CIESC Journal ›› 2023, Vol. 74 ›› Issue (1): 45-59.DOI: 10.11949/0438-1157.20221075
• Reviews and monographs • Previous Articles Next Articles
Zhuotao TAN( ), Siyu QI, Mengjiao XU, Jie DAI, Chenjie ZHU(
), Siyu QI, Mengjiao XU, Jie DAI, Chenjie ZHU( ), Hanjie YING(
), Hanjie YING( )
)
Received:2022-08-01
Revised:2022-10-28
Online:2023-03-20
Published:2023-01-05
Contact:
Chenjie ZHU, Hanjie YING
谭卓涛( ), 齐思雨, 许梦蛟, 戴杰, 朱晨杰(
), 齐思雨, 许梦蛟, 戴杰, 朱晨杰( ), 应汉杰(
), 应汉杰( )
)
通讯作者:
朱晨杰,应汉杰
作者简介:谭卓涛(1991—),男,副研究员,joh_yy@njtech.edu.cn
基金资助:CLC Number:
Zhuotao TAN, Siyu QI, Mengjiao XU, Jie DAI, Chenjie ZHU, Hanjie YING. Application of the redox cascade systems with coenzyme self-cycling in biocatalytic processes: opportunities and challenges[J]. CIESC Journal, 2023, 74(1): 45-59.
谭卓涛, 齐思雨, 许梦蛟, 戴杰, 朱晨杰, 应汉杰. 辅酶自循环的氧化还原级联体系在生物催化过程中的应用:机遇与挑战[J]. 化工学报, 2023, 74(1): 45-59.
Add to citation manager EndNote|Ris|BibTeX
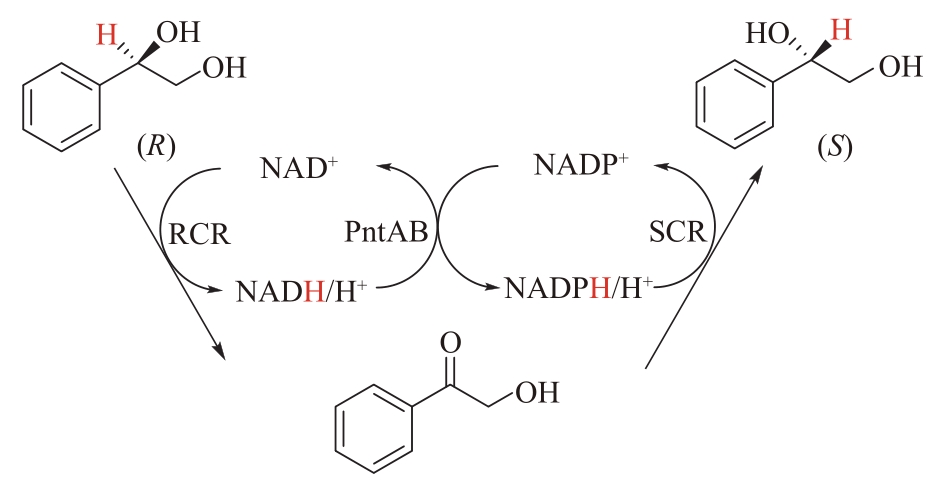
Fig.6 A pair of selectively complementary ADHs catalyze the stereotransformation of (R)-1-phenyl-1,2-ethanediol RCR: NAD+-dependent (R)-ADH; SCR: NADPH-dependent (S)-ADH
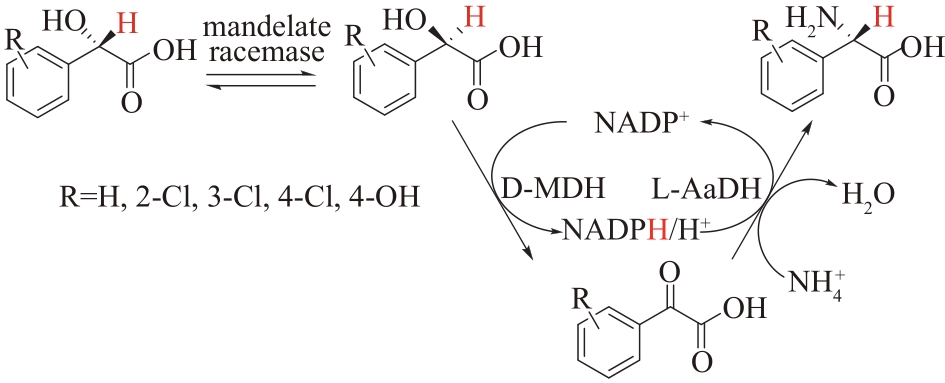
Fig.8 Coupling of mandelate racemase, D-mandelate dehydrogenase (D-MDH) and L-amino acid dehydrogenase (L-AADH) to synthesize L-phenylglycine and its derivatives
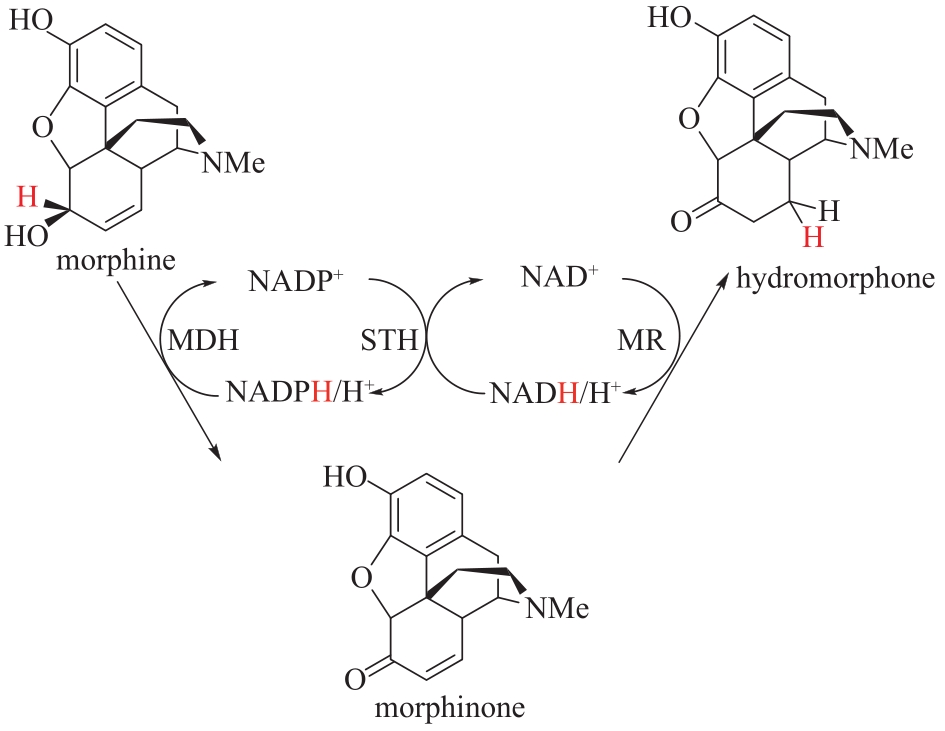
Fig.12 Synthesis of dihydromorphine from morphine catalyzed by morphine dehydrogenase (MDH) coupled with pyridine nucleotide transhydrogenase (STH) and morphone reductase(MR)

Fig.21 Synthesis of lactones by a convergent cascade system consisting of monooxygenase and alcohol dehydrogenase using cyclic ketones and diols as substrates
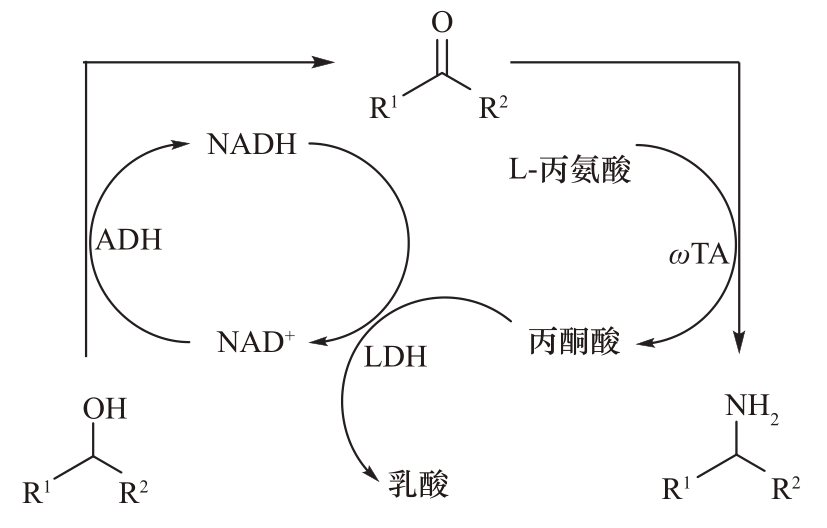
Fig.25 Conversion of sencondary alcohols to chiral amines by alcohol dehydrogenase (ADH) coupled with transaminase (ωTA) and lactate dehydrogenase (LDH)

Fig.26 The production of L-lactic acid from ethanol catalyzed by alcohol dehydrogenase (ADH) coupled decarboxylase (PyDC) and lactate dehydrogenase (LDH)
| 1 | 伍开琳, 陈永正. 氧化还原酶介导生物级联催化研究进展[J]. 合成化学, 2018, 26(4): 292-299. |
| Wu K L, Chen Y Z. Research progress on oxidoreductase mediated biocatalytic cascades[J]. Chinese Journal of Synthetic Chemistry, 2018, 26(4): 292-299. | |
| 2 | Paul C E, Arends I W C E, Hollmann F. Is simpler better? Synthetic nicotinamide cofactor analogues for redox chemistry[J]. ACS Catalysis, 2014, 4(3): 788-797. |
| 3 | 朱晨杰, 付静雯, 谭卓涛, 等. 天然烟酰胺辅因子再生体系及其人工类似物研究进展[J]. 化工学报, 2018, 69(1): 259-271. |
| Zhu C J, Fu J W, Tan Z T, et al. Advances in regeneration system of natural nicotinamide cofactor and its artificial analogues[J]. CIESC Journal, 2018, 69(1): 259-271. | |
