CIESC Journal ›› 2025, Vol. 76 ›› Issue (3): 909-921.DOI: 10.11949/0438-1157.20240830
• Reviews and monographs • Next Articles
Jing ZHANG1,2( ), Yue YUAN1(
), Yue YUAN1( ), Yanmei LIU1, Zhiwen WANG1, Tao CHEN1(
), Yanmei LIU1, Zhiwen WANG1, Tao CHEN1( )
)
Received:2024-07-22
Revised:2024-10-11
Online:2025-03-28
Published:2025-03-25
Contact:
Tao CHEN
张静1,2( ), 元跃1(
), 元跃1( ), 刘艳梅1, 王智文1, 陈涛1(
), 刘艳梅1, 王智文1, 陈涛1( )
)
通讯作者:
陈涛
作者简介:张静(1989—),女,博士研究生,讲师,jingzhang052018@163.com基金资助:CLC Number:
Jing ZHANG, Yue YUAN, Yanmei LIU, Zhiwen WANG, Tao CHEN. Advance on the preparation of itaconic acid by biological method[J]. CIESC Journal, 2025, 76(3): 909-921.
张静, 元跃, 刘艳梅, 王智文, 陈涛. 生物法制备衣康酸研究进展[J]. 化工学报, 2025, 76(3): 909-921.
Add to citation manager EndNote|Ris|BibTeX
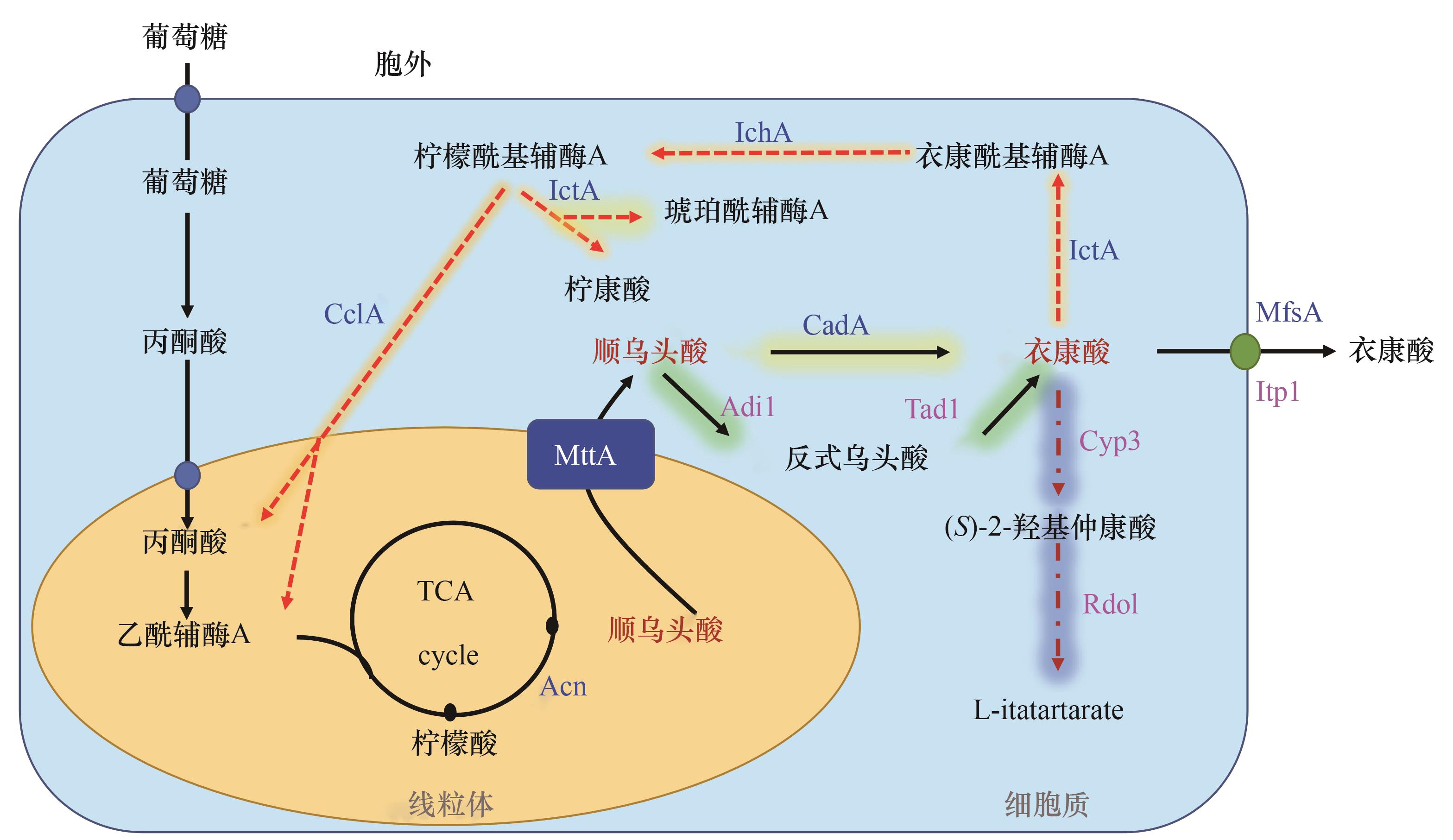
Fig.2 Simplified diagram of itaconic acid biosynthesis and degradation pathways in fungi (enzymes specific to A. terreus are indicated in blue, U. maydis in rose red)
| 菌株 | 策略 | 碳源 | 发酵 方式 | 产量/(g/L) | 得率/(g/g) | 文献 |
|---|---|---|---|---|---|---|
| A. terreus NRRL 265 | 深层发酵 | 蔗糖 | 发酵罐 | 48.7 | — | [ |
| A. terreus NRRL 1960 | 孢子或菌丝固定化发酵 | 木糖和葡萄糖 | 摇瓶 | 30 | 0.54 | [ |
| A. terreus NRRL 1960 | 优化培养基组成包括底物和多种微量元素浓度 | 葡萄糖 | 发酵罐 | 91 | — | [ |
| A. terreus NRRL 1960 | 优化锰离子和葡萄糖浓度 | 葡萄糖 | 发酵罐 | 130 | 0.65 | [ |
| A. terreus DSM 23081 | 培养2.1 d后将pH控制至3 | 葡萄糖 | 发酵罐 | 146 | 0.59 | [ |
| A. terreus DSM 23081 | 将生产阶段pH提高至3.4 | 葡萄糖 | 发酵罐 | 160 150 | 0.46 0.56 | [ |
| A. terreus DSM 23081 | 麦糠进行碱性预处理,50℃、pH 4.8下用10 FPU/g生物量 Biogazyme 2x进行酶糖化,再通过阳离子交换剂进行纯化后用于衣康酸发酵合成 | 麦糠 | 摇瓶 | 27.7 | 0.41 | [ |
| A. terreus NRRL 1960 | 纤维素浆水解液用膜过滤灭菌、碳氮比为20∶1和氧可用性为7.33 (空气体积/培养基体积) | 纤维素浆 水解液 | 摇瓶 | 37.5 | 0.62 | [ |
| A. terreus A156 | 过表达来源于Aspergillus niger的截短的pfkA | 葡萄糖 | 摇瓶 | 31 | — | [ |
| A. terreus LYT10 | 过表达cadA | 葡萄糖 | 发酵罐 | 78.5 | 0.67 | [ |
| A. terreus AN37 | 过表达本源cadA和mfsA突变体 | 葡萄糖 | 摇瓶 | 75 | 0.68 | [ |
| A. terreus CICC 40205 | 过表达A. niger来源的glaA | 淀粉水解物 | 摇瓶 | 77.6 | — | [ |
A. pseudoterreus ATCC32359 | 过表达全局调节因子LaeA | 葡萄糖 | 摇瓶 | 30.4 | — | [ |
| U. zeae | 野生型 | — | — | 15 | — | [ |
| U. maydis MB215 | 优化葡萄糖浓度和NH4Cl浓度 | 葡萄糖 | 发酵罐 | 44.5 | 0.24 | [ |
| U. maydis MB215 | 敲除cyp3并利用启动子P etef 过表达ria1 | 葡萄糖 | 发酵罐 | 54.8 | 0.48 | [ |
| U. maydis MB215 | 敲除cyp3、MEL、UA和dgat并过表达ria1 | 葡萄糖 | 摇瓶 | 53.5 | 0.47 | [ |
| U. maydis MB215 | 过表达本源rai1和mttA并敲除cyp3、fuz7、MEL、UA和dgat | 葡萄糖 | 发酵罐 | 74.9 | 0.54 | [ |
| U. maydis MB215 | 过表达本源rai1和mttA并敲除cyp3和fuz7 | 葡萄糖 | 发酵罐 | 220 | 0.33 | [ |
| U. cynodontis NBRC9727 | 过表达本源rai1和mttA并敲除cyp3和fuz7 | 葡萄糖 | 发酵罐 | 22.3 | 0.42 | [ |
| Candida sp. strain B-1 | 野生型 | 葡萄糖 | 摇瓶 | 35 | — | [ |
| P. antarctica NRRL Y-7808 | 野生型 | 葡萄糖 | 摇瓶 | 30 | 0.29 | [ |
| P. tsukubaensis H488 | 野生型 | 葡萄糖 | 发酵罐 | 74.7 | 0.49 | [ |
| H. mompa TANAKA | 野生型 | 甘薯 | 摇瓶 | 0.5 | — | [ |
Table 1 Itaconic acid production by fermentation of natural microorganisms
| 菌株 | 策略 | 碳源 | 发酵 方式 | 产量/(g/L) | 得率/(g/g) | 文献 |
|---|---|---|---|---|---|---|
| A. terreus NRRL 265 | 深层发酵 | 蔗糖 | 发酵罐 | 48.7 | — | [ |
| A. terreus NRRL 1960 | 孢子或菌丝固定化发酵 | 木糖和葡萄糖 | 摇瓶 | 30 | 0.54 | [ |
| A. terreus NRRL 1960 | 优化培养基组成包括底物和多种微量元素浓度 | 葡萄糖 | 发酵罐 | 91 | — | [ |
| A. terreus NRRL 1960 | 优化锰离子和葡萄糖浓度 | 葡萄糖 | 发酵罐 | 130 | 0.65 | [ |
| A. terreus DSM 23081 | 培养2.1 d后将pH控制至3 | 葡萄糖 | 发酵罐 | 146 | 0.59 | [ |
| A. terreus DSM 23081 | 将生产阶段pH提高至3.4 | 葡萄糖 | 发酵罐 | 160 150 | 0.46 0.56 | [ |
| A. terreus DSM 23081 | 麦糠进行碱性预处理,50℃、pH 4.8下用10 FPU/g生物量 Biogazyme 2x进行酶糖化,再通过阳离子交换剂进行纯化后用于衣康酸发酵合成 | 麦糠 | 摇瓶 | 27.7 | 0.41 | [ |
| A. terreus NRRL 1960 | 纤维素浆水解液用膜过滤灭菌、碳氮比为20∶1和氧可用性为7.33 (空气体积/培养基体积) | 纤维素浆 水解液 | 摇瓶 | 37.5 | 0.62 | [ |
| A. terreus A156 | 过表达来源于Aspergillus niger的截短的pfkA | 葡萄糖 | 摇瓶 | 31 | — | [ |
| A. terreus LYT10 | 过表达cadA | 葡萄糖 | 发酵罐 | 78.5 | 0.67 | [ |
| A. terreus AN37 | 过表达本源cadA和mfsA突变体 | 葡萄糖 | 摇瓶 | 75 | 0.68 | [ |
| A. terreus CICC 40205 | 过表达A. niger来源的glaA | 淀粉水解物 | 摇瓶 | 77.6 | — | [ |
A. pseudoterreus ATCC32359 | 过表达全局调节因子LaeA | 葡萄糖 | 摇瓶 | 30.4 | — | [ |
