.11949/0438-1157.20201243
• Separation engineering • Previous Articles Next Articles
ZHANG Xiao1,2,3( ),JI Zhiyong1,2,3(
),JI Zhiyong1,2,3( ),WANG Jing1,2,3,GUO Xiaofu1,2,3,LIU Jie1,2,3,ZHAO Yingying1,2,3,YUAN Junsheng1,2,3
),WANG Jing1,2,3,GUO Xiaofu1,2,3,LIU Jie1,2,3,ZHAO Yingying1,2,3,YUAN Junsheng1,2,3
Received:2020-08-31
Revised:2020-12-15
Online:2021-04-05
Published:2021-04-05
Contact:
JI Zhiyong
张晓1,2,3( ),纪志永1,2,3(
),纪志永1,2,3( ),汪婧1,2,3,郭小甫1,2,3,刘杰1,2,3,赵颖颖1,2,3,袁俊生1,2,3
),汪婧1,2,3,郭小甫1,2,3,刘杰1,2,3,赵颖颖1,2,3,袁俊生1,2,3
通讯作者:
纪志永
作者简介:张晓(1994—),男,硕士研究生,基金资助:CLC Number:
ZHANG Xiao, JI Zhiyong, WANG Jing, GUO Xiaofu, LIU Jie, ZHAO Yingying, YUAN Junsheng. Exploration and optimization of extraction process of bromine from underground brine by electrooxidation[J]. CIESC Journal, 2021, 72(4): 2123-2131.
张晓, 纪志永, 汪婧, 郭小甫, 刘杰, 赵颖颖, 袁俊生. 电氧化法地下卤水提溴探究及条件优化[J]. 化工学报, 2021, 72(4): 2123-2131.
Add to citation manager EndNote|Ris|BibTeX
| 地区 | 浓度/(g·L-1) | ||||||
|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | Cl- | SO42- | Br- | |
| 四川某地 | 26 | 102 | 10.88 | 1.26 | 201.97 | 0.38 | 1.70 |
| 潍坊某地 | 0.99 | 37.92 | 1.07 | 5.99 | 71.76 | 9.09 | 0.26 |
| 莱州某1号盐场 | 1.52 | 60.3 | 1.02 | 7.85 | 110.1 | 12.35 | 0.401 |
| 莱州某2号盐场 | 1.21 | 47.9 | 1.07 | 6.30 | 88.18 | 9.75 | 0.320 |
| 莱州某3号盐场 | 0.647 | 33.40 | 1.31 | 5.59 | 64.14 | 8.03 | 0.211 |
| 莱州某4号盐场 | 0.461 | 17.33 | 0.843 | 2.27 | 32.73 | 3.34 | 0.113 |
Table 1 The basic chemical composition of underground brines in some parts of China[25-28]
| 地区 | 浓度/(g·L-1) | ||||||
|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | Cl- | SO42- | Br- | |
| 四川某地 | 26 | 102 | 10.88 | 1.26 | 201.97 | 0.38 | 1.70 |
| 潍坊某地 | 0.99 | 37.92 | 1.07 | 5.99 | 71.76 | 9.09 | 0.26 |
| 莱州某1号盐场 | 1.52 | 60.3 | 1.02 | 7.85 | 110.1 | 12.35 | 0.401 |
| 莱州某2号盐场 | 1.21 | 47.9 | 1.07 | 6.30 | 88.18 | 9.75 | 0.320 |
| 莱州某3号盐场 | 0.647 | 33.40 | 1.31 | 5.59 | 64.14 | 8.03 | 0.211 |
| 莱州某4号盐场 | 0.461 | 17.33 | 0.843 | 2.27 | 32.73 | 3.34 | 0.113 |
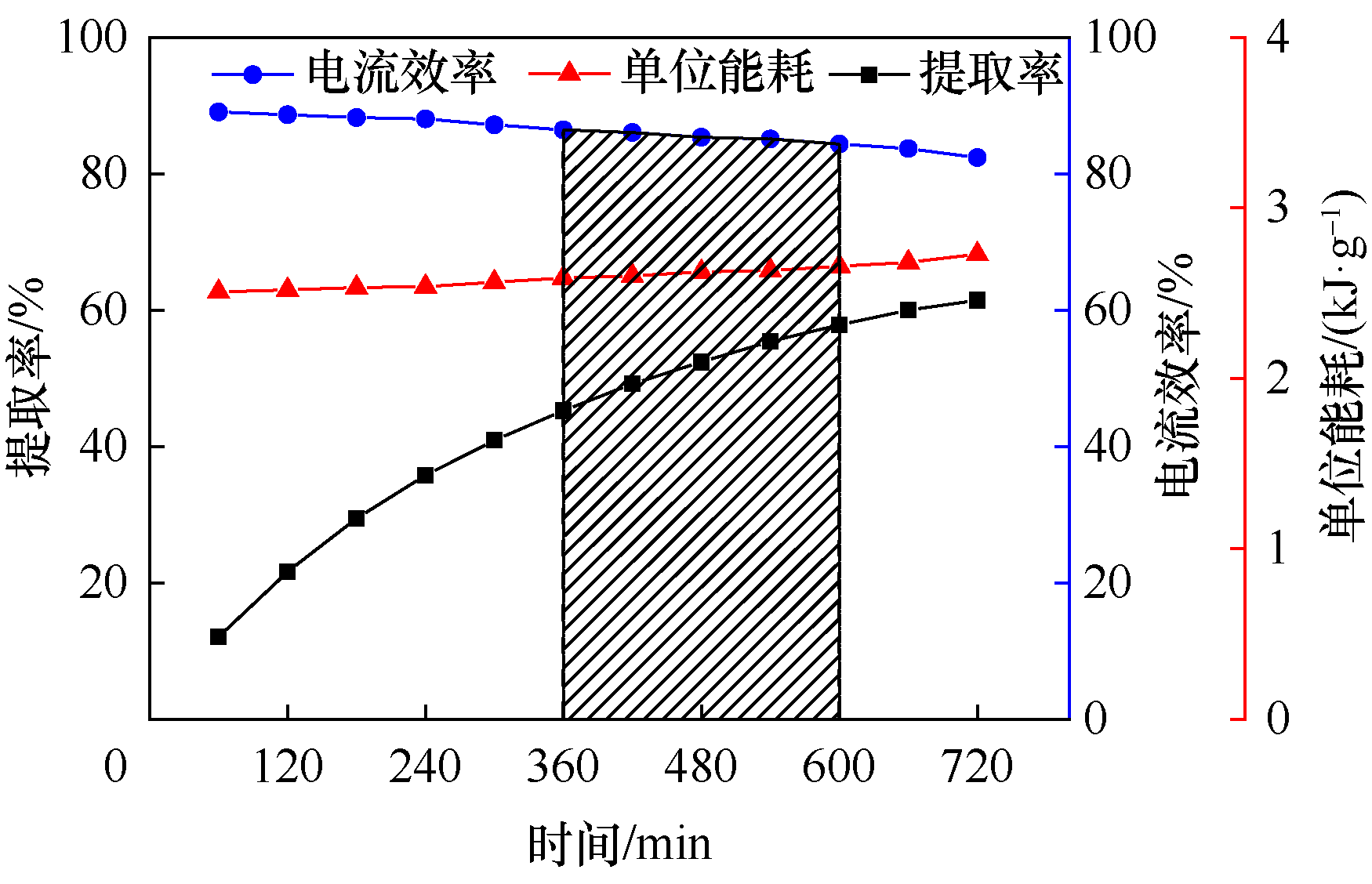
Fig.7 The change of extraction ratio of bromine, current efficiency and energy consumption with reaction time during the process of bromine extraction from underground brine
| 处理时间/h | 产量/kg | 单位产量/(kg·h-1) | 能耗×10-3/kJ | 单位能耗/(kJ·g-1) | 提取率/% |
|---|---|---|---|---|---|
| 6 | 181.65 | 30.28 | 470.30 | 2.59 | 45.3 |
