CIESC Journal ›› 2024, Vol. 75 ›› Issue (11): 4048-4064.DOI: 10.11949/0438-1157.20240753
• Reviews and monographs • Previous Articles Next Articles
Yue MA( ), Dong CAO, Daojian CHENG(
), Dong CAO, Daojian CHENG( )
)
Received:2024-07-03
Revised:2024-09-17
Online:2024-12-26
Published:2024-11-25
Contact:
Daojian CHENG
通讯作者:
程道建
作者简介:马越(2001—),男,硕士研究生,897737462@qq.com
基金资助:CLC Number:
Yue MA, Dong CAO, Daojian CHENG. General synthesis and application of atomically dispersed catalysts[J]. CIESC Journal, 2024, 75(11): 4048-4064.
马越, 曹东, 程道建. 原子级分散催化剂的通用合成及其在热催化中的应用[J]. 化工学报, 2024, 75(11): 4048-4064.
Add to citation manager EndNote|Ris|BibTeX
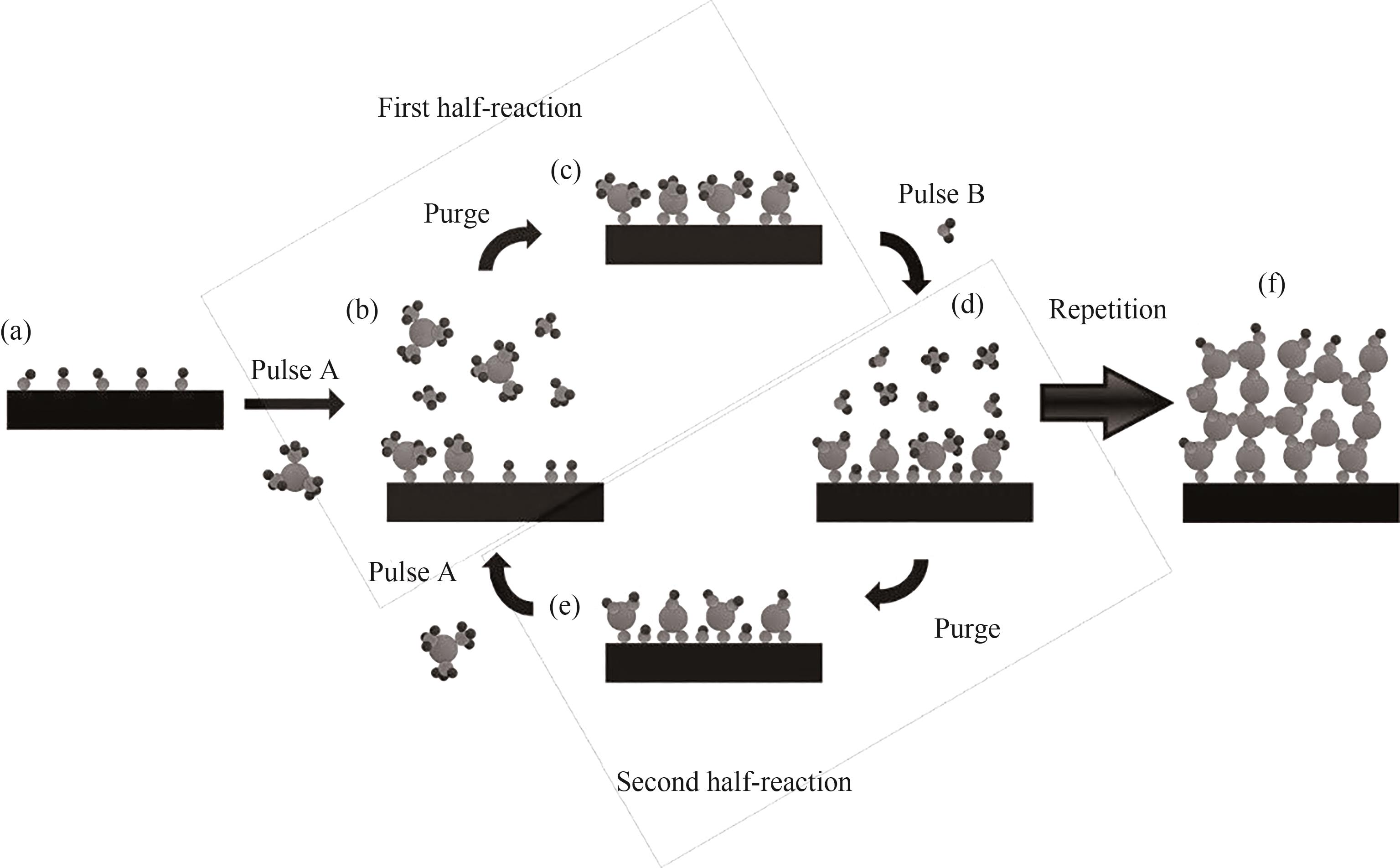
Fig.4 Schematic representation of the ALD technique using a binary (AB) precursor system: (a) Substrate with all active sites available; (b)Exposure to the first precursor vapor; (c) Purging of residual precursor molecules and byproducts; (d) Exposure to the second precursor vapor (counter-reactant); (e) Purging of the residuals of the second precursor vapor and byproducts; (f) Film resulting from several ALD cycles [34]
| 催化剂 | 反应体系 | 反应温度/℃ | 反应压力/MPa | 转化率/% | 选择性/% | TOF/h-1 | 正异比 |
|---|---|---|---|---|---|---|---|
| Pd-SAs [ | 乙炔选择性加氢 | 120 | — | 96.0 | 93.4 | — | — |
| ISA-Pd/MPNC [ | 乙炔选择性加氢 | 110 | — | 83 | 81 | — | — |
| 0.01%-Pd1/ZnO [ | 乙炔选择性加氢 | 80 | — | 100 | 80 | — | — |
| 0.2Pt/m-Al2O3-H2 [ | 苯乙酮加氢 | 50 | — | 69.3 | 98.7 | — | — |
| 0.2Pt/m-Al2O3-H2 [ | 1,3-丁二烯加氢 | 100 | 1.0 | 92.6 | 100 | 432 | — |
| 0.2Pt/m-Al2O3-H2 [ | 硝基苯选择性加氢 | 25 | 1.0 | 99.8 | 100 | — | — |
| Fe1/C-N [ | 硝基苯选择性加氢 | 60 | — | 99 | 99 | 748 | — |
| Pt1@MIL [ | CO2选择性加氢 | 150 | — | — | 90.3 | 117 | — |
| Pt n @MIL [ | CO2选择性加氢 | 150 | — | — | 13.3 | 20.9 | — |
Table 1 Summary of the performance of atomically dispersed catalysts in selective hydrogenation
| 催化剂 | 反应体系 | 反应温度/℃ | 反应压力/MPa | 转化率/% | 选择性/% | TOF/h-1 | 正异比 |
|---|---|---|---|---|---|---|---|
| Pd-SAs [ | 乙炔选择性加氢 | 120 | — | 96.0 | 93.4 | — | — |
| ISA-Pd/MPNC [ | 乙炔选择性加氢 | 110 | — | 83 | 81 | — | — |
| 0.01%-Pd1/ZnO [ | 乙炔选择性加氢 | 80 | — | 100 | 80 | — | — |
| 0.2Pt/m-Al2O3-H2 [ | 苯乙酮加氢 | 50 | — | 69.3 | 98.7 | — | — |
| 0.2Pt/m-Al2O3-H2 [ | 1,3-丁二烯加氢 | 100 | 1.0 | 92.6 | 100 | 432 | — |
| 0.2Pt/m-Al2O3-H2 [ | 硝基苯选择性加氢 | 25 | 1.0 | 99.8 | 100 | — | — |
| Fe1/C-N [ | 硝基苯选择性加氢 | 60 | — | 99 | 99 | 748 | — |
| Pt1@MIL [ | CO2选择性加氢 | 150 | — | — | 90.3 | 117 | — |
| Pt n @MIL [ | CO2选择性加氢 | 150 | — | — | 13.3 | 20.9 | — |
| 催化剂 | 反应体系 | 反应温度/℃ | 反应压力/MPa | 转化率/% | 选择性/% | TOF/h-1 | 正异比 |
|---|---|---|---|---|---|---|---|
| Rh/2.9ReO x -Al2O3 [ | 乙烯羰基化反应 | 150 | 0.1 | — | 33.3 | 10.8 | — |
| Rh-CPOL-1bp&10P [ | 丙烯羰基化反应 | 70 | 0.5 | — | 93 | 1209 | 24.2 |
| Rh@POP-PTBA-HA-50 [ | 丙烯羰基化反应 | 120 | 6 | 83 | — | 396 | 5.67 |
| Rh@POP-PTBA-HA-50 [ | 1-己烯羰基化反应 | 120 | 6 | 91 | — | 434 | 10.1 |
| Rh@POP-PTBA-HA-50 [ | 1-辛烯羰基化反应 | 120 | 6 | 88 | 99 | 801 | 11.5 |
Table 2 Summary of the performance of atomically dispersed catalysts in hydroformylation
| 催化剂 | 反应体系 | 反应温度/℃ | 反应压力/MPa | 转化率/% | 选择性/% | TOF/h-1 | 正异比 |