| 4 | Guarneri A, van Berkel W J H, Paul C E. Alternative coenzymes for biocatalysis[J]. Current Opinion in Biotechnology, 2019, 60: 63-71. |
| 5 | Wu H, Tian C Y, Song X K, et al. Methods for the regeneration of nicotinamide coenzymes[J]. Green Chemistry, 2013, 15(7): 1773-1789. |
| 6 | Mordhorst S, Andexer J N. Round, round we go—strategies for enzymatic cofactor regeneration[J]. Natural Product Reports, 2020, 37(10): 1316-1333. |
| 7 | Seelbach K, Riebel B, Hummel W, et al. A novel, efficient regenerating method of NADPH using a new formate dehydrogenase[J]. Tetrahedron Letters, 1996, 37(9): 1377-1380. |
| 8 | Pire C, Esclapez J, Díaz S, et al. Alteration of coenzyme specificity in halophilic NAD(P)+ glucose dehydrogenase by site-directed mutagenesis[J]. Journal of Molecular Catalysis B: Enzymatic, 2009, 59(4): 261-265. |
| 9 | Johannes T W, Woodyer R D, Zhao H M. Directed evolution of a thermostable phosphite dehydrogenase for NAD(P)H regeneration[J]. Applied and Environmental Microbiology, 2005, 71(10): 5728-5734. |
| 10 | Matsuda T, Yamagishi Y, Koguchi S, et al. An effective method to use ionic liquids as reaction media for asymmetric reduction by Geotrichum candidum [J]. Tetrahedron Letters, 2006, 47(27): 4619-4622. |
| 11 | Tassano E, Hall M. Enzymatic self-sufficient hydride transfer processes[J]. Chemical Society Reviews, 2019, 48(23): 5596-5615. |
| 12 | Koesoema A A, Standley D M, Senda T, et al. Impact and relevance of alcohol dehydrogenase enantioselectivities on biotechnological applications[J]. Applied Microbiology and Biotechnology, 2020, 104(7): 2897-2909. |
| 13 | Musa M M, Phillips R S, Laivenieks M, et al. Racemization of enantiopure secondary alcohols by Thermoanaerobacter ethanolicus secondary alcohol dehydrogenase[J]. Organic & Biomolecular Chemistry, 2013, 11(17): 2911-2915. |
| 14 | Musa M M, Patel J M, Nealon C M, et al. Thermoanaerobacter ethanolicus secondary alcohol dehydrogenase mutants with improved racemization activity[J]. Journal of Molecular Catalysis B: Enzymatic, 2015, 115: 155-159. |
| 15 | Musa M M. Racemization of enantiopure alcohols using two mutants of Thermoanaerobacter pseudoethanolicus secondary alcohol dehydrogenase[J]. ChemistrySelect, 2021, 6(46): 13261-13264. |
| 16 | Liu Y C, Merten C, Deska J. Enantioconvergent biocatalytic redox isomerization[J]. Angewandte Chemie International Edition, 2018, 57(37): 12151-12156. |
| 17 | Gruber C, Nestl B, Gross J, et al. Emulation of racemase activity by employing a pair of stereocomplementary biocatalysts[J]. Chemistry-A European Journal, 2007, 13(29): 8271-8276. |
| 18 | Bodlenner A, Glueck S M, Nestl B M, et al. Biocatalytic racemization of α-hydroxycarboxylic acids using a stereo-complementary pair of α-hydroxycarboxylic acid dehydrogenases[J]. Tetrahedron, 2009, 65(36): 7752-7755. |