| 8 | 210.12 | 26.27 | 551.58 | 2.63 | 52.4 |
| 10 | 231.78 | 23.18 | 616.07 | 2.66 | 57.8 |
Table 2 Indicators at different treating time during the treatment of concentrated seawater
| 处理时间/h | 产量/kg | 单位产量/(kg·h-1) | 能耗×10-3/kJ | 单位能耗/(kJ·g-1) | 提取率/% |
|---|---|---|---|---|---|
| 6 | 181.65 | 30.28 | 470.30 | 2.59 | 45.3 |
| 8 | 210.12 | 26.27 | 551.58 | 2.63 | 52.4 |
| 10 | 231.78 | 23.18 | 616.07 | 2.66 | 57.8 |
| 1 | 张慧峰, 王国强, 蔡荣华, 等. 提溴技术研究进展与展望[J]. 化学工业与工程, 2010, 27(5): 450-456. |
| Zhang H F, Wang G Q, Cai R H, et al. Progress in bromine extracting technology[J]. Chemical Industry and Engineering, 2010, 27(5): 450-456. | |
| 2 | 陈向锋, 曹春燕. 中国工业溴现状及前景展望[J]. 化工科技市场, 2010, 33(7): 5-8. |
| Chen X F, Cao C Y. Current status and prospect forecast of Chinese industrial bromine[J]. Chemical Technology Market, 2010, 33(7): 5-8. | |
| 3 | 柴子华, 李明明. 我国溴工业生产技术现状与展望[J]. 盐科学与化工, 2018, 47(6): 1-4. |
| Chai Z H, Li M M. Domestic manufacturing process and expectation of bromine industry[J]. Journal of Salt Science and Chemical Industry, 2018, 47(6): 1-4. | |
| 4 | 陈向楠, 王海增. 溴素资源与产业发展分析[J]. 盐业与化工, 2013, 42(6): 4-7. |
| Chen X N, Wang H Z. Bromine resource and analysis of the industry development[J]. Journal of Salt and Chemical Industry, 2013, 42(6): 4-7. | |
| 5 | 黄西平. 国内外盐湖(地下)卤水资源综合利用综述[J]. 海洋技术, 2002, 21(4): 66-72. |
| Huang X P. Multipurpose utilization of bittern resource in salt lake[J]. Ocean Technology, 2002, 21(4): 66-72. | |
| 6 | 韩积斌, 许建新, 安朝, 等. 盐湖地下卤水的开采技术及其展望[J]. 盐湖研究, 2015, 23(1): 67-72. |
| Han J B, Xu J X, An Z, et al. Research advance of the salt lake underground brine extraction technology[J]. Journal of Salt Lake Research, 2015, 23(1): 67-72. | |
| 7 | 李海民, 程怀德, 张全有. 卤水资源开发利用技术述评[J]. 盐湖研究, 2003, 11(3): 51-64. |
| Li H M, Cheng H D, Zhang Q Y. Evaluation of the technologies of comprehensive utilization and exploitation salt resource[J]. Journal of Salt Lake Research, 2003, 11(3): 51-64. | |
| 8 | 李海民, 程怀德, 张全有. 卤水资源开发利用技术述评(续完)[J]. 盐湖研究, 2004, 12(1): 62-72, 56. |
| Li H M, Cheng H D, Zhang Q Y. Evaluation of the technologies of comprehensive utilizationand exploitation brine resources[J]. Journal of Salt Lake Research, 2004, 12(1): 62-72, 56. | |
| 9 | 张琳娜, 刘有智, 焦纬洲, 等. 卤水提溴技术的发展与研究现状[J]. 盐湖研究, 2009, 17(1): 68-72. |
| Zhang L N, Liu Y Z, Jiao W Z, et al. Development and research status of bromine extracting technology from the brine[J]. Journal of Salt Lake Research, 2009, 17(1): 68-72. | |
