CIESC Journal ›› 2024, Vol. 75 ›› Issue (2): 647-658.DOI: 10.11949/0438-1157.20231088
• Energy and environmental engineering • Previous Articles Next Articles
Yanping JIA( ), Dongxu YIN, Jingyi XU, Haifeng ZHANG, Lanhe ZHANG(
), Dongxu YIN, Jingyi XU, Haifeng ZHANG, Lanhe ZHANG( )
)
Received:2023-10-24
Revised:2023-12-24
Online:2024-04-10
Published:2024-02-25
Contact:
Lanhe ZHANG
通讯作者:
张兰河
作者简介:贾艳萍(1973—),女,博士,教授,jiayanping1111@sina.com
基金资助:CLC Number:
Yanping JIA, Dongxu YIN, Jingyi XU, Haifeng ZHANG, Lanhe ZHANG. Mechanism study of oxytetracycline hydrochloride degradation through activating sulfite by Fe2+/Mn2+[J]. CIESC Journal, 2024, 75(2): 647-658.
贾艳萍, 阴东旭, 徐静仪, 张海丰, 张兰河. Fe2+/Mn2+活化亚硫酸盐降解盐酸土霉素的机理研究[J]. 化工学报, 2024, 75(2): 647-658.
Add to citation manager EndNote|Ris|BibTeX
| 产物 | 保留 时间/min | m/z | 分子式 | 分子结构 | 产物 | 保留 时间/min | m/z | 分子式 | 分子结构 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.295 | 106 | C8H10 | 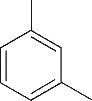 | 10 | 9.585 | 138 | C8H10O2 | 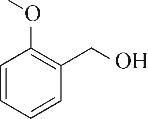 |
| 2 | 4.900 | 108 | C7H8O | 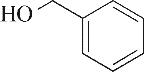 | 11 | 13.525 | 206 | C14H22O | 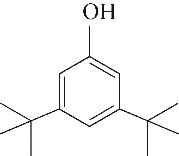 |
| 3 | 5.955 | 144 | C8H16O2 | 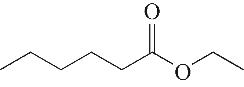 | 12 | 18.705 | 278 | C16H22O4 | 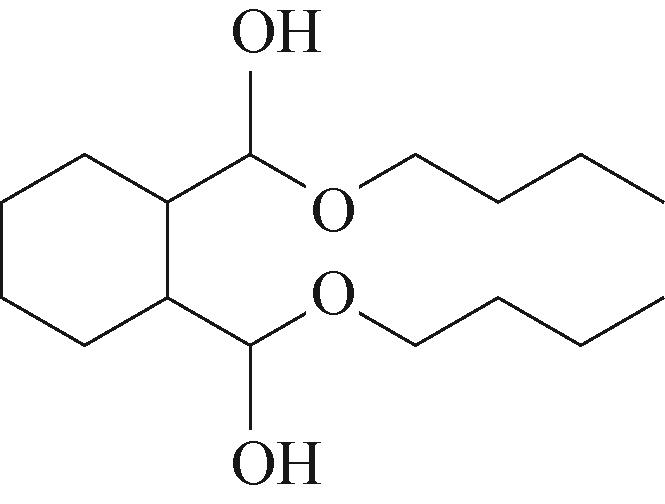 |
| 4 | 6.385 | 134 | C10H14 | 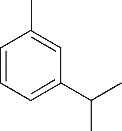 | 13 | 20.050 | 196 | C10H12O4 | 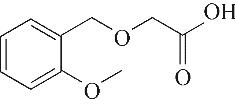 |
| 5 | 7.260 | 108 | C7H8O | 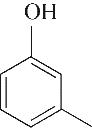 | 14 | 23.385 | 154 | C9H14O2 | 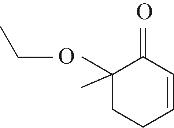 |
| 6 | 7.360 | 136 | C10H16 | 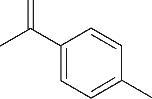 | 15 | 24.215 | 424 | C29H44O2 | 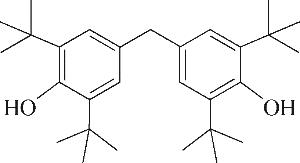 |
| 7 | 7.940 | 152 | C10H16O | 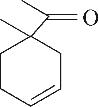 | 16 | 24.640 | 208 | C13H20O2 | 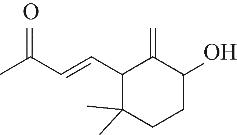 |
| 8 | 8.975 | 134 | C9H10O | 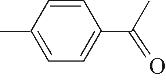 | 17 | 26.645 | 180 | C11H16O2 | 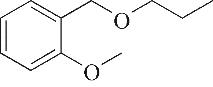 |
| 9 | 9.150 | 138 | C9H14O | 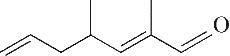 |
Table 1 Intermediate products in the degradation process of OTH
| 产物 | 保留 时间/min | m/z | 分子式 | 分子结构 | 产物 | 保留 时间/min | m/z | 分子式 | 分子结构 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.295 | 106 | C8H10 |  | 10 | 9.585 | 138 | C8H10O2 |  |
| 2 | 4.900 | 108 | C7H8O |  | 11 | 13.525 | 206 | C14H22O |  |
| 3 | 5.955 | 144 | C8H16O2 |  | 12 | 18.705 | 278 | C16H22O4 |  |
| 4 | 6.385 | 134 | C10H14 |  | 13 | 20.050 | 196 | C10H12O4 |  |
| 5 | 7.260 | 108 | C7H8O |  | 14 | 23.385 | 154 | C9H14O2 |  |
| 6 | 7.360 | 136 | C10H16 |  | 15 | 24.215 | 424 | C29H44O2 |  |
| 7 | 7.940 | 152 | C10H16O |  | 16 | 24.640 | 208 | C13H20O2 |  |
| 8 | 8.975 | 134 | C9H10O |  | 17 | 26.645 | 180 | C11H16O2 |  |
| 9 | 9.150 | 138 | C9H14O |  |