|---|---|---|---|---|---|---|---|
| Rh/2.9ReO x -Al2O3 [ | 乙烯羰基化反应 | 150 | 0.1 | — | 33.3 | 10.8 | — |
| Rh-CPOL-1bp&10P [ | 丙烯羰基化反应 | 70 | 0.5 | — | 93 | 1209 | 24.2 |
| Rh@POP-PTBA-HA-50 [ | 丙烯羰基化反应 | 120 | 6 | 83 | — | 396 | 5.67 |
| Rh@POP-PTBA-HA-50 [ | 1-己烯羰基化反应 | 120 | 6 | 91 | — | 434 | 10.1 |
| Rh@POP-PTBA-HA-50 [ | 1-辛烯羰基化反应 | 120 | 6 | 88 | 99 | 801 | 11.5 |
| 催化剂 | 反应体系 | 反应温度/℃ | 反应压力/MPa | 转化率/% | 选择性/% | TOF/h-1 | 正异比 |
|---|---|---|---|---|---|---|---|
| Pd/N-MSC-30 [ | 甲酸脱氢 | 60 | — | — | — | 8414 | — |
| Co1.0-N-C(800) [ | 甲酸脱氢 | — | — | — | — | 47.1 | — |
| R-PtNi/NiO@SiO2 [ | 氨硼烷脱氢 | — | — | — | — | 74418 | — |
| Pt1/Co3O4-c [ | 氨硼烷脱氢 | — | — | — | — | 362100 | — |
| Ni1/A-TiO2 [ | 丙烷脱氢 | 580 | 0.1 | — | 90 | 115 | — |
| Pt/Cu SAA [ | 丙烷脱氢 | 520 | 0.1 | — | 90 | 622.2 | — |
Table 3 Summary of the performance of atomically dispersed catalysts in dehydrogenation
| 催化剂 | 反应体系 | 反应温度/℃ | 反应压力/MPa | 转化率/% | 选择性/% | TOF/h-1 | 正异比 |
|---|---|---|---|---|---|---|---|
| Pd/N-MSC-30 [ | 甲酸脱氢 | 60 | — | — | — | 8414 | — |
| Co1.0-N-C(800) [ | 甲酸脱氢 | — | — | — | — | 47.1 | — |
| R-PtNi/NiO@SiO2 [ | 氨硼烷脱氢 | — | — | — | — | 74418 | — |
| Pt1/Co3O4-c [ | 氨硼烷脱氢 | — | — | — | — | 362100 | — |
| Ni1/A-TiO2 [ | 丙烷脱氢 | 580 | 0.1 | — | 90 | 115 | — |
| Pt/Cu SAA [ | 丙烷脱氢 | 520 | 0.1 | — | 90 | 622.2 | — |
| 1 | Wang Y, Wang D S, Li Y D. Rational design of single-atom site electrocatalysts: from theoretical understandings to practical applications[J]. Advanced Materials, 2021, 33(34): e2008151. |
| 2 | Li R Z, Wang D S. Understanding the structure-performance relationship of active sites at atomic scale[J]. Nano Research, 2022, 15(8): 6888-6923. |
| 3 | He F, Li Y L. Advances on theory and experiments of the energy applications in graphdiyne[J]. CCS Chemistry, 2023, 5(1): 72-94. |
| 4 | Li Z, Ji S F, Liu Y W, et al. Well-defined materials for heterogeneous catalysis: from nanoparticles to isolated single-atom sites[J]. Chemical Reviews, 2020, 120(2): 623-682. |
| 5 | Yang J R, Li W H, Tang H T, et al. CO2-mediated organocatalytic chlorine evolution under industrial conditions[J]. Nature, 2023, 617(7961): 519-523. |
| 6 | Zheng X B, Li B B, Wang Q S, et al. Emerging low-nuclearity supported metal catalysts with atomic level precision for efficient heterogeneous catalysis[J]. Nano Research, 2022, 15(9): 7806-7839. |
| 7 | Chen Y, Lin J, Jia B H, et al. Isolating single and few atoms for enhanced catalysis[J]. Advanced Materials, 2022, 34(39): e2201796. |
| 8 | Wang A Q, Li J, Zhang T. Heterogeneous single-atom catalysis[J]. Nature Reviews Chemistry, 2018, 2: 65-81. |
| 9 | Zhu R, Zheng W Q, Yan R, et al. Modulating bond interactions and interface microenvironments between polysulfide and catalysts toward advanced metal-sulfur batteries[J]. Advanced Functional Materials, 2022, 32(45): 2207021. |
| 10 | Li W H, Yang J R, Wang D S, et al. Striding the threshold of an atom era of organic synthesis by single-atom catalysis[J]. Chem, 2022, 8(1): 119-140. |