| 10 | 詹进先. 试论威远气田水提溴技术[J]. 石油与天然气化工, 1996, 25(4): 239-242. |
| Zhan J X. Discussion on bromine recovery process for Weiyuan gas field formation water[J]. Chemical Engineering of Oil and Gas, 1996, 25(4): 239-242. | |
| 11 | 郭如新. 美国溴产品近况和前景[J]. 盐湖研究, 2006, 14(1): 66-72. |
| Guo R X. Present situation and prospect of bromine and its derivatives in USA[J]. Journal of Salt Lake Research, 2006, 14(1): 66-72. | |
| 12 | Fan M H, Xu S Y. Adsorption and desorption properties of macroreticular resins for salidroside from Rhodiola sachalinensis A. Bor[J]. Separation and Purification Technology, 2008, 61(2): 211-216. |
| 13 | 朱昌洛, 寇建军. 树脂吸附法由卤水中提溴[J]. 矿产综合利用, 2003, (5): 13-16. |
| Zhu C L, Kou J J. Extraction of bromine from brine by RIP method[J]. Multipurpose Utilization of Mineral Resources, 2003, (5): 13-16. | |
| 14 | Ensafi A A, Eskandari H. Selective extraction of bromide with liquid organic membrane[J]. Separation Science and Technology, 2001, 36(1): 81-89. |
| 15 | 王国强, 张淑芬, 王俐聪, 等. 气态膜法海水提溴影响因素的研究[J]. 海洋技术, 2004, 23(1): 77-80. |
| Wang G Q, Zhang S F, Wang L C, et al. Study on extraction of bromine from seawater with gas membrane[J]. Ocean Technology, 2004, 23(1): 77-80. | |
| 16 | Kosaka Y, Fujita T, Nanun N. Recovery of bromine: JP58-041703[P]. 1983. |
| 17 | 孙志娟, 张心亚, 黄洪, 等. 乳状液膜分离技术的发展与应用[J]. 现代化工, 2006, 26(9): 63-66. |
| Sun Z J, Zhang X Y, Huang H, et al. Development of emulsion liquid membrane separation and its application[J]. Modern Chemical Industry, 2006, 26(9): 63-66. | |
| 18 | 万印华, 王向德, 张秀娟. 乳状液膜用表面活性剂研究进展[J]. 化工进展, 1998, 17(5): 12-15, 28. |
| Wan Y H, Wang X D, Zhang X J. Progress in the study of surfactant used for emulsion liquid membrane[J]. Chemical Industry and Engineering Progress, 1998, 17(5): 12-15, 28. | |
| 19 | 王红, 王巍杰, 李红霞. 乳状液膜法提取溴[J]. 河北理工学院学报, 2005, 27(3): 110-112. |
| Wang H, Wang W J, Li H X. The extraction of bromine with liquid membrane[J]. Journal of Hebei Institute of Technology, 2005, 27(3): 110-112. | |
| 20 | 张良晓, 孔望清, 杨祥. 表面活性剂在化学分离中的应用[J]. 河北化工, 2005, 28(4): 20-21, 46. |
| Zhang L X, Kong W Q, Yang X. Application of surfactant in chemical separation[J]. Hebei Chemical Engineering and Industry, 2005, 28(4): 20-21, 46. | |
| 21 | Bard A J, Jordan J, Parsons R. Standard Potentials in Aqueous Solutions[M]. New York: Marcel Dekker, 1985. |