| 1 | Moudgil P, Bedi J S, Aulakh R S, et al. Validation of HPLC multi-residue method for determination of fluoroquinolones, tetracycline, sulphonamides and chloramphenicol residues in bovine milk[J]. Food Analytical Methods, 2019, 12(2): 338-346. |
| 2 | Petković H, Lukežič T, Šušković J. Biosynthesis of oxytetracycline by Streptomyces rimosus: past, present and future directions in the development of tetracycline antibiotics[J]. Food Technology and Biotechnology, 2017, 55(1): 3-13. |
| 3 | 展海银, 周启星. 环境中四环素类抗生素污染处理技术研究进展[J]. 环境工程技术学报, 2021, 11(3): 571-581. |
| Zhan H Y, Zhou Q X. Research progress on treatment technology of tetracycline antibiotics pollution in the environment[J]. Journal of Environmental Engineering Technology, 2021, 11(3): 571-581. | |
| 4 | 陈明如, 马可可, 周律. 基于亚硫酸盐的高级氧化活化方法最新进展[J]. 工业水处理, 2022, 42(6): 109-115, 124. |
| Chen M R, Ma K K, Zhou L. Recent progress in sulfite advanced oxidation activation approaches[J]. Industrial Water Treatment, 2022, 42(6): 109-115, 124. | |
| 5 | Yuan Y N, Luo T, Xu J, et al. Enhanced oxidation of aniline using Fe(Ⅲ)-S(Ⅳ) system: role of different oxysulfur radicals[J]. Chemical Engineering Journal, 2019, 362: 183-189. |
| 6 | 李阳, 关小红, 董红钰. Fe(Ⅱ)活化亚硫酸盐降解卡马西平的动力学及机制研究[J]. 土木与环境工程学报(中英文), 2021, 43(6): 165-171. |
| Li Y, Guan X H, Dong H Y. Kinetics and mechanism of carbamazepine degradation through activating sulfite by Fe(Ⅱ)[J]. Journal of Civil and Environmental Engineering, 2021, 43(6): 165-171. | |
| 7 | Grgić I, Hudnik V, Bizjak M, et al. Aqueous S(Ⅳ) oxidation(Ⅰ): Catalytic effects of some metal ions[J]. Atmospheric Environment. Part A. General Topics, 1991, 25(8): 1591-1597. |
| 8 | Berglund J, Elding L I. Manganese-catalysed autoxidation of dissolved sulfur dioxide in the atmospheric aqueous phase[J]. Atmospheric Environment, 1995, 29(12): 1379-1391. |
| 9 | Zhao X D, Wu W J, Yan Y G. Efficient abatement of an iodinated X-ray contrast media iohexol by Co(Ⅱ) or Cu(Ⅱ) activated sulfite autoxidation process[J]. Environmental Science and Pollution Research, 2019, 26(24): 24707-24719. |
| 10 | Podkrajšek B, Berčič G, Turšič J, et al. Aqueous oxidation of sulfur(Ⅳ) catalyzed by manganese(Ⅱ): a generalized simple kinetic model[J]. Journal of Atmospheric Chemistry, 2004, 47(3): 287-303. |
| 11 | Guo Y G, Lou X Y, Fang C L, et al. Novel photo-sulfite system: toward simultaneous transformations of inorganic and organic pollutants[J]. Environmental Science & Technology, 2013, 47(19): 11174-11181. |
| 12 | Ling L, Zhang D P, Fan C, et al. A Fe(Ⅱ)/citrate/UV/PMS process for carbamazepine degradation at a very low Fe(Ⅱ)/PMS ratio and neutral pH: the mechanisms[J]. Water Research, 2017, 124: 446-453. |
| 13 | Berglund J, Werndrup P, Elding L I. Kinetics and mechanism for the redox reaction between hexaaquathallium(Ⅲ) and sulfur dioxide in acidic aqueous solution[J]. Journal of the Chemical Society, Dalton Transactions, 1994(9): 1435-1439. |