| 11 | Gao Y, Wang D S. Atomically dispersed catalysts: precise synthesis, structural regulation, and structure-activity relationship[J]. CCS Chemistry, 2024, 6(4): 833-855. |
| 12 | Zhang N Q, Ye C L, Yan H, et al. Single-atom site catalysts for environmental catalysis[J]. Nano Research, 2020, 13(12): 3165-3182. |
| 13 | Chandio I, Ai Y J, Wu L, et al. Recent progress in MOFs-based nanozymes for biosensing[J]. Nano Research, 2024, 17(1): 39-64. |
| 14 | Gan T, Wang D S. Atomically dispersed materials: ideal catalysts in atomic era[J]. Nano Research, 2024, 17(1): 18-38. |
| 15 | Zhu P, Xiong X, Wang D S. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction[J]. Nano Research, 2022, 15(7): 5792-5815. |
| 16 | Cao T, Lin R, Liu S J, et al. Atomically dispersed Ni anchored on polymer-derived mesh-like N-doped carbon nanofibers as an efficient CO2 electrocatalytic reduction catalyst[J]. Nano Research, 2022, 15(5): 3959-3963. |
| 17 | Chang B S, Zhang L Q, Wu S L, et al. Engineering single-atom catalysts toward biomedical applications[J]. Chemical Society Reviews, 2022, 51(9): 3688-3734. |
| 18 | Wang B Q, Cheng C, Jin M M, et al. A site distance effect induced by reactant molecule matchup in single-atom catalysts for Fenton-like reactions[J]. Angewandte Chemie International Edition, 2022, 61(33): e202207268. |
| 19 | Wang G, Wu Y, Li Z J, et al. Engineering a copper single-atom electron bridge to achieve efficient photocatalytic CO2 conversion[J]. Angewandte Chemie International Edition, 2023, 62(13): e202218460. |
| 20 | Zhou M, Wang Z Q, Mei A H, et al. Photocatalytic CO2 reduction using La-Ni bimetallic sites within a covalent organic framework[J]. Nature Communications, 2023, 14(1): 2473. |
| 21 | Gao Y, Liu B Z, Wang D S. Microenvironment engineering of single/dual-atom catalysts for electrocatalytic application[J]. Advanced Materials, 2023, 35(31): e2209654. |
| 22 | Qin R X, Liu P X, Fu G, et al. Strategies for stabilizing atomically dispersed metal catalysts[J]. Small Methods, 2018, 2(1): 1700286. |
| 23 | Zhuang Z C, Xia L X, Huang J Z, et al. Continuous modulation of electrocatalytic oxygen reduction activities of single-atom catalysts through p-n junction rectification[J]. Angewandte Chemie International Edition, 2023, 135(5): e202212335. |
| 24 | Marcinkowski M D, Darby M T, Liu J L, et al. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C—H activation[J]. Nature Chemistry, 2018, 10(3): 325-332. |
| 25 | Nie L, Mei D H, Xiong H F, et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation [J]. Science, 2017, 358(6369): 1419-1423. |
| 26 | Yang H Z, Shang L, Zhang Q H, et al. A universal ligand mediated method for large scale synthesis of transition metal single atom catalysts[J]. Nature Communications, 2019, 10(1): 4585. |
| 27 | Guo J, Yan X M, Liu Q, et al. The synthesis and synergistic catalysis of iron phthalocyanine and its graphene-based axial complex for enhanced oxygen reduction[J]. Nano Energy, 2018, 46: 347-355. |
| 28 | Fei H L, Dong J C, Arellano-Jiménez M J, et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation[J]. Nature Communications, 2015, 6: 8668. |
