CIESC Journal ›› 2025, Vol. 76 ›› Issue (4): 1604-1616.DOI: 10.11949/0438-1157.20241136
• Separation engineering • Previous Articles Next Articles
Feng ZHU( ), Yue ZHAO, Fengxiang MA, Wei LIU
), Yue ZHAO, Fengxiang MA, Wei LIU
Received:2024-10-14
Revised:2024-11-21
Online:2025-05-12
Published:2025-04-25
Contact:
Feng ZHU
通讯作者:
朱峰
作者简介:朱峰(1990—),男,硕士,高级工程师,feng342524@126.com
基金资助:CLC Number:
Feng ZHU, Yue ZHAO, Fengxiang MA, Wei LIU. Adsorption properties of modified UIO-66 for SF6/N2 gas mixture and its decomposition products[J]. CIESC Journal, 2025, 76(4): 1604-1616.
朱峰, 赵跃, 马凤翔, 刘伟. 改性UIO-66对SF6/N2混合气体及其分解产物的吸附特性[J]. 化工学报, 2025, 76(4): 1604-1616.
Add to citation manager EndNote|Ris|BibTeX
| 放电持续时间/h | 主要分解产物/(µl·L-1) | |||||
|---|---|---|---|---|---|---|
| SOF2 | SO2F2 | SO2 | N2O | H2S | CF4 | |
| 2 | 58.2 | 27.0 | 2.6 | 0 | 0 | 4.0 |
| 4 | 78.0 | 41.8 | 8.2 | 0 | 11.6 | 7.2 |
| 6 | 112.0 | 80.9 | 22.6 | 1.0 | 25.4 | 10.5 |
| 8 | 174.2 | 107.5 | 32.5 | 1.4 | 42.4 | 21.3 |
| 10 | 192.5 | 113.8 | 43.2 | 2.2 | 58.4 | 32.5 |
Table 1 SF6/N2 gas mixture decomposition product data for local release faults at 500 kPa gas pressure
| 放电持续时间/h | 主要分解产物/(µl·L-1) | |||||
|---|---|---|---|---|---|---|
| SOF2 | SO2F2 | SO2 | N2O | H2S | CF4 | |
| 2 | 58.2 | 27.0 | 2.6 | 0 | 0 | 4.0 |
| 4 | 78.0 | 41.8 | 8.2 | 0 | 11.6 | 7.2 |
| 6 | 112.0 | 80.9 | 22.6 | 1.0 | 25.4 | 10.5 |
| 8 | 174.2 | 107.5 | 32.5 | 1.4 | 42.4 | 21.3 |
| 10 | 192.5 | 113.8 | 43.2 | 2.2 | 58.4 | 32.5 |
| 气体组分 | 红外特征峰强度(km·mol-1) | 吸附率/% | |
|---|---|---|---|
| 吸附前 | 吸附后 | ||
| SF6 | 1134.28 | 1070.65 | 5.61 |
| N2 | — | — | 1.52 |
| SO2 | 199.54 | 49.41 | 75.24 |
| N2O | 302.73 | 296.13 | 2.18 |
| SOF2 | 193.69 | 58.15 | 69.98 |
| SO2F2 | 218.78 | 71.43 | 67.35 |
| CF4 | 31.99 | 30.96 | 3.21 |
| H2S | 40.31 | 11.22 | 72.17 |
Table 2 Adsorption rate of gas components before and after adsorption
| 气体组分 | 红外特征峰强度(km·mol-1) | 吸附率/% | |
|---|---|---|---|
| 吸附前 | 吸附后 | ||
| SF6 | 1134.28 | 1070.65 | 5.61 |
| N2 | — | — | 1.52 |
| SO2 | 199.54 | 49.41 | 75.24 |
| N2O | 302.73 | 296.13 | 2.18 |
| SOF2 | 193.69 | 58.15 | 69.98 |
| SO2F2 | 218.78 | 71.43 | 67.35 |
| CF4 | 31.99 | 30.96 | 3.21 |
| H2S | 40.31 | 11.22 | 72.17 |
| 分子结构式 | 键 | 键长/Å | 键 | 键角/(°) |
|---|---|---|---|---|
| SF6 | S—F | 1.164 | F—S—F | 90.00 |
| N2 | N—N | 1.107 | — | — |