| 14 | Fronaeus S, Berglund J, Elding L I. Iron-manganese redox processes and synergism in the mechanism for manganese-catalyzed autoxidation of hydrogen sulfite[J]. Inorganic Chemistry, 1998, 37(19): 4939-4944. |
| 15 | Zhang J M, Ma J, Song H R, et al. Organic contaminants degradation from the S(Ⅳ) autoxidation process catalyzed by ferrous-manganous ions: a noticeable Mn(Ⅲ) oxidation process[J]. Water Research, 2018, 133: 227-235. |
| 16 | Zhang J M, Song H R, Liu Y L, et al. Remarkable enhancement of a photochemical Fenton-like system (UV-A/Fe(Ⅱ)/PMS) at near-neutral pH and low Fe(Ⅱ)/peroxymonosulfate ratio by three alpha hydroxy acids: mechanisms and influencing factors[J]. Separation and Purification Technology, 2019, 224: 142-151. |
| 17 | Morgan J J. Kinetics of reaction between O2 and Mn(Ⅱ) species in aqueous solutions[J]. Geochimica et Cosmochimica Acta, 2005, 69(1): 35-48. |
| 18 | Springer S D, Butler A. Magnetic susceptibility of Mn(Ⅲ) complexes of hydroxamate siderophores[J]. Journal of Inorganic Biochemistry, 2015, 148: 22-26. |
| 19 | Wang Z M, Xiong W, Tebo B M, et al. Oxidative UO2 dissolution induced by soluble Mn(Ⅲ)[J]. Environmental Science & Technology, 2014, 48(1): 289-298. |
| 20 | Feng J W, Zheng Z, Sun Y B, et al. Degradation of diuron in aqueous solution by dielectric barrier discharge[J]. Journal of Hazardous Materials, 2008, 154(1/2/3): 1081-1089. |
| 21 | Klewicki J K, Morgan J J. Kinetic behavior of Mn(Ⅲ) complexes of pyrophosphate, EDTA, and citrate[J]. Environmental Science and Technology, 1998, 32(19): 2916-2922. |
| 22 | Xie P C, Zhang L, Chen J H, et al. Enhanced degradation of organic contaminants by zero-valent iron/sulfite process under simulated sunlight irradiation[J]. Water Research, 2019, 149: 169-178. |
| 23 | Luo C W, Jiang J, Ma J, et al. Oxidation of the odorous compound 2, 4, 6-trichloroanisole by UV activated persulfate: kinetics, products, and pathways[J]. Water Research, 2016, 96: 12-21. |
| 24 | Sun P Z, Lee W N, Zhang R C, et al. Degradation of DEET and caffeine under UV/chlorine and simulated sunlight/chlorine conditions[J]. Environmental Science & Technology, 2016, 50(24): 13265-13273. |
| 25 | Fang G D, Dionysiou D D, Wang Y, et al. Sulfate radical-based degradation of polychlorinated biphenyls: effects of chloride ion and reaction kinetics[J]. Journal of Hazardous Materials, 2012, 227/228: 394-401. |
| 26 | Wu Z H, Guo K H, Fang J Y, et al. Factors affecting the roles of reactive species in the degradation of micropollutants by the UV/chlorine process[J]. Water Research, 2017, 126: 351-360. |
| 27 | Chen Y, Liu Z Z, Wang Z P, et al. Photodegradation of propranolol by Fe(Ⅲ)-citrate complexes: kinetics, mechanism and effect of environmental media[J]. Journal of Hazardous Materials, 2011, 194: 202-208. |
| 28 | Wang X H, Yao J Y, Wang S Y, et al. Phototransformation of estrogens mediated by Mn(Ⅲ), not by reactive oxygen species, in the presence of humic acids[J]. Chemosphere, 2018, 201: 224-233. |