| 29 | Fei H L, Dong J C, Feng Y X, et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities[J]. Nature Catalysis, 2018, 1: 63-72. |
| 30 | Pan Y, Lin R, Chen Y J, et al. Design of single-atom co-N5 catalytic site: a robust electrocatalyst for CO2 reduction with nearly 100% CO selectivity and remarkable stability[J]. Journal of the American Chemical Society, 2018, 140(12): 4218-4221. |
| 31 | Tian S B, Fu Q, Chen W X, et al. Carbon nitride supported Fe2 cluster catalysts with superior performance for alkene epoxidation[J]. Nature Communications, 2018, 9(1): 2353. |
| 32 | Xie S H, Tsunoyama H, Kurashige W, et al. Enhancement in aerobic alcohol oxidation catalysis of Au25 clusters by single Pd atom doping[J]. ACS Catalysis, 2012, 2(7): 1519-1523. |
| 33 | 高亚, 徐丹, 王树元, 等. 原子层沉积构建高性能催化剂的研究进展[J]. 化工进展, 2021, 40(8): 4242-4252. |
| Gao Y, Xu D, Wang S Y, et al. Recent progress in fabrication of high efficient catalysts by atomic layer deposition[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4242-4252. | |
| 34 | Fonseca J, Lu J L. Single-atom catalysts designed and prepared by the atomic layer deposition technique[J]. ACS Catalysis, 2021, 11(12): 7018-7059. |
| 35 | Sun S H, Zhang G X, Gauquelin N, et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition[J]. Scientific Reports, 2013, 3: 1775. |
| 36 | Yan H, Lin Y, Wu H, et al. Bottom-up precise synthesis of stable platinum dimers on graphene[J]. Nature Communications, 2017, 8(1): 1070. |
| 37 | Zhang L, Si R T, Liu H S, et al. Atomic layer deposited Pt-Ru dual-metal dimers and identifying their active sites for hydrogen evolution reaction[J]. Nature Communications, 2019, 10(1): 4936. |
| 38 | Yi F Y, Zhang R, Wang H L, et al. Metal-organic frameworks and their composites: synthesis and electrochemical applications[J]. Small Methods, 2017, 1(11): 1700187. |
| 39 | Yang H B, Hung S F, Liu S, et al. Atomically dispersed Ni(Ⅰ) as the active site for electrochemical CO2 reduction[J]. Nature Energy, 2018, 3: 140-147. |
| 40 | Zhao L, Zhang Y, Huang L B, et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts[J]. Nature Communications, 2019, 10(1): 1278. |
| 41 | Jiao L, Wang Y, Jiang H L, et al. Metal-organic frameworks as platforms for catalytic applications[J]. Advanced Materials, 2018, 30(37): e1703663. |
| 42 | Wang X, Chen W X, Zhang L, et al. Uncoordinated amine groups of metal-organic frameworks to anchor single Ru sites as chemoselective catalysts toward the hydrogenation of quinoline[J]. Journal of the American Chemical Society, 2017, 139(28): 9419-9422. |
| 43 | Chen W X, Pei J J, He C T, et al. Single tungsten atoms supported on MOF-derived N-doped carbon for robust electrochemical hydrogen evolution[J]. Advanced Materials, 2018, 30(30): e1800396. |
| 44 | Geng Z G, Liu Y, Kong X D, et al. Achieving a record-high yield rate of 120.9 for N2 electrochemical reduction over Ru single-atom catalysts[J]. Advanced Materials, 2018, 30(40): 1803498. |
| 45 | Yin P Q, Yao T, Wu Y E, et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts[J]. Angewandte Chemie International Edition, 2016, 55(36): 10800-10805. |