| 19 | Zhang R Z, Xu Y, Xiao R, et al. Efficient one-step production of (S)-1-phenyl-1,2-ethanediol from (R)-enantiomer plus NAD(+)-NADPH in situ regeneration using engineered Escherichia coli [J]. Microbial Cell Factories, 2012, 11: 167. |
| 20 | Kostyanovsky R G, Kadorkina G K, Lyssenko K A, et al. Chiral drugs via the spontaneous resolution[J]. Mendeleev Communications, 2002, 12(1): 6-8. |
| 21 | Perdih A, Sollner Dolenc M. Recent advances in the synthesis of unnatural α-amino acids—an updated version[J]. Current Organic Chemistry, 2011, 15(22): 3750-3799. |
| 22 | Wandrey C, Fiolitakis E, Wichmann U, et al. L-amino acids from a racemic mixture of α-hydroxy acids[J]. Annals of the New York Academy of Sciences, 1984, 434(1): 91-94. |
| 23 | Bossow B, Wandrey C. Continuous enzymatically catalyzed production of L-leucine from the corresponding racemic hydroxy acid[J]. Annals of the New York Academy of Sciences, 1987, 506(1): 325-336. |
| 24 | Resch V, Fabian W M F, Kroutil W. Deracemisation of mandelic acid to optically pure non-natural L-phenylglycine via a redox-neutral biocatalytic cascade[J]. Advanced Synthesis & Catalysis, 2010, 352(6): 993-997. |
| 25 | Fan C W, Xu G C, Ma B D, et al. A novel D-mandelate dehydrogenase used in three-enzyme cascade reaction for highly efficient synthesis of non-natural chiral amino acids[J]. Journal of Biotechnology, 2015, 195: 67-71. |
| 26 | Wittcoff H A, Reuben B G, Plotkin J S. Industrial Organic Chemicals[M]. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2012. |
| 27 | Nugent T C. Chiral Amine Synthesis: Methods, Developments and Applications[M]. New York: John Wiley & Sons Inc., 2010. |
| 28 | Ducrot L, Bennett M, Grogan G, et al. NAD(P)H-dependent enzymes for reductive amination: active site description and carbonyl-containing compound spectrum[J]. Advanced Synthesis & Catalysis, 2021, 363(2): 328-351. |
| 29 | Wang Z L, Sundara Sekar B, Li Z. Recent advances in artificial enzyme cascades for the production of value-added chemicals[J]. Bioresource Technology, 2021, 323: 124551. |
| 30 | Mutti F G, Knaus T, Scrutton N S, et al. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades[J]. Science, 2015, 349(6255): 1525-1529. |
| 31 | Chen F F, Liu Y Y, Zheng G W, et al. Asymmetric amination of secondary alcohols by using a redox-neutral two-enzyme cascade[J]. ChemCatChem, 2015, 7(23): 3838-3841. |
| 32 | Thompson M P, Turner N J. Two-enzyme hydrogen-borrowing amination of alcohols enabled by a cofactor-switched alcohol dehydrogenase[J]. ChemCatChem, 2017, 9(20): 3833-3836. |
| 33 | Aleku G A, France S P, Man H, et al. A reductive aminase from Aspergillus oryzae [J]. Nature Chemistry, 2017, 9(10): 961-969. |