| 29 | Xu K, Ben W W, Ling W C, et al. Impact of humic acid on the degradation of levofloxacin by aqueous permanganate: kinetics and mechanism[J]. Water Research, 2017, 123: 67-74. |
| 30 | Yang Y, Jiang J, Lu X L, et al. Production of sulfate radical and hydroxyl radical by reaction of ozone with peroxymonosulfate: a novel advanced oxidation process[J]. Environmental Science & Technology, 2015, 49(12): 7330-7339. |
| 31 | Feng M B, Sharma V K. Enhanced oxidation of antibiotics by ferrate ( Ⅵ ) - s u l f u r ( Ⅳ ) system: elucidating multi-oxidant mechanism[J]. Chemical Engineering Journal, 2018, 341: 137-145. |
| 32 | Neta P, Huie R E. Free-radical chemistry of sulfite[J]. Environmental Health Perspectives, 1985, 64: 209-217. |
| 33 | Neta P, Huie R E, Ross A B. Rate constants for reactions of inorganic radicals in aqueous solution[J]. Journal of Physical and Chemical Reference Data, 1988, 17(3): 1027-1284. |
| 34 | Chen L, Peng X Z, Liu J H, et al. Decolorization of orange Ⅱ in aqueous solution by an F e ( Ⅱ ) /sulfite system: replacement of persulfate[J]. Industrial & Engineering Chemistry Research, 2012, 51(42): 13632-13638. |
| 35 | Anipsitakis G P, Dionysiou D D. Radical generation by the interaction of transition metals with common oxidants[J]. Environmental Science & Technology, 2004, 38(13): 3705-3712. |
| 36 | Zou J, Ma J, Chen L W, et al. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting F e ( Ⅲ ) / F e ( Ⅱ ) cycle with hydroxylamine[J]. Environmental Science & Technology, 2013, 47(20): 11685-11691. |
| 37 | 孙绍芳, 李佳龙, 邱琪, 等. Fe(Ⅵ)/Na2SO3体系降解阿特拉津效能[J]. 中国环境科学, 2021, 41(1): 192-198. |
| Sun S F, Li J L, Qiu Q, et al. Degradation efficiency of atrazine by Fe(Ⅵ)/Na2SO3 system[J]. China Environmental Science, 2021, 41(1): 192-198. | |
| 38 | 唐海, 张昊楠, 段升飞, 等. S O 3 2 - 活化 S 2 O 8 2 - 降解偶氮染料废水的机制研究[J]. 中国环境科学, 2018, 38(3): 959-967. |
| Tang H, Zhang H N, Duan S F, et al. Mechanism research for degradation of azo dying wastewater based on persulfate activated by sulphite[J]. China Environmental Science, 2018, 38(3): 959-967. | |
| 39 | 于怀东, 方茹, 陈士明, 等. 锰离子参与的类Fenton反应的HPLC和ESR波谱研究[J]. 化学学报, 2005, 63(14): 1357-1360, 1244. |
| Yu H D, Fang R, Chen S M, et al. Manganous participation in Fenton like reaction studied by HPLC and ESR spectroscopy[J]. Acta Chimica Sinica, 2005, 63(14): 1357-1360, 1244. | |
| 40 | Rao D D, Sun Y K, Shao B B, et al. Activation of oxygen with sulfite for enhanced removal of Mn(Ⅱ): the involvement of S O 4 • - [J]. Water Research, 2019, 157: 435-444. |
| 41 | Sun S F, Pang S Y, Jiang J, et al. The combination of ferrate(Ⅵ) and sulfite as a novel advanced oxidation process for enhanced degradation of organic contaminants[J]. Chemical Engineering Journal, 2018, 333: 11-19. |
| 42 | Wang Y, Zhang H, Chen L. Ultrasound enhanced catalytic ozonation of tetracycline in a rectangular air-lift reactor[J]. Catalysis Today, 2011, 175(1): 283-292. |