| U. zeae | 野生型 | — | — | 15 | — | [ |
| U. maydis MB215 | 优化葡萄糖浓度和NH4Cl浓度 | 葡萄糖 | 发酵罐 | 44.5 | 0.24 | [ |
| U. maydis MB215 | 敲除cyp3并利用启动子P etef 过表达ria1 | 葡萄糖 | 发酵罐 | 54.8 | 0.48 | [ |
| U. maydis MB215 | 敲除cyp3、MEL、UA和dgat并过表达ria1 | 葡萄糖 | 摇瓶 | 53.5 | 0.47 | [ |
| U. maydis MB215 | 过表达本源rai1和mttA并敲除cyp3、fuz7、MEL、UA和dgat | 葡萄糖 | 发酵罐 | 74.9 | 0.54 | [ |
| U. maydis MB215 | 过表达本源rai1和mttA并敲除cyp3和fuz7 | 葡萄糖 | 发酵罐 | 220 | 0.33 | [ |
| U. cynodontis NBRC9727 | 过表达本源rai1和mttA并敲除cyp3和fuz7 | 葡萄糖 | 发酵罐 | 22.3 | 0.42 | [ |
| Candida sp. strain B-1 | 野生型 | 葡萄糖 | 摇瓶 | 35 | — | [ |
| P. antarctica NRRL Y-7808 | 野生型 | 葡萄糖 | 摇瓶 | 30 | 0.29 | [ |
| P. tsukubaensis H488 | 野生型 | 葡萄糖 | 发酵罐 | 74.7 | 0.49 | [ |
| H. mompa TANAKA | 野生型 | 甘薯 | 摇瓶 | 0.5 | — | [ |
| 菌株 | 策略 | 碳源 | 发酵方式 | 产量/(g/L) | 得率/(g/g) | 文献 |
|---|---|---|---|---|---|---|
| A. niger AB 1.13 | 过表达异源cadA | 葡萄糖 | 摇瓶 | 0.7 | 0.01 | [ |
| A. niger ATCC 1015 | 线粒体定位表达acoA和cadA | 葡萄糖 | 摇瓶 | 1.2 | — | [ |
| A. niger ATCC 1015 | 过表达mttA、mfsA、adi1、tad1、itp1、acoA并敲除ictA | 葡萄糖 | 摇瓶 | 9.08 | 0.09 | [ |
| A. niger YX-1217 | 过表达由P glaA 启动子调控的下肽接头连接的acoA和cadA | 玉米粉 | 发酵罐 | 7.2 | — | [ |
| A. niger NW186 | oahA和goxC突变,过表达异源cadA、mttA 和mfsA | 山梨醇和木糖 | 发酵罐 | 7.1 | — | [ |
| A. niger AB 1.13 | 过表达citB、cadA、mttA和mfsA | 葡萄糖 | 发酵罐 | 26.2 | 0.37 | [ |
| A. niger AB 1.13 | 过表达acl12、citB、cadA、mttA和mfsA | 葡萄糖 | 发酵罐 | 42.7 | 0.26 | [ |
| S. cerevisiae BY4741 | 过表达A. terreus来源的cadA | 葡萄糖 | 摇瓶 | 0.168 | — | [ |
| S. cerevisiae XYY27 | 过表达cadA、cyc1t和AAC2 | 乙醇 | 摇瓶 | 0.142 | — | [ |
| P. kudriavzevii YB4010 | 过表达异源cadA、本源pk和mttA并敲除icd | 葡萄糖 | 发酵罐 | 1.23 | 0.029 | [ |
| Y. lipolytica PO1f | 胞浆中共表达cadA和ACOnoMLS | 葡萄糖 | 发酵罐 | 4.6 | 0.058 | [ |
| Y. lipolytica PO1f | 过表达A. terreus来源的cadA、mttA、mfsA和acoA | 葡萄糖 | 发酵罐 | 22 | 0.056 | [ |
| Y. lipolytica Po1g | 过氧化物酶体中表达cadA,过表达POT1并敲除icl | 废弃食用油 | 发酵罐 | 54.6 | 0.3 | [ |
| C. glutamicum | 过表达cadA与MBP的融合基因;突变异柠檬酸脱氢酶基因 | 葡萄糖 | 摇瓶 | 7.8 | 0.29 | [ |
| C. glutamicum | 过表达异源的cadA并突变icd | 乙酸盐 | 发酵罐 | 29.2 | 0.16 | [ |
| E. coli BW25113 | 过表达异源的cadA和acnB,敲除icd | 葡萄糖 | 摇瓶 | 4.34 | — | [ |
| E. coli XL1-Blue | 过表达高可溶性表达的同义密码子变体scv_cadA | 甘油 | 发酵罐 | 7.2 | — | [ |
E. coli MG1655 (ita23) | 过表达cadA和gltA,下调icd,敲除aceA、sucCD、pta、pykA和pykF | 葡萄糖 | 发酵罐 | 32 | 0.43 | [ |
E. coli MG1655 (ita36A) | 过表达cadA和gltA,替换icd启动子为λ启动子,敲除aceA、sucCD、pta、pykA和pykF | 葡萄糖 | 发酵罐 | 47 | 0.45 | [ |
| E. coli MG1655 | 过表达由GA、ACN和CAD组成的多酶复合体,敲除icd | 葡萄糖 | 摇瓶 | 3.06 | — | [ |
| N. crassa FGSC9720 | 过表达异源的cadA | 玉米秸秆 | 摇瓶 | 0.02 | — | [ |
| P. putida | 过表达由生物传感器PurtA∶T7pol∶lysY+调控的cadA,敲除PHA合成酶 | 碱预处理 木质素 | 摇瓶 | 1.3 | — | [ |
| Synechocystis sp. PCC6803 | 过表达异源的cadA | CO2 | 摇瓶 | 0.014 | — | [ |
Table 2 Itaconic acid production by fermentation of heterologous microorganisms
| 菌株 | 策略 | 碳源 | 发酵方式 | 产量/(g/L) | 得率/(g/g) | 文献 |
|---|---|---|---|---|---|---|
| A. niger AB 1.13 | 过表达异源cadA | 葡萄糖 | 摇瓶 | 0.7 | 0.01 | [ |
| A. niger ATCC 1015 | 线粒体定位表达acoA和cadA | 葡萄糖 | 摇瓶 | 1.2 | — | [ |
| A. niger ATCC 1015 | 过表达mttA、mfsA、adi1、tad1、itp1、acoA并敲除ictA | 葡萄糖 | 摇瓶 | 9.08 | 0.09 | [ |
| A. niger YX-1217 | 过表达由P glaA 启动子调控的下肽接头连接的acoA和cadA | 玉米粉 | 发酵罐 | 7.2 | — | [ |
| A. niger NW186 | oahA和goxC突变,过表达异源cadA、mttA 和mfsA | 山梨醇和木糖 | 发酵罐 | 7.1 | — | [ |
| A. niger AB 1.13 | 过表达citB、cadA、mttA和mfsA | 葡萄糖 | 发酵罐 | 26.2 | 0.37 | [ |
| A. niger AB 1.13 | 过表达acl12、citB、cadA、mttA和mfsA | 葡萄糖 | 发酵罐 | 42.7 | 0.26 | [ |
| S. cerevisiae BY4741 | 过表达A. terreus来源的cadA | 葡萄糖 | 摇瓶 | 0.168 | — | [ |
| S. cerevisiae XYY27 | 过表达cadA、cyc1t和AAC2 | 乙醇 | 摇瓶 | 0.142 | — | [ |
| P. kudriavzevii YB4010 | 过表达异源cadA、本源pk和mttA并敲除icd | 葡萄糖 | 发酵罐 | 1.23 | 0.029 | [ |
| Y. lipolytica PO1f | 胞浆中共表达cadA和ACOnoMLS | 葡萄糖 | 发酵罐 | 4.6 | 0.058 | [ |
| Y. lipolytica PO1f | 过表达A. terreus来源的cadA、mttA、mfsA和acoA | 葡萄糖 | 发酵罐 | 22 | 0.056 | [ |
| Y. lipolytica Po1g | 过氧化物酶体中表达cadA,过表达POT1并敲除icl | 废弃食用油 | 发酵罐 | 54.6 | 0.3 | [ |
| C. glutamicum | 过表达cadA与MBP的融合基因;突变异柠檬酸脱氢酶基因 | 葡萄糖 | 摇瓶 | 7.8 | 0.29 | [ |