| 34 | Montgomery S L, Mangas-Sanchez J, Thompson M P, et al. Direct alkylation of amines with primary and secondary alcohols through biocatalytic hydrogen borrowing[J]. Angewandte Chemie International Edition, 2017, 56(35): 10491-10494. |
| 35 | Lenz M, Borlinghaus N, Weinmann L, et al. Recent advances in imine reductase-catalyzed reactions[J]. World Journal of Microbiology & Biotechnology, 2017, 33(11): 199. |
| 36 | Roiban G D, Kern M, Liu Z, et al. Efficient biocatalytic reductive aminations by extending the imine reductase toolbox[J]. ChemCatChem, 2017, 9(24): 4475-4479. |
| 37 | Scholtissek A, Tischler D, Westphal A, et al. Old yellow enzyme-catalysed asymmetric hydrogenation: linking family roots with improved catalysis[J]. Catalysts, 2017, 7(12): 130. |
| 38 | Boonstra B, Rathbone D A, French C E, et al. Cofactor regeneration by a soluble pyridine nucleotide transhydrogenase for biological production of hydromorphone[J]. Applied and Environmental Microbiology, 2000, 66(12): 5161-5166. |
| 39 | Gargiulo S, Opperman D J, Hanefeld U, et al. A biocatalytic redox isomerisation[J]. Chemical Communications, 2012, 48(53): 6630-6632. |
| 40 | Reich S, Nestl B M, Hauer B. Loop-grafted old yellow enzymes in the bienzymatic cascade reduction of allylic alcohols[J]. ChemBioChem, 2016, 17(7): 561-565. |
| 41 | Oberleitner N, Peters C, Muschiol J, et al. An enzymatic toolbox for cascade reactions: a showcase for an in vivo redox sequence in asymmetric synthesis[J]. ChemCatChem, 2013, 5(12): 3524-3528. |
| 42 | Okamoto Y, Köhler V, Paul C E, et al. Efficient in situ regeneration of NADH mimics by an artificial metalloenzyme[J]. ACS Catalysis, 2016, 6(6): 3553-3557. |
| 43 | Köhler V, Wilson Y M, Dürrenberger M, et al. Synthetic cascades are enabled by combining biocatalysts with artificial metalloenzymes[J]. Nature Chemistry, 2013, 5(2): 93-99. |
| 44 | Gurak J A, Yang K S, Liu Z, et al. Directed, regiocontrolled hydroamination of unactivated alkenes via protodepalladation[J]. Journal of the American Chemical Society, 2016, 138(18): 5805-5808. |
| 45 | Winkler T, Gröger H, Hummel W. Enantioselective rearrangement coupled with water addition: direct synthesis of enantiomerically pure saturated carboxylic acids from α,β-unsaturated aldehydes[J]. ChemCatChem, 2014, 6(4): 961-964. |
| 46 | Knaus T, Mutti F G, Humphreys L D, et al. Systematic methodology for the development of biocatalytic hydrogen-borrowing cascades: application to the synthesis of chiral α-substituted carboxylic acids from α-substituted α,β-unsaturated aldehydes[J]. Organic & Biomolecular Chemistry, 2015, 13(1): 223-233. |
| 47 | Ngo T A, Nakata E, Saimura M, et al. Spatially organized enzymes drive cofactor-coupled cascade reactions[J]. Journal of the American Chemical Society, 2016, 138(9): 3012-3021. |