| SO2 | S—O | 1.479 | O—S—O | 120.03 |
| CF4 | C—F | 1.340 | F—C—F | 109.44 |
| H2S | H—S | 1.354 | H—S—H | 91.29 |
| SOF2 | S—O | 1.459 | F—S—O | 107.22 |
| S—F | 1.609 | F—S—F | 93.15 | |
| SO2F2 | S—O | 1.440 | O—S—O | 126.84 |
| S—F | 1.609 | F—S—F | 107.52 | |
| N2O | N—N | 1.140 | N—N—O | 180.00 |
| N—O | 1.195 |
Table 3 Structural parameters of SF6/N2 and major decomposition products molecules
| 分子结构式 | 键 | 键长/Å | 键 | 键角/(°) |
|---|---|---|---|---|
| SF6 | S—F | 1.164 | F—S—F | 90.00 |
| N2 | N—N | 1.107 | — | — |
| SO2 | S—O | 1.479 | O—S—O | 120.03 |
| CF4 | C—F | 1.340 | F—C—F | 109.44 |
| H2S | H—S | 1.354 | H—S—H | 91.29 |
| SOF2 | S—O | 1.459 | F—S—O | 107.22 |
| S—F | 1.609 | F—S—F | 93.15 | |
| SO2F2 | S—O | 1.440 | O—S—O | 126.84 |
| S—F | 1.609 | F—S—F | 107.52 | |
| N2O | N—N | 1.140 | N—N—O | 180.00 |
| N—O | 1.195 |
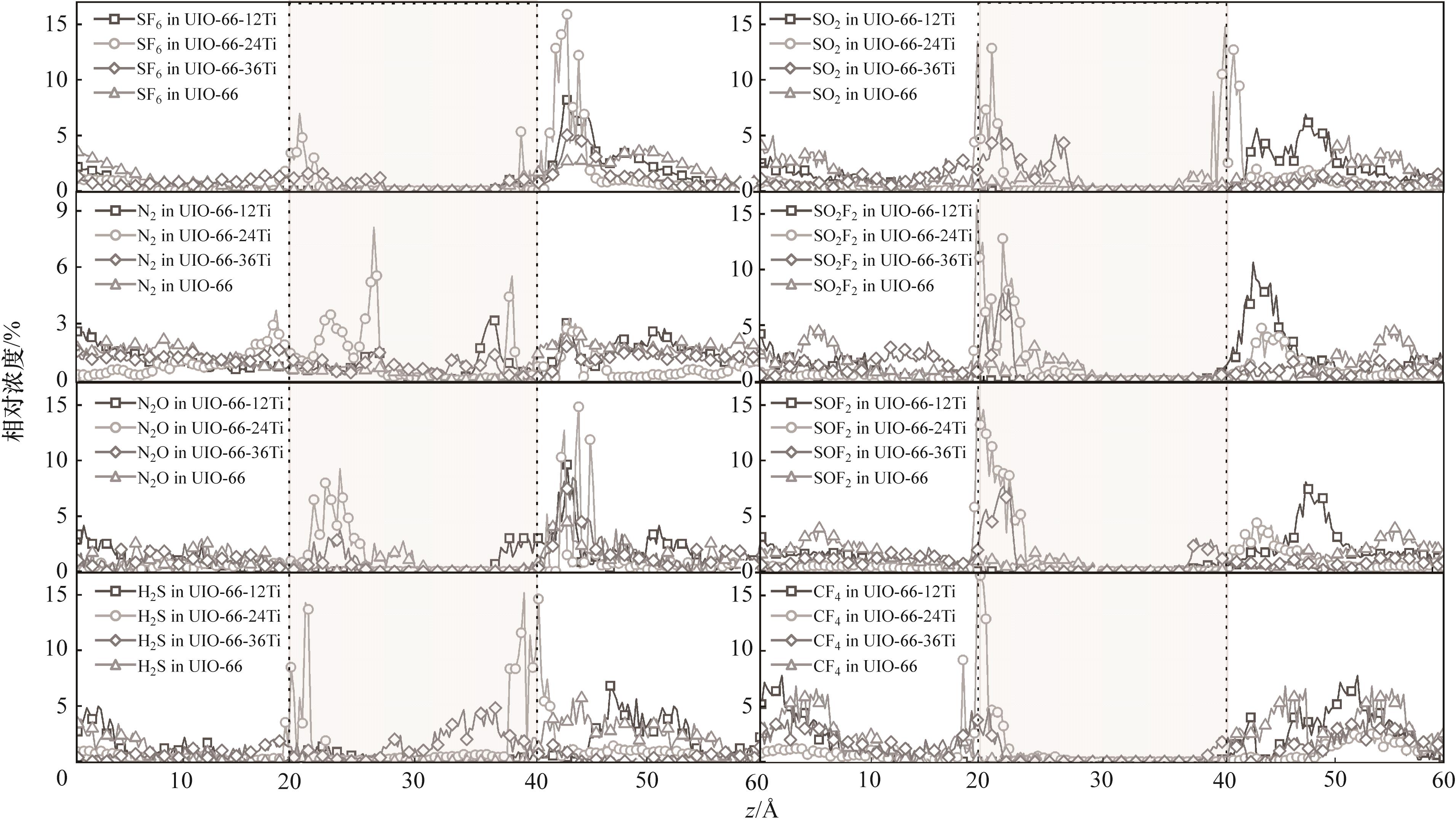
Fig.8 Concentration distribution curve of SF6/N2 and its decomposition product gas molecules in the vertical direction on the surface of organometallic architecture before and after modification
| 组分 | 有机金属框架 | D /(10-8 m2·s-1) | R2 | 组分 | 有机金属框架 | D /(10-8 m2·s-1) | R2 |
|---|---|---|---|---|---|---|---|
| SF6 | UIO-66 | 3.27509 | 0.99801 | SOF2 | UIO-66 | 2.41781 | 0.99120 |
| UIO-66-12Ti | 0.70361 | 0.95610 | UIO-66-12Ti | 0.40866 | 0.98160 | ||
| UIO-66-24Ti | 1.02379 | 0.99704 | UIO-66-24Ti | 0.35311 | 0.99409 | ||
| UIO-66-36Ti | 2.00478 | 0.99652 | UIO-66-36Ti | 0.51946 | 0.98553 | ||
| N2 | UIO-66 | 5.54245 | 0.99902 | SO2F2 | UIO-66 | 1.90605 | 0.99628 |
| UIO-66-12Ti | 2.53479 | 0.99968 | UIO-66-12Ti | 0.85346 | 0.96210 | ||
| UIO-66-24Ti | 1.61016 | 0.97939 | UIO-66-24Ti | 0.77260 | 0.99889 | ||
| UIO-66-36Ti | 3.83695 | 0.99949 | UIO-66-36Ti | 1.47711 | 0.98894 | ||
| SO2 | UIO-66 | 2.07137 | 0.99749 | CF4 | UIO-66 | 6.29844 | 0.99147 |
| UIO-66-12Ti | 0.66923 | 0.95147 | UIO-66-12Ti | 1.51485 | 0.99509 | ||
| UIO-66-24Ti | 0.54071 | 0.99905 | UIO-66-24Ti | 0.64479 | 0.97562 | ||
| UIO-66-36Ti | 2.72807 | 0.99369 | UIO-66-36Ti | 3.05387 | 0.99740 | ||
| N2O | UIO-66 | 4.40870 | 0.98702 | H2S | UIO-66 | 1.59684 | 0.99561 |
| UIO-66-12Ti | 1.74265 | 0.99703 | UIO-66-12Ti | 1.21616 | 0.99852 | ||
| UIO-66-24Ti | 0.50103 | 0.99946 | UIO-66-24Ti | 0.45336 | 0.99311 | ||
| UIO-66-36Ti | 2.32431 | 0.99723 | UIO-66-36Ti | 0.98093 | 0.99502 |