| 22 | Cohen I, Shapira B, Avraham E, et al. Bromide ions specific removal and recovery by electrochemical desalination[J]. Environmental Science & Technology, 2018, 52(11): 6275-6281. |
| 23 | Sun M, Lowry G V, Gregory K B. Selective oxidation of bromide in wastewater brines from hydraulic fracturing[J]. Water Research, 2013, 47(11): 3723-3731. |
| 24 | Zhang X, Ji Z Y, Liu F, et al. Investigation of electrochemical oxidation technology for selective bromine extraction in comprehensive utilization of concentrated seawater[J]. Separation and Purification Technology, 2020, 248: 117108. |
| 25 | 杨能红, 孙成高, 彭建忠. 地下卤水综合利用新工艺研究[J]. 盐业与化工, 2016, 45(5): 24-27. |
| Yang N H, Sun C G, Peng J Z. New technology for comprehensive utilization of underground brine[J]. Journal of Salt and Chemical Industry, 2016, 45(5): 24-27. | |
| 26 | 王振, 要璇. 地下卤水综合利用工艺探究[J]. 化工设计通讯, 2017, 43(5): 185. |
| Wang Z, Yao X. Study on comprehensive utilization of underground brine[J]. Chemical Engineering Design Communications, 2017, 43(5): 185. | |
| 27 | 康兴伦, 程作联. 山东渤海沿岸地下卤水的成分研究[J]. 海洋通报, 1990, 9(6): 25-29. |
| Kang X L, Cheng Z L. Composition research for underground brine along Shandong coastal flatlands on Bohai sea[J]. Marine Science Bulletin, 1990, 9(6): 25-29. | |
| 28 | 贾梦秋, 杨文胜. 应用电化学[M]. 北京: 高等教育出版社, 2004. |
| Jia M Q, Yang W S. Applied Electrochemistry [M]. Beijing: Higher Education Press, 2004. | |
| 29 | 刘建兰, 李冀蜀, 郭会明, 等. 物理化学(上) [M]. 北京: 化学工业出版社, 2013. |
| Liu J L, Li J S, Guo H M, et al. Physical Chemistry [M]. Beijing: Chemical Industry Press, 2013. | |
| 30 | Xu P, Drewes J E, Heil D, et al. Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology[J]. Water Research, 2008, 42(10/11): 2605-2617. |
| 31 | Wang L, Lin S H. Theoretical framework for designing a desalination plant based on membrane capacitive deionization[J]. Water Research, 2019, 158: 359-369. |
| [1] | Cheng CHENG, Zhongdi DUAN, Haoran SUN, Haitao HU, Hongxiang XUE. Lattice Boltzmann simulation of surface microstructure effect on crystallization fouling [J]. CIESC Journal, 2023, 74(S1): 74-86. |
| [2] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [3] | Xuejin YANG, Jintao YANG, Ping NING, Fang WANG, Xiaoshuang SONG, Lijuan JIA, Jiayu FENG. Research progress in dry purification technology of highly toxic gas PH3 [J]. CIESC Journal, 2023, 74(9): 3742-3755. |