| 43 | Wang Y, Zhang H, Zhang J H, et al. Degradation of tetracycline in aqueous media by ozonation in an internal loop-lift reactor[J]. Journal of Hazardous Materials, 2011, 192(1): 35-43. |
| 44 | Zhou J, Hou M F, Pan D Y. Degradation of oxytetracycline by the iron wire/H2O2 system[C]//Proceedings of the 2016 International Conference on Biological Engineering and Pharmacy (BEP 2016). Paris, France: Atlantis Press, 2017. |
| 45 | Zhu X D, Wang Y J, Sun R J, et al. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2 [J]. Chemosphere, 2013, 92(8): 925-932. |
| 46 | Chen B L, Zhou D D, Zhu L Z. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures[J]. Environmental Science & Technology, 2008, 42(14): 5137-5143. |
| 47 | Jia M Y, Wang F, Bian Y R, et al. Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar[J]. Bioresource Technology, 2013, 136: 87-93. |
| 48 | 徐惟馨, 夏静静, 韦芸, 等. 红外光谱对牛预混料中违禁添加盐酸土霉素的快速定量[J]. 光谱学与光谱分析, 2023, 43(3): 842-847. |
| Xu W X, Xia J J, Wei Y, et al. Rapid determination of oxytetracycline hydrochloride illegally added in cattle premix by ATR-FTIR[J]. Spectroscopy and Spectral Analysis, 2023, 43(3): 842-847. | |
| 49 | Shi Z Y, Jin C, Zhang J, et al. Insight into mechanism of arsanilic acid degradation in permanganate-sulfite system: role of reactive species[J]. Chemical Engineering Journal, 2019, 359: 1463-1471. |
| 50 | Anipsitakis G P, Dionysiou D D, Gonzalez M A. Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions[J]. Environmental Science & Technology, 2006, 40(3): 1000-1007. |
| 51 | 黄智辉, 纪志永, 陈希, 等. 过硫酸盐高级氧化降解水体中有机污染物研究进展[J]. 化工进展, 2019, 38(5): 2461-2470. |
| Huang Z H, Ji Z Y, Chen X, et al. Degradation of organic pollutants in water by persulfate advanced oxidation[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2461-2470. | |
| 52 | 吴文瞳, 张玲玲, 李子富, 等. 高级氧化技术降解抗生素及去除耐药性的研究进展[J]. 化工进展, 2021, 40(8): 4551-4561. |
| Wu W T, Zhang L L, Li Z F, et al. Research progress of advanced oxidation technology in degradation of antibiotics and removal of antibiotic resistance[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4551-4561. | |
| 53 | 叶林静, 关卫省, 李宇亮. 高级氧化技术降解双酚A的研究进展[J]. 化工进展, 2013, 32(4): 909-918. |
| Ye L J, Guan W S, Li Y L. Research advances in bisphenol A degraded by advanced oxidation processes[J]. Chemical Industry and Engineering Progress, 2013, 32(4): 909-918. | |
| 54 | Hu E D, Zhang Y, Wu S Y, et al. Role of dissolved Mn(Ⅲ) in transformation of organic contaminants: non-oxidative versus oxidative mechanisms[J]. Water Research, 2017, 111: 234-243. |
| [1] | Chao HU, Yuming DONG, Wei ZHANG, Hongling ZHANG, Peng ZHOU, Hongbin XU. Preparation of high-concentration positive electrolyte of vanadium redox flow battery by activating vanadium pentoxide with highly concentrated sulfuric acid [J]. CIESC Journal, 2023, 74(S1): 338-345. |