Table 4 Linear fitting results of the mean square displacement of each gas molecule in the organometallic architecture before and after modification
| 组分 | 有机金属框架 | D /(10-8 m2·s-1) | R2 | 组分 | 有机金属框架 | D /(10-8 m2·s-1) | R2 |
|---|---|---|---|---|---|---|---|
| SF6 | UIO-66 | 3.27509 | 0.99801 | SOF2 | UIO-66 | 2.41781 | 0.99120 |
| UIO-66-12Ti | 0.70361 | 0.95610 | UIO-66-12Ti | 0.40866 | 0.98160 | ||
| UIO-66-24Ti | 1.02379 | 0.99704 | UIO-66-24Ti | 0.35311 | 0.99409 | ||
| UIO-66-36Ti | 2.00478 | 0.99652 | UIO-66-36Ti | 0.51946 | 0.98553 | ||
| N2 | UIO-66 | 5.54245 | 0.99902 | SO2F2 | UIO-66 | 1.90605 | 0.99628 |
| UIO-66-12Ti | 2.53479 | 0.99968 | UIO-66-12Ti | 0.85346 | 0.96210 | ||
| UIO-66-24Ti | 1.61016 | 0.97939 | UIO-66-24Ti | 0.77260 | 0.99889 | ||
| UIO-66-36Ti | 3.83695 | 0.99949 | UIO-66-36Ti | 1.47711 | 0.98894 | ||
| SO2 | UIO-66 | 2.07137 | 0.99749 | CF4 | UIO-66 | 6.29844 | 0.99147 |
| UIO-66-12Ti | 0.66923 | 0.95147 | UIO-66-12Ti | 1.51485 | 0.99509 | ||
| UIO-66-24Ti | 0.54071 | 0.99905 | UIO-66-24Ti | 0.64479 | 0.97562 | ||
| UIO-66-36Ti | 2.72807 | 0.99369 | UIO-66-36Ti | 3.05387 | 0.99740 | ||
| N2O | UIO-66 | 4.40870 | 0.98702 | H2S | UIO-66 | 1.59684 | 0.99561 |
| UIO-66-12Ti | 1.74265 | 0.99703 | UIO-66-12Ti | 1.21616 | 0.99852 | ||
| UIO-66-24Ti | 0.50103 | 0.99946 | UIO-66-24Ti | 0.45336 | 0.99311 | ||
| UIO-66-36Ti | 2.32431 | 0.99723 | UIO-66-36Ti | 0.98093 | 0.99502 |
分子 结构式 | 吸附热/(kJ·mol-1) | |||
|---|---|---|---|---|
| UIO-66 | UIO-66-12Ti | UIO-66-24Ti | UIO-66-36Ti | |
| SF6 | 28.59 | 27.73 | 27.22 | 27.55 |
| N2 | 13.98 | 14.19 | 14.18 | 14.03 |
| SO2 | 30.62 | 30.41 | 31.13 | 30.98 |
| N2O | 19.99 | 20.10 | 20.36 | 20.10 |
| SOF2 | 33.08 | 47.70 | 32.51 | 31.44 |
| SO2F2 | 43.22 | 39.07 | 39.64 | 39.46 |
| CF4 | 27.95 | 27.37 | 28.54 | 28.50 |
| H2S | 29.41 | 28.57 | 29.57 | 29.96 |
Table 5 Heat of adsorption of SF6/N2 and major impurity molecules on UIO-66 modified materials
分子 结构式 | 吸附热/(kJ·mol-1) | |||
|---|---|---|---|---|
| UIO-66 | UIO-66-12Ti | UIO-66-24Ti | UIO-66-36Ti | |
| SF6 | 28.59 | 27.73 | 27.22 | 27.55 |
| N2 | 13.98 | 14.19 | 14.18 | 14.03 |
| SO2 | 30.62 | 30.41 | 31.13 | 30.98 |
| N2O | 19.99 | 20.10 | 20.36 | 20.10 |
| SOF2 | 33.08 | 47.70 | 32.51 | 31.44 |
| SO2F2 | 43.22 | 39.07 | 39.64 | 39.46 |
| CF4 | 27.95 | 27.37 | 28.54 | 28.50 |