| C. glutamicum | 过表达异源的cadA并突变icd | 乙酸盐 | 发酵罐 | 29.2 | 0.16 | [ |
| E. coli BW25113 | 过表达异源的cadA和acnB,敲除icd | 葡萄糖 | 摇瓶 | 4.34 | — | [ |
| E. coli XL1-Blue | 过表达高可溶性表达的同义密码子变体scv_cadA | 甘油 | 发酵罐 | 7.2 | — | [ |
E. coli MG1655 (ita23) | 过表达cadA和gltA,下调icd,敲除aceA、sucCD、pta、pykA和pykF | 葡萄糖 | 发酵罐 | 32 | 0.43 | [ |
E. coli MG1655 (ita36A) | 过表达cadA和gltA,替换icd启动子为λ启动子,敲除aceA、sucCD、pta、pykA和pykF | 葡萄糖 | 发酵罐 | 47 | 0.45 | [ |
| E. coli MG1655 | 过表达由GA、ACN和CAD组成的多酶复合体,敲除icd | 葡萄糖 | 摇瓶 | 3.06 | — | [ |
| N. crassa FGSC9720 | 过表达异源的cadA | 玉米秸秆 | 摇瓶 | 0.02 | — | [ |
| P. putida | 过表达由生物传感器PurtA∶T7pol∶lysY+调控的cadA,敲除PHA合成酶 | 碱预处理 木质素 | 摇瓶 | 1.3 | — | [ |
| Synechocystis sp. PCC6803 | 过表达异源的cadA | CO2 | 摇瓶 | 0.014 | — | [ |
| 菌株 | 策略 | 产量/(g/L) | 得率/(g/g) | 文献 |
|---|---|---|---|---|
| E. coli Rosetta (DE3) | 过表达ACO和CAD通过蛋白肽相互作用的自组装酶 | 8.7 | 0.1 | [ |
| E. coli JY002 | 过表达acn和多拷贝cadA | 41.6 | 0.48 | [ |
| E. coli BL-CAR470E-DS/A-CS | 优化CAD表达并用蛋白支架共定位表达ACO和CAD | 51.79 | 0.56 | [ |
| E. coli AtCg | 过表达双T7启动子调控的AtCadA和CgAcnA,优化催化条件和培养基 | 67 | 0.35 | [ |
| E. coli Lemo21 (DE3) | 过表达acn和cadA,基因组上整合表达分子伴侣基因GroESL,催化前细胞冷处理24 h | 98.17 | 0.51 | [ |
| H. bluephagenesis TDZI-08 | 过表达cadA、acn和分子伴侣基因GroESL,增加acn表达量,弱化icd表达,优化细胞培养和催化条件 | 58.73 | 0.66 | [ |
| H. bluephagenesis TDZI-08 | 一锅法合成 | 40.50 | 0.68 | [ |
Table 3 Production of itaconic acid by whole cell catalytic synthesis of citric acid
| 菌株 | 策略 | 产量/(g/L) | 得率/(g/g) | 文献 |
|---|---|---|---|---|
| E. coli Rosetta (DE3) | 过表达ACO和CAD通过蛋白肽相互作用的自组装酶 | 8.7 | 0.1 | [ |
| E. coli JY002 | 过表达acn和多拷贝cadA | 41.6 | 0.48 | [ |
| E. coli BL-CAR470E-DS/A-CS | 优化CAD表达并用蛋白支架共定位表达ACO和CAD | 51.79 | 0.56 | [ |
| E. coli AtCg | 过表达双T7启动子调控的AtCadA和CgAcnA,优化催化条件和培养基 | 67 | 0.35 | [ |
| E. coli Lemo21 (DE3) | 过表达acn和cadA,基因组上整合表达分子伴侣基因GroESL,催化前细胞冷处理24 h | 98.17 | 0.51 | [ |
| H. bluephagenesis TDZI-08 | 过表达cadA、acn和分子伴侣基因GroESL,增加acn表达量,弱化icd表达,优化细胞培养和催化条件 | 58.73 | 0.66 | [ |
| H. bluephagenesis TDZI-08 | 一锅法合成 | 40.50 | 0.68 | [ |
| 1 | Diankristanti P A, Ng I S. Microbial itaconic acid bioproduction towards sustainable development: insights, challenges, and prospects[J]. Bioresource Technology, 2023, 384: 129280. |
| 2 | Zhang R W, Liu H, Ning Y C, et al. Recent advances on the production of itaconic acid via the fermentation and metabolic engineering[J]. Fermentation, 2023, 9(1): 71. |
| 3 | Werpy T A, Petersen G, Added T. Top value added chemicals from biomass(volume Ⅰ): Results of screening for potential candidates from sugars and synthesis gas[R]. Golden, CO, United States: National Renewable Energy Laboratory, 2004. |
| 4 | Willke T, Vorlop K D. Biotechnological production of itaconic acid[J]. Applied Microbiology and Biotechnology, 2001, 56(3/4): 289-295. |
| 5 | Park D S, Abdelrahman O A, Vinter K P, et al. Multifunctional cascade catalysis of itaconic acid hydrodeoxygenation to 3-methyl-tetrahydrofuran[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 9394-9402. |
| 6 | Liu C S, Wang Y L, Liu J H, et al. One-step synthesis of 4-octyl itaconate through the structure control of lipase[J]. Journal of Organic Chemistry, 2021, 86(12): 7895-7903. |
| 7 | Sano M, Tanaka T, Ohara H, et al. Itaconic acid derivatives: structure, function, biosynthesis, and perspectives[J]. Applied Microbiology and Biotechnology, 2020, 104(21): 9041-9051. |
| 8 | Cunha da Cruz J, Machado de Castro A, Camporese Sérvulo E F. World market and biotechnological production of itaconic acid[J]. 3 Biotech, 2018, 8(3): 138. |
| 9 | Yang J, Xu H, Jiang J C, et al. Production of itaconic acid through microbiological fermentation of inexpensive materials[J]. Journal of Bioresources and Bioproducts, 2019, 4(3): 135-142. |
| 10 | Gopaliya D, Kumar V, Khare S K. Recent advances in itaconic acid production from microbial cell factories[J]. Biocatalysis and Agricultural Biotechnology, 2021, 36: 102130. |
| 11 | Kuenz A, Krull S. Biotechnological production of itaconic acid-things you have to know[J]. Applied Microbiology and Biotechnology, 2018, 102(9): 3901-3914. |