| 46 | Qu Y T, Li Z J, Chen W X, et al. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms[J]. Nature Catalysis, 2018, 1: 781-786. |
| 47 | Chen Z G, Xu Y F, Ding D, et al. Thermal migration towards constructing W-W dual-sites for boosted alkaline hydrogen evolution reaction[J]. Nature Communications, 2022, 13(1): 763. |
| 48 | 郭子薇, 刘运浔, 李烨, 等. 电化学沉积法制备纳米铂催化剂的定量分析[J]. 广州化工, 2023, 51(20): 66-68. |
| Guo Z W, Liu Y X, Li Y, et al. Quantitative analysis of nano-Pt catalysts prepared by electrochemical deposition method[J]. Guangzhou Chemical Industry, 2023, 51(20): 66-68. | |
| 49 | 陈家一, 高帷韬, 阴亚楠, 等. 电化学沉积法制备质子交换膜燃料电池催化剂[J]. 化工进展, 2024, 43(4): 1796-1809. |
| Chen J Y, Gao W T, Yin Y N, et al. Preparation of PEMFC catalysts by electrodeposition[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1796-1809. | |
| 50 | Zhang Z R, Feng C, Liu C X, et al. Electrochemical deposition as a universal route for fabricating single-atom catalysts[J]. Nature Communications, 2020, 11(1): 1215. |
| 51 | Cao D, Zhang Z R, Cui Y H, et al. One-step approach for constructing high-density single-atom catalysts toward overall water splitting at industrial current densities[J]. Angewandte Chemie International Edition, 2023, 62(9): e202214259. |
| 52 | Yang S X, Du R Q, Yu Y H, et al. One-step electrodeposition of carbon quantum dots and transition metal ions for N-doped carbon coupled with NiFe oxide clusters: a high-performance electrocatalyst for oxygen evolution[J]. Nano Energy, 2020, 77: 105057. |
| 53 | Zu X L, Li X D, Liu W, et al. Efficient and robust carbon dioxide electroreduction enabled by atomically dispersed Sn δ + sites[J]. Advanced Materials, 2019, 31(15): e1808135. |
| 54 | Yang Z K, Chen B X, Chen W X, et al. Directly transforming copper (Ⅰ) oxide bulk into isolated single-atom copper sites catalyst through gas-transport approach[J]. Nature Communications, 2019, 10: 3734. |
| 55 | Zhao Y X, Sun Q Y, Zhou X Y, et al. Scalable synthesis of Ir cluster anchored on porous hollow carbon nanobowls for enhancing pH-universal hydrogen evolution[J]. Small, 2023, 19(52): e2305343. |
| 56 | He X H, Deng Y C, Zhang Y, et al. Mechanochemical kilogram-scale synthesis of noble metal single-atom catalysts[J]. Cell Reports Physical Science, 2020, 1(1): 100004. |
| 57 | He X H, Zhang H, Zhang X C, et al. Building up libraries and production line for single atom catalysts with precursor-atomization strategy[J]. Nature Communications, 2022, 13(1): 5721. |
| 58 | Yan B, Song H L, Yang G W. A facile and green large-scale fabrication of single atom catalysts for high photocatalytic H2 evolution activity[J]. Chemical Engineering Journal, 2022, 427: 131795. |
| 59 | 陈志强, 车春霞, 吴登峰, 等. 乙炔选择性加氢催化剂研究进展[J]. 化工进展, 2022, 41(10): 5390-5405. |
| Chen Z Q, Che C X, Wu D F, et al. Advances in catalysts for selective hydrogenation of acetylene[J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5390-5405. | |