| 48 | Staudt S, Burda E, Giese C, et al. Direct oxidation of cycloalkanes to cycloalkanones with oxygen in water[J]. Angewandte Chemie International Edition, 2013, 52(8): 2359-2363. |
| 49 | Loida P J, Sligar S G. Molecular recognition in cytochrome P-450: mechanism for the control of uncoupling reactions[J]. Biochemistry, 1993, 32(43): 11530-11538. |
| 50 | Schulz S, Girhard M, Gaßmeyer S K, et al. Selective enzymatic synthesis of the grapefruit flavor (+)-nootkatone[J]. ChemCatChem, 2015, 7(4): 601-604. |
| 51 | Hofer M, Strittmatter H, Sieber V. Biocatalytic synthesis of a diketobornane as a building block for bifunctional camphor derivatives[J]. ChemCatChem, 2013, 5(11): 3351-3357. |
| 52 | Müller C A, Dennig A, Welters T, et al. Whole-cell double oxidation of n-heptane[J]. Journal of Biotechnology, 2014, 191: 196-204. |
| 53 | O’Reilly E, Köhler V, Flitsch S L, et al. Cytochromes P450 as useful biocatalysts: addressing the limitations[J]. Chemical Communications, 2011, 47(9): 2490-2501. |
| 54 | Willetts A J, Knowles C J, Levitt M S, et al. Biotransformation of endo-bicyclo[2.2.1]heptan-2-ols and endo-bicyclo[3.2.0]hept-2-en-6-ol into the corresponding lactones[J]. Journal of the Chemical Society, Perkin Transactions 1, 1991(6): 1608. |
| 55 | Grogan G, Roberts S, Willetts A. Biotransformations by microbial Baeyer-Villiger monooxygenases stereoselective lactone formation in vitro by coupled enzyme systems[J]. Biotechnology Letters, 1992, 14(12): 1125-1130. |
| 56 | Gagnon R, Grogan G, Roberts S M, et al. Enzymatic Baeyer-Villiger oxidations of some bicyclo[2.2.1]heptan-2-ones using monooxygenases from Pseudomonas putida NCIMB 10007: enantioselective preparation of a precursor of azadirachtin[J]. Journal of the Chemical Society, Perkin Transactions 1, 1995(12): 1505-1511. |
| 57 | Mallin H, Wulf H, Bornscheuer U T. A self-sufficient Baeyer-Villiger biocatalysis system for the synthesis of ɛ-caprolactone from cyclohexanol[J]. Enzyme & Microbial Technology, 2013, 53(4): 283-287. |
| 58 | Staudt S, Bornscheuer U T, Menyes U, et al. Direct biocatalytic one-pot-transformation of cyclohexanol with molecular oxygen into ɛ-caprolactone[J]. Enzyme & Microbial Technology, 2013, 53(4): 288-292. |
| 59 | Kara S, Spickermann D, Schrittwieser J H, et al. More efficient redox biocatalysis by utilising 1,4-butanediol as a ‘smart cosubstrate’[J]. Green Chemistry, 2013, 15(2): 330-335. |
| 60 | Bornadel A, Hatti-Kaul R, Hollmann F, et al. A bi-enzymatic convergent cascade for ε-caprolactone synthesis employing 1, 6-hexanediol as a ‘double-smart cosubstrate’[J]. ChemCatChem, 2015, 7(16): 2442-2445. |