| 12 | Diankristanti P A, Effendi S S W, Hsiang C C, et al. High-level itaconic acid (IA) production using engineered Escherichia coli Lemo21(DE3) toward sustainable biorefinery[J]. Enzyme and Microbial Technology, 2023, 167: 110231. |
| 13 | Hsiang C C, Diankristanti P A, Tan S I, et al. Tailoring key enzymes for renewable and high-level itaconic acid production using genetic Escherichia coli via whole-cell bioconversion[J]. Enzyme and Microbial Technology, 2022, 160: 110087. |
| 14 | Kinoshita K. Über die produktion von itaconsäure und mannit durch einen neuen schimmelpilz Aspergillus itaconicus [J]. Acta Phytochimica, 1932, 5: 271-287. |
| 15 | Bentley R, Thiessen C P. Biosynthesis of itaconic acid in Aspergillus terreus(Ⅰ): Tracer studies with C14-labeled substrates[J]. Journal of Biological Chemistry, 1957, 226(2): 673-687. |
| 16 | Bonnarme P, Gillet B, Sepulchre A M, et al. Itaconate biosynthesis in Aspergillus terreus [J]. Journal of Bacteriology, 1995, 177(12): 3573-3578. |
| 17 | Li A, van Luijk N, ter Beek M, et al. A clone-based transcriptomics approach for the identification of genes relevant for itaconic acid production in Aspergillus [J]. Fungal Genetics and Biology, 2011, 48(6): 602-611. |
| 18 | Chen M, Huang X N, Zhong C W, et al. Identification of an itaconic acid degrading pathway in itaconic acid producing Aspergillus terreus [J]. Applied Microbiology and Biotechnology, 2016, 100(17): 7541-7548. |
| 19 | Geiser E, Przybilla S K, Friedrich A, et al. Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate[J]. Microbial Biotechnology, 2016, 9(1): 116-126. |
| 20 | Geiser E, Hosseinpour Tehrani H, Meyer S, et al. Evolutionary freedom in the regulation of the conserved itaconate cluster by Ria1 in related Ustilaginaceae[J]. Fungal Biology and Biotechnology, 2018, 5: 14. |
| 21 | Steiger M G. Itaconic acid—an emerging building block[M]//Wierckx N, Blank L M. Industrial Biotechnology: Products and Processes. New York: John Wiley & Sons Inc., 2017: 453-472. |
| 22 | Calam C T, Oxford A E, Raistrick H. Studies in the biochemistry of micro-organisms: itaconic acid, a metabolic product of a strain of Aspergillus terreus thom[J]. Biochemical Journal, 1939, 33(9): 1488-1495. |
| 23 | Lockwood L B, Nelson G E. Some factors affecting the production of itaconic acid by Aspergillus terreus in agitated cultures[J]. Archives of Biochemistry, 1946, 10: 365-374. |
| 24 | Mario B, Schweiger L B. Process for the production of itaconic acid: US3162582[P]. 1964-12-22. |
| 25 | da Cruz J C, Camporese Sérvulo E F, de Castro A M. Microbial production of itaconic acid[M]//Microbial Production of Food Ingredients and Additives. Amsterdam: Elsevier, 2017: 291-316. |
| 26 | Hevekerl A, Kuenz A, Vorlop K D. Influence of the pH on the itaconic acid production with Aspergillus terreus [J]. Applied Microbiology and Biotechnology, 2014, 98(24): 10005-10012. |
| 27 | Krull S, Hevekerl A, Kuenz A, et al. Process development of itaconic acid production by a natural wild type strain of Aspergillus terreus to reach industrially relevant final titers[J]. Applied Microbiology and Biotechnology, 2017, 101(10): 4063-4072. |
| 28 | Mariana J, Joaquín O, Ester L M. Study of itaconic acid production by Aspergillus terrus MJL05 strain with different variable[J]. Revista Colombiana de Biotecnología, 2010, 12(2): 187. |
| 29 | Narisetty V, Prabhu A A, Al-Jaradah K, et al. Microbial itaconic acid production from starchy food waste by newly isolated thermotolerant Aspergillus terreus strain[J]. Bioresource Technology, 2021, 337: 125426. |
| 30 | Krull S, Eidt L, Hevekerl A, et al. Itaconic acid production from wheat chaff by Aspergillus terreus [J]. Process Biochemistry, 2017, 63: 169-176. |
| 31 | Kerssemakers A A J, Doménech P, Cassano M, et al. Production of itaconic acid from cellulose pulp: feedstock feasibility and process strategies for an efficient microbial performance[J]. Energies, 2020, 13(7): 1654. |