| 60 | Sun Z Y, Wang S, Chen W X. Metal single-atom catalysts for selective hydrogenation of unsaturated bonds[J]. Journal of Materials Chemistry A, 2021, 9(9): 5296-5319. |
| 61 | Armbrüster M, Kovnir K, Behrens M, et al. Pd-Ga intermetallic compounds as highly selective semihydrogenation catalysts[J]. Journal of the American Chemical Society, 2010, 132(42): 14745-14747. |
| 62 | Armbrüster M, Kovnir K, Friedrich M, et al. Al13Fe4 as a low-cost alternative for palladium in heterogeneous hydrogenation[J]. Nature Materials, 2012, 11(8): 690-693. |
| 63 | Sulman E M, Valetsky P M, Sulman M G, et al. Nanosized catalysts as a basis for intensifications of technologies[J]. Chemical Engineering and Processing: Process Intensification, 2011, 50(10): 1041-1053. |
| 64 | More S R, Yadav G D. Effect of supercritical CO2 as reaction medium for selective hydrogenation of acetophenone to 1-phenylethanol[J]. ACS Omega, 2018, 3(6): 7124-7132. |
| 65 | Huang X H, Xia Y J, Cao Y J, et al. Enhancing both selectivity and coking-resistance of a single-atom Pd1/C3N4 catalyst for acetylene hydrogenation[J]. Nano Research, 2017, 10(4): 1302-1312. |
| 66 | Qiao B T, Wang A Q, Yang X F, et al. Single-atom catalysis of CO oxidation using Pt1/FeO x [J]. Nature Chemistry, 2011, 3(8): 634-641. |
| 67 | Zhang X, Shi H, Xu B Q. Catalysis by gold: isolated surface Au3+ ions are active sites for selective hydrogenation of 1,3-butadiene over Au/ZrO2 catalysts[J]. Angewandte Chemie International Edition, 2005, 44(43): 7132-7135. |
| 68 | Zhuang Z C, Kang Q, Wang D S, et al. Single-atom catalysis enables long-life, high-energy lithium-sulfur batteries[J]. Nano Research, 2020, 13(7): 1856-1866. |
| 69 | Wei S J, Li A, Liu J C, et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms[J]. Nature Nanotechnology, 2018, 13(9): 856-861. |
| 70 | Feng Q C, Zhao S, Xu Q, et al. Mesoporous nitrogen-doped carbon-nanosphere-supported isolated single-atom Pd catalyst for highly efficient semihydrogenation of acetylene[J]. Advanced Materials, 2019, 31(36): e1901024. |
| 71 | Zhou H R, Yang X F, Wang A Q, et al. Pd/ZnO catalysts with different origins for high chemoselectivity in acetylene semi-hydrogenation[J]. Chinese Journal of Catalysis, 2016, 37(5): 692-699. |
| 72 | Zhang Z L, Zhu Y H, Asakura H, et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation[J]. Nature Communications, 2017, 8: 16100. |
| 73 | Cheong W C, Yang W J, Zhang J, et al. Isolated iron single-atomic site-catalyzed chemoselective transfer hydrogenation of nitroarenes to arylamines[J]. ACS Applied Materials & Interfaces, 2019, 11(37): 33819-33824. |
| 74 | Chen Y Z, Li H L, Zhao W H, et al. Optimizing reaction paths for methanol synthesis from CO2 hydrogenation via metal-ligand cooperativity[J]. Nature Communications, 2019, 10(1): 1885. |
| 75 | Liu B Y, Wang Y, Huang N, et al. Heterogeneous hydroformylation of alkenes by Rh-based catalysts[J]. Chem, 2022, 8(10): 2630-2658. |
| 76 | Franke R, Selent D, Börner A. Applied hydroformylation[J]. Chemical Reviews, 2012, 112(11): 5675-5732. |
| 77 | Peng J B, Geng H Q, Wu X F. The chemistry of CO: carbonylation[J]. Chem, 2019, 5(3): 526-552. |