| H2S | 29.41 | 28.57 | 29.57 | 29.96 |
| 气体组分 | 相对于纯UIO-66吸附提高量/% | 误差/% | |
|---|---|---|---|
| 模拟 | 实验 | ||
| SF6 | 16.30 | 18.41 | -11.48 |
| N2 | 88.37 | 73.77 | 19.78 |
| SO2 | 59.33 | 50.43 | 17.65 |
| N2O | 67.06 | 57.00 | 17.65 |
| SOF2 | 56.96 | 50.70 | 12.35 |
| SO2F2 | 49.59 | 43.15 | 14.94 |
| CF4 | 86.55 | 83.10 | 4.17 |
| H2S | 83.48 | 85.15 | -1.97 |
Table 6 Improvement of adsorption performance of SF6/N2 and main decomposition product molecules in UIO-66-50%Ti
| 气体组分 | 相对于纯UIO-66吸附提高量/% | 误差/% | |
|---|---|---|---|
| 模拟 | 实验 | ||
| SF6 | 16.30 | 18.41 | -11.48 |
| N2 | 88.37 | 73.77 | 19.78 |
| SO2 | 59.33 | 50.43 | 17.65 |
| N2O | 67.06 | 57.00 | 17.65 |
| SOF2 | 56.96 | 50.70 | 12.35 |
| SO2F2 | 49.59 | 43.15 | 14.94 |
| CF4 | 86.55 | 83.10 | 4.17 |
| H2S | 83.48 | 85.15 | -1.97 |
| 1 | Maiss M, Brenninkmeijer C A. Atmospheric SF6: trends, sources, and prospects[J]. Environmental Science & Technology, 1998, 32(20): 3077-3086. |
| 2 | Chiang Y C, Wu P Y. Adsorption equilibrium of sulfur hexafluoride on multi-walled carbon nanotubes[J]. Journal of Hazardous Materials, 2010, 178(1/2/3): 729-738. |
| 3 | Fang X, Hu X, Janssens-Maenhout G, et al. Sulfur hexafluoride (SF6) emission estimates for China: an inventory for 1990—2010 and a projection to 2020[J]. Environmental Science & Technology, 2013, 47(8): 3848-3855. |
| 4 | Christophorou L G, Van Brunt R J. SF6/N2 mixtures: basic and HV insulation properties[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2002, 2(5): 952-1003. |
| 5 | Montzka S A, Dlugokencky E J, Butler J H. Non-CO2 greenhouse gases and climate change[J]. Nature, 2011, 476(7358): 43-50. |
| 6 | Beroual A, Haddad A M. Recent advances in the quest for a new insulation gas with a low impact on the environment to replace sulfur hexafluoride (SF6) gas in high-voltage power network applications[J]. Energies, 2017, 10(8): 1216. |
| 7 | Wang Y, Huang D Q, Liu J, et al. Alternative environmentally friendly insulating gases for SF6 [J]. Processes, 2019, 7(4): 216. |
| 8 | Yamamoto O, Takuma T, Kinouchi M. Recovery of SF6 from N2/SF6 gas mixtures by using a polymer membrane[J]. IEEE Electrical Insulation Magazine, 2002, 18(3): 32-37. |
| 9 | Zhao H, Deng Y K, Tian Z Y. Dielectric breakdown properties of CF3I-N2 mixtures containing a small amount of SF6 [J]. AIP Advances, 2019, 9(5): 055031. |