| 32 | Huang X N, Lu X F, Li Y M, et al. Improving itaconic acid production through genetic engineering of an industrial Aspergillus terreus strain[J]. Microbial Cell Factories, 2014, 13: 119. |
| 33 | Tevz G, Bencina M, Legisa M. Enhancing itaconic acid production by Aspergillus terreus [J]. Applied Microbiology and Biotechnology, 2010, 87(5): 1657-1664. |
| 34 | Shin W S, Park B, Lee D, et al. Enhanced production of itaconic acid through development of transformed fungal strains of Aspergillus terreus [J]. Journal of Microbiology and Biotechnology, 2017, 27(2): 306-315. |
| 35 | Krull S, Lünsmann M, Prüße U, et al. Ustilago rabenhorstiana—an alternative natural itaconic acid producer[J]. Fermentation, 2020, 6(1): 4. |
| 36 | Hosseinpour Tehrani H, Saur K, Tharmasothirajan A, et al. Process engineering of pH tolerant Ustilago cynodontis for efficient itaconic acid production[J]. Microbial Cell Factories, 2019, 18(1): 213. |
| 37 | Zambanini T, Hosseinpour Tehrani H, Geiser E, et al. Efficient itaconic acid production from glycerol with Ustilago vetiveriae TZ1[J]. Biotechnology for Biofuels, 2017, 10: 131. |
| 38 | Haskins R H, Thorn J A, Boothroyd B. Biochemistry of the ustilaginales(Ⅺ): Metabolic products of Ustilago zeae in submerged culture[J]. Canadian Journal of Microbiology, 1955, 1(9): 749-756. |
| 39 | Maassen N, Panakova M, Wierckx N, et al. Influence of carbon and nitrogen concentration on itaconic acid production by the smut fungus Ustilago maydis [J]. Engineering in Life Sciences, 2014, 14(2): 129-134. |
| 40 | Geiser E, Przybilla S K, Engel M, et al. Genetic and biochemical insights into the itaconate pathway of Ustilago maydis enable enhanced production[J]. Metabolic Engineering, 2016, 38: 427-435. |
| 41 | Becker J, Hosseinpour Tehrani H, Gauert M, et al. An Ustilago maydis chassis for itaconic acid production without by-products[J]. Microbial Biotechnology, 2020, 13(2): 350-362. |
| 42 | Tehrani H H, Tharmasothirajan A, Track E, et al. Engineering the morphology and metabolism of pH tolerant Ustilago cynodontis for efficient itaconic acid production[J]. Metabolic Engineering, 2019, 54: 293-300. |
| 43 | Tehrani H H, Becker J, Bator I, et al. Integrated strain-and process design enable production of 220 g·L-1 itaconic acid with Ustilago maydis [J]. Biotechnology for Biofuels, 2019, 12(1): 263. |
| 44 | Tabuchi T, Sugisawa T, Ishidori T, et al. Itaconic acid fermentation by a yeast belonging to the genus Candida [J]. Agricultural and Biological Chemistry, 1981, 45(2): 475-479. |
| 45 | Levinson W E, Kurtzman C P, Kuo T M. Production of itaconic acid by Pseudozyma antarctica NRRL Y-7808 under nitrogen-limited growth conditions[J]. Enzyme and Microbial Technology, 2006, 39(4): 824-827. |
| 46 | Araki T, Yamazaki Y, Suzuki N. Production of itaconic acid by Helicobasidium mompa TANAKA[J]. Japanese Journal of Phytopathology, 1957, 22(2): 83-87. |
| 47 | Kautola H, Vahvaselkä M, Linko Y Y, et al. Itaconic acid production by immobilized Aspergillus terreus from xylose and glucose[J]. Biotechnology Letters, 1985, 7(3): 167-172. |
| 48 | Karaffa L, Díaz R, Papp B, et al. A deficiency of manganese ions in the presence of high sugar concentrations is the critical parameter for achieving high yields of itaconic acid by Aspergillus terreus [J]. Applied Microbiology and Biotechnology, 2015, 99(19): 7937-7944. |
| 49 | Huang X N, Chen M, Lu X F, et al. Direct production of itaconic acid from liquefied corn starch by genetically engineered Aspergillus terreus [J]. Microbial Cell Factories, 2014, 13: 108. |
| 50 | Becker J, Tehrani H H, Ernst P, et al. An optimized Ustilago maydis for itaconic acid production at maximal theoretical yield[J]. Journal of Fungi, 2020, 7(1): 20. |
| 51 | Blumhoff M L, Steiger M G, Mattanovich D, et al. Targeting enzymes to the right compartment: metabolic engineering for itaconic acid production by Aspergillus niger [J]. Metabolic Engineering, 2013, 19: 26-32. |