| 78 | Ro I, Xu M J, Graham G W, et al. Synthesis of heteroatom Rh–ReO x atomically dispersed species on Al2O3 and their tunable catalytic reactivity in ethylene hydroformylation[J]. ACS Catalysis, 2019, 9(12): 10899-10912. |
| 79 | Li C Y, Yan L, Lu L L, et al. Single atom dispersed Rh-biphephos&PPh3@porous organic copolymers: highly efficient catalysts for continuous fixed-bed hydroformylation of propene[J]. Green Chemistry, 2016, 18(10): 2995-3005. |
| 80 | Zhao K, Wang H L, Wang X Z, et al. Confinement of atomically dispersed Rh catalysts within porous monophosphine polymers for regioselective hydroformylation of alkenes[J]. Journal of Catalysis, 2021, 401: 321-330. |
| 81 | Eppinger J, Huang K W. Formic acid as a hydrogen energy carrier[J]. ACS Energy Letters, 2017, 2(1): 188-195. |
| 82 | Bi Q Y, Du X L, Liu Y M, et al. Efficient subnanometric gold-catalyzed hydrogen generation via formic acid decomposition under ambient conditions[J]. Journal of the American Chemical Society, 2012, 134(21): 8926-8933. |
| 83 | Mori K, Miyawaki K, Yamashita H. Ru and Ru-Ni nanoparticles on TiO2 support as extremely active catalysts for hydrogen production from ammonia-borane[J]. ACS Catalysis, 2016, 6(5): 3128-3135. |
| 84 | Chandra M, Xu Q. Room temperature hydrogen generation from aqueous ammonia-borane using noble metal nano-clusters as highly active catalysts[J]. Journal of Power Sources, 2007, 168(1): 135-142. |
| 85 | Li Z P, Yang X C, Tsumori N, et al. Tandem nitrogen functionalization of porous carbon: toward immobilizing highly active palladium nanoclusters for dehydrogenation of formic acid[J]. ACS Catalysis, 2017, 7(4): 2720-2724. |
| 86 | Zhao X G, Wang Y P, Shang M W, et al. Mechanism difference between nanoparticles and single-atom sites on aqueous formic acid dehydrogenation over coblat catalyst[J]. Molecular Catalysis, 2022, 531: 112671. |
| 87 | Ge Y Z, Ye W Y, Shah Z H, et al. PtNi/NiO clusters coated by hollow sillica: novel design for highly efficient hydrogen production from ammonia-borane[J]. ACS Applied Materials & Interfaces, 2017, 9(4): 3749-3756. |
| 88 | Sun Q D, Wang X Y, Wang H, et al. Crystal facet effects of platinum single-atom catalysts in hydrolytic dehydrogenation of ammonia borane[J]. Journal of Materials Chemistry A, 2022, 10(20): 10837-10843. |
| 89 | Zhang Q, Jiang X Z, Su Y, et al. Catalytic propane dehydrogenation by anatase supported Ni single-atom catalysts[J]. Chinese Journal of Catalysis, 2024, 57: 105-113. |
| 90 | Sun G D, Zhao Z J, Mu R T, et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation[J]. Nature Communications, 2018, 9(1): 4454. |
| [1] | Meilin SHI, Lianda ZHAO, Xingjian DENG, Jingsong WANG, Haibin ZUO, Qingguo XUE. Research progress on catalytic methane reforming process [J]. CIESC Journal, 2024, 75(S1): 25-39. |
| [2] | Shuzhen WANG, Yuting WANG, Mengxi MA, Wei ZHANG, Jiangnan XIANG, Haiying LU, Yan WANG, Binbin FAN, Jiajun ZHENG, Weijiong DAI, Ruifeng LI. Synthesis of ZSM-22 molecular sieve by two-step crystallization and its hydroisomerization performance [J]. CIESC Journal, 2024, 75(9): 3176-3187. |