| [4] | Baiyu YANG, Yue KOU, Juntao JIANG, Yali ZHAN, Qinghong WANG, Chunmao CHEN. Chemical conversion of dissolved organic matter in petrochemical spent caustic along a wet air oxidation pretreatment process [J]. CIESC Journal, 2023, 74(9): 3912-3920. |
| [5] | Linzheng WANG, Yubing LU, Ruizhi ZHANG, Yonghao LUO. Analysis on thermal oxidation characteristics of VOCs based on molecular dynamics simulation [J]. CIESC Journal, 2023, 74(8): 3242-3255. |
| [6] | Yali HU, Junyong HU, Suxia MA, Yukun SUN, Xueyi TAN, Jiaxin HUANG, Fengyuan YANG. Development of novel working fluid and study on electrochemical characteristics of reverse electrodialysis heat engine [J]. CIESC Journal, 2023, 74(8): 3513-3521. |
| [7] | Jintong LI, Shun QIU, Wenshou SUN. Oxalic acid and UV enhanced arsenic leaching from coal in flue gas desulfurization by coal slurry [J]. CIESC Journal, 2023, 74(8): 3522-3532. |
| [8] | Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent [J]. CIESC Journal, 2023, 74(8): 3375-3385. |
| [9] | Kaixuan LI, Wei TAN, Manyu ZHANG, Zhihao XU, Xuyu WANG, Hongbing JI. Design of cobalt-nitrogen-carbon/activated carbon rich in zero valent cobalt active site and application of catalytic oxidation of formaldehyde [J]. CIESC Journal, 2023, 74(8): 3342-3352. |
| [10] | Yuming TU, Gaoyan SHAO, Jianjie CHEN, Feng LIU, Shichao TIAN, Zhiyong ZHOU, Zhongqi REN. Advances in the design, synthesis and application of calcium-based catalysts [J]. CIESC Journal, 2023, 74(7): 2717-2734. |
| [11] | Jiali GE, Tuxiang GUAN, Xinmin QIU, Jian WU, Liming SHEN, Ningzhong BAO. Synthesis of FeF3 nanoparticles covered by vertical porous carbon for high performance Li-ion battery cathode [J]. CIESC Journal, 2023, 74(7): 3058-3067. |
| [12] | Yuanhao QU, Wenyi DENG, Xiaodan XIE, Yaxin SU. Study on electro-osmotic dewatering of sludge assisted by activated carbon/graphite [J]. CIESC Journal, 2023, 74(7): 3038-3050. |
| [13] | Pan LI, Junyang MA, Zhihao CHEN, Li WANG, Yun GUO. Effect of the morphology of Ru/α-MnO2 on NH3-SCO performance [J]. CIESC Journal, 2023, 74(7): 2908-2918. |
| [14] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| [15] | Bin LI, Zhenghu XU, Shuang JIANG, Tianyong ZHANG. Clean and efficient synthesis of accelerator CBS by hydrogen peroxide catalytic oxidation method [J]. CIESC Journal, 2023, 74(7): 2919-2925. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||