| 52 | Wang Y Q, Guo Y F, Cao W, et al. Synergistic effects on itaconic acid production in engineered Aspergillus niger expressing the two distinct biosynthesis clusters from Aspergillus terreus and Ustilago maydis [J]. Microbial Cell Factories, 2022, 21(1): 158. |
| 53 | Xie H, Ma Q Y, Wei D Z, et al. Metabolic engineering of an industrial Aspergillus niger strain for itaconic acid production[J]. 3 Biotech, 2020, 10(3): 113. |
| 54 | van der Straat L, Vernooij M, Lammers M, et al. Expression of the Aspergillus terreus itaconic acid biosynthesis cluster in Aspergillus niger [J]. Microbial Cell Factories, 2014, 13: 11. |
| 55 | Hossain A H, Li A, Brickwedde A, et al. Rewiring a secondary metabolite pathway towards itaconic acid production in Aspergillus niger [J]. Microbial Cell Factories, 2016, 15(1): 130. |
| 56 | Hossain A H, van Gerven R, Overkamp K M, et al. Metabolic engineering with ATP-citrate lyase and nitrogen source supplementation improves itaconic acid production in Aspergillus niger [J]. Biotechnology for Biofuels, 2019, 12: 233. |
| 57 | Blazeck J, Miller J, Pan A, et al. Metabolic engineering of Saccharomyces cerevisiae for itaconic acid production[J]. Applied Microbiology and Biotechnology, 2014, 98(19): 8155-8164. |
| 58 | Xu Y Y, Li Z M. Utilization of ethanol for itaconic acid biosynthesis by engineered Saccharomyces cerevisiae [J]. FEMS Yeast Research, 2021, 21(6): foab043. |
| 59 | Sun W, Vila-Santa A, Liu N, et al. Metabolic engineering of an acid-tolerant yeast strain Pichia kudriavzevii for itaconic acid production[J]. Metabolic Engineering Communications, 2020, 10: e00124. |
| 60 | Blazeck J, Hill A, Jamoussi M, et al. Metabolic engineering of Yarrowia lipolytica for itaconic acid production[J]. Metabolic Engineering, 2015, 32: 66-73. |
| 61 | Zhao C, Cui Z Y, Zhao X Y, et al. Enhanced itaconic acid production in Yarrowia lipolytica via heterologous expression of a mitochondrial transporter MTT[J]. Applied Microbiology and Biotechnology, 2019, 103(5): 2181-2192. |
| 62 | Rong L X, Miao L, Wang S H, et al. Engineering Yarrowia lipolytica to produce itaconic acid from waste cooking oil[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 888869. |
| 63 | Otten A, Brocker M, Bott M. Metabolic engineering of Corynebacterium glutamicum for the production of itaconate[J]. Metabolic Engineering, 2015, 30: 156-165. |
| 64 | Merkel M, Kiefer D, Schmollack M, et al. Acetate-based production of itaconic acid with Corynebacterium glutamicum using an integrated pH-coupled feeding control[J]. Bioresource Technology, 2022, 351: 126994. |
| 65 | Okamoto S, Chin T, Hiratsuka K, et al. Production of itaconic acid using metabolically engineered Escherichia coli [J]. Journal of General and Applied Microbiology, 2014, 60(5): 191-197. |
| 66 | Jeon H G, Cheong D E, Han Y, et al. Itaconic acid production from glycerol using Escherichia coli harboring a random synonymous codon-substituted 5'-coding region variant of the cadA gene[J]. Biotechnology and Bioengineering, 2016, 113(7): 1504-1510. |
| 67 | Harder B J, Bettenbrock K, Klamt S. Model-based metabolic engineering enables high yield itaconic acid production by Escherichia coli [J]. Metabolic Engineering, 2016, 38: 29-37. |
| 68 | Harder B J, Bettenbrock K, Klamt S. Temperature-dependent dynamic control of the TCA cycle increases volumetric productivity of itaconic acid production by Escherichia coli [J]. Biotechnology and Bioengineering, 2018, 115(1): 156-164. |
| 69 | Yang Z W, Wang H L, Wang Y X, et al. Manufacturing multienzymatic complex reactors in vivo by self-assembly to improve the biosynthesis of itaconic acid in Escherichia coli [J]. ACS Synthetic Biology, 2018, 7(5): 1244-1250. |
| 70 | Zhao C, Chen S L, Fang H. Consolidated bioprocessing of lignocellulosic biomass to itaconic acid by metabolically engineering Neurospora crassa [J]. Applied Microbiology and Biotechnology, 2018, 102(22): 9577-9584. |