| 61 | Bornadel A, Hatti-Kaul R, Hollmann F, et al. Enhancing the productivity of the bi-enzymatic convergent cascade for ɛ-caprolactone synthesis through design of experiments and a biphasic system[J]. Tetrahedron, 2016, 72(46): 7222-7228. |
| 62 | Engel J, Mthethwa K S, Opperman D J, et al. Characterization of new Baeyer-Villiger monooxygenases for lactonizations in redox-neutral cascades[J]. Molecular Catalysis, 2019, 468: 44-51. |
| 63 | Huang L, Romero E, Ressmann A K, et al. Nicotinamide adenine dinucleotide-dependent redox-neutral convergent cascade for lactonizations with type Ⅱ flavin-containing monooxygenase[J]. Advanced Synthesis & Catalysis, 2017, 359(12): 2142-2148. |
| 64 | Qi S Y, Tan Z T, Na Q, et al. Constructing a multienzyme cascade redox-neutral system for the synthesis of halogenated indoles[J]. Chemical Communications, 2022, 58(40): 6016-6019. |
| 65 | Lo H C, Fish R H. Biomimetic NAD+ models for tandem cofactor regeneration, horse liver alcohol dehydrogenase recognition of 1, 4-NADH derivatives, and chiral synthesis[J]. Angewandte Chemie International Edition, 2002, 41(3): 478-481. |
| 66 | Paul C E, Tischler D, Riedel A, et al. Nonenzymatic regeneration of styrene monooxygenase for catalysis[J]. ACS Catalysis, 2015, 5(5): 2961-2965. |
| 67 | Simon R C, Richter N, Busto E, et al. Recent developments of cascade reactions involving ω-transaminases[J]. ACS Catalysis, 2014, 4(1): 129-143. |
| 68 | Sattler J H, Fuchs M, Tauber K, et al. Redox self-sufficient biocatalyst network for the amination of primary alcohols[J]. Angewandte Chemie, 2012, 124(36): 9290-9293. |
| 69 | Tauber K, Fuchs M, Sattler J H, et al. Artificial multi-enzyme networks for the asymmetric amination of sec-alcohols[J]. Chemistry-A European Journal, 2013, 19(12): 4030-4035. |
| 70 | Tong X D, El-Zahab B, Zhao X Y, et al. Enzymatic synthesis of L-lactic acid from carbon dioxide and ethanol with an inherent cofactor regeneration cycle[J]. Biotechnology and Bioengineering, 2011, 108(2): 465-469. |
| 71 | Sinha R, Shukla P. Current trends in protein engineering: updates and progress[J]. Current Protein & Peptide Science, 2019, 20(5): 398-407. |
| 72 | Hyster T K, Ward T R. Genetic optimization of metalloenzymes: enhancing enzymes for non-natural reactions[J]. Angewandte Chemie International Edition, 2016, 55(26): 7344-7357. |
| 73 | Turner N J, O'Reilly E. Biocatalytic retrosynthesis[J]. Nature Chemical Biology, 2013, 9(5): 285-288. |
| 74 | Schrittwieser J H, Velikogne S, Hall M, et al. Artificial biocatalytic linear cascades for preparation of organic molecules[J]. Chemical Reviews, 2018, 118(1): 270-348. |
| [1] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [2] | Lingding MENG, Ruqing CHONG, Feixue SUN, Zihui MENG, Wenfang LIU. Immobilization of carbonic anhydrase on modified polyethylene membrane and silica [J]. CIESC Journal, 2023, 74(8): 3472-3484. |