| [2] | Baiyu YANG, Yue KOU, Juntao JIANG, Yali ZHAN, Qinghong WANG, Chunmao CHEN. Chemical conversion of dissolved organic matter in petrochemical spent caustic along a wet air oxidation pretreatment process [J]. CIESC Journal, 2023, 74(9): 3912-3920. |
| [3] | Xuejin YANG, Jintao YANG, Ping NING, Fang WANG, Xiaoshuang SONG, Lijuan JIA, Jiayu FENG. Research progress in dry purification technology of highly toxic gas PH3 [J]. CIESC Journal, 2023, 74(9): 3742-3755. |
| [4] | Linzheng WANG, Yubing LU, Ruizhi ZHANG, Yonghao LUO. Analysis on thermal oxidation characteristics of VOCs based on molecular dynamics simulation [J]. CIESC Journal, 2023, 74(8): 3242-3255. |
| [5] | Jintong LI, Shun QIU, Wenshou SUN. Oxalic acid and UV enhanced arsenic leaching from coal in flue gas desulfurization by coal slurry [J]. CIESC Journal, 2023, 74(8): 3522-3532. |
| [6] | Kaixuan LI, Wei TAN, Manyu ZHANG, Zhihao XU, Xuyu WANG, Hongbing JI. Design of cobalt-nitrogen-carbon/activated carbon rich in zero valent cobalt active site and application of catalytic oxidation of formaldehyde [J]. CIESC Journal, 2023, 74(8): 3342-3352. |
| [7] | Xiaokun HE, Rui LIU, Yuan XUE, Ran ZUO. Review of gas phase and surface reactions in AlN MOCVD [J]. CIESC Journal, 2023, 74(7): 2800-2813. |
| [8] | Chao KANG, Jinpeng QIAO, Shengchao YANG, Chao PENG, Yuanpeng FU, Bin LIU, Jianrong LIU, Aleksandrova TATIANA, Chenlong DUAN. Research progress on activation extraction of valuable metals in coal gangue [J]. CIESC Journal, 2023, 74(7): 2783-2799. |
| [9] | Pan LI, Junyang MA, Zhihao CHEN, Li WANG, Yun GUO. Effect of the morphology of Ru/α-MnO2 on NH3-SCO performance [J]. CIESC Journal, 2023, 74(7): 2908-2918. |
| [10] | Bin LI, Zhenghu XU, Shuang JIANG, Tianyong ZHANG. Clean and efficient synthesis of accelerator CBS by hydrogen peroxide catalytic oxidation method [J]. CIESC Journal, 2023, 74(7): 2919-2925. |
| [11] | Yuming TU, Gaoyan SHAO, Jianjie CHEN, Feng LIU, Shichao TIAN, Zhiyong ZHOU, Zhongqi REN. Advances in the design, synthesis and application of calcium-based catalysts [J]. CIESC Journal, 2023, 74(7): 2717-2734. |
| [12] | Xueyan WEI, Yong QIAN. Experimental study on the low to medium temperature oxidation characteristics and kinetics of micro-size iron powder [J]. CIESC Journal, 2023, 74(6): 2624-2638. |
| [13] | Chen WANG, Xiufeng SHI, Xianfeng WU, Fangjia WEI, Haohong ZHANG, Yin CHE, Xu WU. Preparation of Mn3O4 catalyst by redox method and study on its catalytic oxidation performance and mechanism of toluene [J]. CIESC Journal, 2023, 74(6): 2447-2457. |
| [14] | Jipeng ZHOU, Wenjun HE, Tao LI. Reaction engineering calculation of deactivation kinetics for ethylene catalytic oxidation over irregular-shaped catalysts [J]. CIESC Journal, 2023, 74(6): 2416-2426. |
| [15] | Xu GUO, Yongzheng ZHANG, Houbing XIA, Na YANG, Zhenzhen ZHU, Jingyao QI. Research progress in the removal of water pollutants by carbon-based materials via electrooxidation [J]. CIESC Journal, 2023, 74(5): 1862-1874. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||