| 71 | Elmore J R, Dexter G N, Salvachúa D, et al. Production of itaconic acid from alkali pretreated lignin by dynamic two stage bioconversion[J]. Nature Communications, 2021, 12(1): 2261. |
| 72 | Chin T, Sano M, Takahashi T, et al. Photosynthetic production of itaconic acid in Synechocystis sp. PCC6803[J]. Journal of Biotechnology, 2015, 195: 43-45. |
| 73 | Cairns T C, Barthel L, Meyer V. Something old, something new: challenges and developments in Aspergillus niger biotechnology[J]. Essays in Biochemistry, 2021, 65(2): 213-224. |
| 74 | Li A, Pfelzer N, Zuijderwijk R, et al. Reduced by-product formation and modified oxygen availability improve itaconic acid production in Aspergillus niger [J]. Applied Microbiology and Biotechnology, 2013, 97(9): 3901-3911. |
| 75 | Porro D, Branduardi P. Production of organic acids by yeasts and filamentous fungi[M]//Sibirny A A. Biotechnology of Yeasts and Filamentous Fungi. Cham: Springer International Publishing, 2017: 205-223. |
| 76 | Beopoulos A, Nicaud J M, Gaillardin C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes[J]. Applied Microbiology and Biotechnology, 2011, 90(4): 1193-1206. |
| 77 | Joo Y C, You S K, Shin S K, et al. Bio-based production of dimethyl itaconate from rice wine waste-derived itaconic acid[J]. Biotechnology Journal, 2017, 12(11): 1700114. |
| 78 | Pontrelli S, Chiu T Y, Lan E I, et al. Escherichia coli as a host for metabolic engineering[J]. Metabolic Engineering, 2018, 50: 16-46. |
| 79 | Liu H, Song R R, Liang Y, et al. Genetic manipulation of Escherichia coli central carbon metabolism for efficient production of fumaric acid[J]. Bioresource Technology, 2018, 270: 96-102. |
| 80 | de Carvalho C C C R. Whole cell biocatalysts: essential workers from nature to the industry[J]. Microbial Biotechnology, 2017, 10(2): 250-263. |
| 81 | Madavi T B, Chauhan S, Keshri A, et al. Whole-cell biocatalysis: advancements toward the biosynthesis of fuels[J]. Biofuels, Bioproducts and Biorefining, 2022, 16(3): 859-876. |
| 82 | Lin B X, Tao Y. Whole-cell biocatalysts by design[J]. Microbial Cell Factories, 2017, 16(1): 106. |
| 83 | Yang Z W, Gao X, Xie H, et al. Enhanced itaconic acid production by self-assembly of two biosynthetic enzymes in Escherichia coli [J]. Biotechnology and Bioengineering, 2017, 114(2): 457-462. |
| 84 | Kim J, Seo H M, Bhatia S K, et al. Production of itaconate by whole-cell bioconversion of citrate mediated by expression of multiple cis-aconitate decarboxylase (cadA) genes in Escherichia coli [J]. Scientific Reports, 2017, 7: 39768. |
| 85 | Feng J, Li C Q, He H, et al. Construction of cell factory through combinatorial metabolic engineering for efficient production of itaconic acid[J]. Microbial Cell Factories, 2022, 21(1): 275. |
| 86 | Zhang J, Jin B, Hong K Q, et al. Cell catalysis of citrate to itaconate by engineered Halomonas bluephagenesis [J]. ACS Synthetic Biology, 2021, 10(11): 3017-3027. |
| 87 | 张静, 元跃, 王智文, 等. 基于工程化盐单胞菌TDZI-08一锅法合成衣康酸[J]. 生物工程学报, 2024, 40(8): 2666-2677. |
| Zhang J, Yuan Y, Wang Z W, et al. One-pot synthesis of itaconic acid by engineered Halomonas bluephagenesis TDZI-08[J]. Chinese Journal of Biotechnology, 2024, 40(8): 2666-2677. | |
| 88 | Luo G, Fujino M, Nakano S, et al. Accelerating itaconic acid production by increasing membrane permeability of whole-cell biocatalyst based on a psychrophilic bacterium Shewanella livingstonensis Ac10[J]. Journal of Biotechnology, 2020, 312: 56-62. |
| 89 | Hanko E K R, Minton N P, Malys N. A transcription factor-based biosensor for detection of itaconic acid[J]. ACS Synthetic Biology, 2018, 7(5): 1436-1446. |
| 90 | Zhao M, Li Y T, Wang F Q, et al. A CRISPRi mediated self-inducible system for dynamic regulation of TCA cycle and improvement of itaconic acid production in Escherichia coli [J]. Synthetic and Systems Biotechnology, 2022, 7(3): 982-988. |