| [3] | Ran WANG, Huan WANG, Xiaoyun XIONG, Huimin GUAN, Yunfeng ZHENG, Cailin CHEN, Yucai QIN, Lijuan SONG. Visual analysis of mass transfer enhanced active site utilization efficiency of FCC catalyst [J]. CIESC Journal, 2024, 75(9): 3198-3209. |
| [4] | Yachao LIU, Xiaojie TAN, Xudong LI, Rui WANG, Hui WANG, Xuan HAN, Qingshan ZHAO. Synthesis of efficient cobalt carbonate nanosheets based on DES for oxygen evolution reaction [J]. CIESC Journal, 2024, 75(9): 3320-3328. |
| [5] | Mengting ZHANG, Shulin WANG, Xi SANG, Xinghao YUAN, Gang XU. Artificial Cu-TM1459 metalloenzyme catalyzes asymmetric Michael addition reaction [J]. CIESC Journal, 2024, 75(9): 3255-3265. |
| [6] | Shaojun DOU, Liang HAO. Mesoscale simulation of coupled gas charge transfer process in PEMFC catalyst layer [J]. CIESC Journal, 2024, 75(8): 3002-3010. |
| [7] | Yin WANG, Pengfei CHU, Hu LIU, Jing LYU, Shouying HUANG, Shengping WANG, Xinbin MA. Influence of aluminum sol with different pH on performance of shaped mordenite catalyst for dimethyl ether carbonylation [J]. CIESC Journal, 2024, 75(7): 2533-2543. |
| [8] | Lu YANG, Congcong LIU, Tongtong MENG, Boyuan ZHANG, Tengfei YANG, Wen’an DENG, Xiaobin WANG. Hydrogenation and coke-suppression performance of dispersed catalyst in coal/heavy oil co-processing reactions [J]. CIESC Journal, 2024, 75(7): 2556-2564. |
| [9] | Xusheng LIU, Zeyang LI, Yusen YANG, Min WEI. Research progress on electrocatalytic carbon dioxide reduction to gaseous products [J]. CIESC Journal, 2024, 75(7): 2385-2408. |
| [10] | Li LUO, Wenyao CHEN, Jing ZHANG, Gang QIAN, Xinggui ZHOU, Xuezhi DUAN. Alumina structure and surface property regulation for catalyzing methanol dehydration to dimethyl ether [J]. CIESC Journal, 2024, 75(7): 2522-2532. |
| [11] | Han ZHANG, Shuning ZHANG, Ke LIU, Guanlong DENG. Particle size prediction of cobalt oxalate synthesis process based on slow feature analysis and least squares support vector regression [J]. CIESC Journal, 2024, 75(6): 2313-2321. |
| [12] | Tianwen WANG, Su YAN, Mengyuan ZHAO, Tianrang YANG, Jianguo LIU. Mechanisms of chromium poisoning in solid oxide cell air electrodes and research advances in enhancing chromium-resistivity [J]. CIESC Journal, 2024, 75(6): 2091-2108. |
| [13] | Yu DING, Changze YANG, Jun LI, Huidong SUN, Hui SHANG. Research progress and prospects of atomic-scale molybdenum-based hydrodesulfurization catalysts [J]. CIESC Journal, 2024, 75(5): 1735-1749. |
| [14] | Tingting ZHAO, Lixiang YAN, Fuli TANG, Minzhi XIAO, Ye TAN, Liubin SONG, Zhongliang XIAO, Lingjun LI. Research progress on design strategies and reaction mechanisms of photo-assisted Li-CO2 battery catalysts [J]. CIESC Journal, 2024, 75(5): 1750-1764. |
| [15] | Jinhong MO, Xue HAN, Yixiang ZHU, Jing LI, Xuyu WANG, Hongbing JI. Investigation of Pt-Ga/CeO2-ZrO2-Al2O3 bifunctional catalyst for the catalytic conversion of n-butane into olefins [J]. CIESC Journal, 2024, 75(5): 1855-1869. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||