| [3] | Yaxin CHEN, Hang YUAN, Guanzhang LIU, Lei MAO, Chun YANG, Ruifang ZHANG, Guangya ZHANG. Advances in enzyme self-immobilization mediated by protein nanocages [J]. CIESC Journal, 2023, 74(7): 2773-2782. |
| [4] | Xiaoling TANG, Jiarui WANG, Xuanye ZHU, Renchao ZHENG. Biosynthesis of chiral epichlorohydrin by halohydrin dehalogenase based on Pickering emulsion system [J]. CIESC Journal, 2023, 74(7): 2926-2934. |
| [5] | Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics [J]. CIESC Journal, 2023, 74(6): 2589-2598. |
| [6] | Lanhe ZHANG, Qingyi LAI, Tiezheng WANG, Xiaozhuo GUAN, Mingshuang ZHANG, Xin CHENG, Xiaohui XU, Yanping JIA. Effect of H2O2 on nitrogen removal and sludge properties in SBR [J]. CIESC Journal, 2023, 74(5): 2186-2196. |
| [7] | Lufan JIA, Yiying WANG, Yuman DONG, Qinyuan LI, Xin XIE, Hao YUAN, Tao MENG. Aqueous two-phase system based adherent droplet microfluidics for enhanced enzymatic reaction [J]. CIESC Journal, 2023, 74(3): 1239-1246. |
| [8] | Yang HU, Yan SUN. Self-propulsion of enzyme and enzyme-induced micro-/nanomotor [J]. CIESC Journal, 2023, 74(1): 116-132. |
| [9] | Xin LIU, Jun GE, Chun LI. Light-driven microbial hybrid systems improve level of biomanufacturing [J]. CIESC Journal, 2023, 74(1): 330-341. |
| [10] | Shaojie AN, Hongfeng XU, Si LI, Yuanhang XU, Jiaxi LI. Construction of pH sensitive artificial glutathione peroxidase based on the formation and dissociation of molecular machine [J]. CIESC Journal, 2022, 73(8): 3669-3678. |
| [11] | Mai ZHANG, Yao TIAN, Zhiqi GUO, Ye WANG, Guangjin DOU, Hao SONG. Design and optimization of photocatalysis-biological hybrid system for green synthesis of fuels and chemicals [J]. CIESC Journal, 2022, 73(7): 2774-2789. |
| [12] | Jiachen SUN, Wentao SUN, Hui SUN, Bo LYU, Chun LI. Licorice flavone synthase Ⅱ catalyzes liquiritigenin to specifically synthesize 7,4′-dihydroxyflavone [J]. CIESC Journal, 2022, 73(7): 3202-3211. |
| [13] | Xinzhe ZHANG, Wentao SUN, Bo LYU, Chun LI. Oxidative modification of plant natural products and microbial manufacturing [J]. CIESC Journal, 2022, 73(7): 2790-2805. |
| [14] | Yinlong XU, Wenchieh CHENG, Lin WANG, Zhongfei XUE, Yixin XIE. Implication and enhancement mechanism of chitosan-assisted enzyme- induced carbonate precipitation for copper wastewater treatment [J]. CIESC Journal, 2022, 73(5): 2222-2232. |
| [15] | Haihang TONG, Dezhi SHI, Jiayu LIU, Huayi CAI, Dan LUO, Fei CHEN. Research progress on dark fermentative bio-hydrogen production from lignocellulose assisted by metal nanoparticles [J]. CIESC Journal, 2022, 73(4): 1417-1435. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||