| 91 | Okabe M, Ohta N, Park Y S. Itaconic acid production in an air-lift bioreactor using a modified draft tube[J]. Journal of Fermentation and Bioengineering, 1993, 76(2): 117-122. |
| 92 | Lin Y H, Li Y F, Huang M C, et al. Intracellular expression of Vitreoscilla hemoglobin in Aspergillus terreus to alleviate the effect of a short break in aeration during culture[J]. Biotechnology Letters, 2004, 26(13): 1067-1072. |
| 93 | Songserm P, Thitiprasert S, Tolieng V, et al. Regulating pyruvate carboxylase in the living culture of Aspergillus terreus NRRL 1960 by L-aspartate for enhanced itaconic acid production[J]. Applied Biochemistry and Biotechnology, 2015, 177(3): 595-609. |
| 94 | Li A, Pfelzer N, Zuijderwijk R, et al. Enhanced itaconic acid production in Aspergillus niger using genetic modification and medium optimization[J]. BMC Biotechnology, 2012, 12: 57. |
| 95 | Vassilev N, Kautola H, Linko Y Y. Immobilized Aspergillus terreus in itaconic acid production from glucose[J]. Biotechnology Letters, 1992, 14(3): 201-206. |
| 96 | Zhao M L, Lu X Y, Zong H, et al. Itaconic acid production in microorganisms[J]. Biotechnology Letters, 2018, 40(3): 455-464. |
| [1] | Mengting ZHANG, Shulin WANG, Xi SANG, Xinghao YUAN, Gang XU. Artificial Cu-TM1459 metalloenzyme catalyzes asymmetric Michael addition reaction [J]. CIESC Journal, 2024, 75(9): 3255-3265. |
| [2] | Xuemei NA, Yu WANG, Yaozhu JIANG, Nan JIA, Ying WANG, Chun LI. Expression optimization of heterologous CYP450 enzyme promotes the synthesis of ursolic acid in engineering Saccharomyces cerevisiae [J]. CIESC Journal, 2024, 75(7): 2624-2632. |
| [3] | Zheming WU, Biyun ZHANG, Renchao ZHENG. Engineering of nitrilase enantioselectivity for efficient synthesis of brivaracetam [J]. CIESC Journal, 2024, 75(7): 2633-2643. |
| [4] | Rufeng XU, Yucheng CHEN, Dan GAO, Jingyu JIAO, Dong GAO, Haibin WANG, Shanjing YAO, Dongqiang LIN. Model-assisted process optimization of ion-exchange chromatography for monoclonal antibody charge variant separation [J]. CIESC Journal, 2024, 75(5): 1903-1911. |
| [5] | Shupeng WANG, Jianjun DU, Yao YAO, Jiangli FAN, Xiaojun PENG. Mitochondria-targeted rhodamine photosensitizers for tumor fluorescence imaging [J]. CIESC Journal, 2024, 75(4): 1679-1686. |
| [6] | Tao SUN, Meili SUN, Ran LU, Yizi YU, Kaifeng WANG, Xiaojun JI. Synthetic biology of yeasts drives green biomanufacturing of succinic acid [J]. CIESC Journal, 2024, 75(4): 1382-1393. |
| [7] | Shihao LI, Zhenhua WU, Zhanfeng ZHAO, Hong WU, Dong YANG, Jiafu SHI, Zhongyi JIANG. Electron transfer, proton transfer and molecule transfer in chemical processes [J]. CIESC Journal, 2024, 75(3): 1052-1064. |
| [8] | Zhidong MA, Yapeng ZHANG, Huipeng GAO, Wenqiang LI, Bo LYU, Lei QIN, Quan ZHANG, Chun LI. Optimization of biomanufacturing process of high-energy fuel precursor α-bisabolene [J]. CIESC Journal, 2024, 75(12): 4702-4711. |
| [9] | Wenzhe MA, Wei SONG, Wanqing WEI, Jing WU, Liming LIU. Mechanism analysis of β-lactam synthase in synthesizing piracetam intermediate based on quantum mechanics [J]. CIESC Journal, 2024, 75(12): 4712-4722. |
| [10] | Xueying WANG, Yongjin ZHOU, Zongbao ZHAO. Non-natural redox cofactors empowered biomanufacturing [J]. CIESC Journal, 2024, 75(11): 4037-4047. |
| [11] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [12] | Xiaoling TANG, Jiarui WANG, Xuanye ZHU, Renchao ZHENG. Biosynthesis of chiral epichlorohydrin by halohydrin dehalogenase based on Pickering emulsion system [J]. CIESC Journal, 2023, 74(7): 2926-2934. |
| [13] | Yaxin CHEN, Hang YUAN, Guanzhang LIU, Lei MAO, Chun YANG, Ruifang ZHANG, Guangya ZHANG. Advances in enzyme self-immobilization mediated by protein nanocages [J]. CIESC Journal, 2023, 74(7): 2773-2782. |
| [14] | Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics [J]. CIESC Journal, 2023, 74(6): 2589-2598. |
| [15] | Yang HU, Yan SUN. Self-propulsion of enzyme and enzyme-induced micro-/nanomotor [J]. CIESC Journal, 2023, 74(1): 116-132. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||