| 10 | Inami K, Maeda Y, Habuchi Y, et al. Problems of the application of N2/SF6 mixtures to gas-insulated bus[J]. Electrical Engineering in Japan, 2001, 137(4): 25-31. |
| 11 | Builes S, Roussel T, Vega L F. Optimization of the separation of sulfur hexafluoride and nitrogen by selective adsorption using Monte Carlo simulations[J]. AIChE Journal, 2011, 57(4): 962-974. |
| 12 | Kim K, Kim K S, Lee J E, et al. Status of SF6 separation/refining technology development for electric industry in Korea[J]. Separation and Purification Technology, 2018, 200: 29-35. |
| 13 | Stenning A H, Martin C B. An analytical and experimental study of air-lift pump performance[J]. Journal of Engineering for Power, 1968, 90(2): 106-110. |
| 14 | 韩沛, 李金键, 柯天, 等. 新型多孔材料吸附分离六氟化硫/氮气研究进展[J]. 化工进展, DOI:10.16085/j.issn.1000-6613.2024-0671 . |
| Han P, Li J J, Ke T, et al. Progress of adsorption separation of sulfur hexafluoride/nitrogen by novel porous materials[J]. Chemical Industry and Engineering Progress, DOI:10.16085/j.issn.1000-6613.2024-0671 . | |
| 15 | 常苗, 刘磊, 阳庆元, 等. 水热稳定金属-有机骨架材料用于高效分离SF6/N2混合物的研究[J]. 化工学报, 2020, 71(1): 320-328. |
| Chang M, Liu L, Yang Q Y, et al. Study on efficient separation of SF6/N2 mixture using a hydrothermally stable metal-organic framework[J]. CIESC Journal, 2020, 71(1): 320-328. | |
| 16 | Kim M B, Yoon T U, Hong D Y, et al. High SF6/N2 selectivity in a hydrothermally stable zirconium-based metal–organic framework[J]. Chemical Engineering Journal, 2015, 276: 315-321. |
| 17 | Kim M B, Kim T H, Yoon T U, et al. Efficient SF6/N2 separation at high pressures using a zirconium-based mesoporous metal-organic framework[J]. Journal of Industrial and Engineering Chemistry, 2020, 84: 179-184. |
| 18 | Kim M B, Kim K M, Kim T H, et al. Highly selective adsorption of SF6 over N2 in a bromine-functionalized zirconium-based metal-organic framework[J]. Chemical Engineering Journal, 2018, 339: 223-229. |
| 19 | Webber M E. Diode laser measurements of NH3 and CO2 for combustion and bioreactor applications[D]. Stanford :Stanford University, 2001. |
| 20 | Fuss S P, Hamins A. Determination of Planck mean absorption coefficients for HBr, HCl, and HF[J]. Journal of Heat Transfer, 2002, 124(1): 26-29. |
| 21 | 唐炬, 曾福平, 梁鑫, 等. 吸附剂对局部放电下SF6分解特征组分的吸附研究[J]. 中国电机工程学报, 2014, 34(3): 486-494. |
| Tang J, Zeng F P, Liang X, et al. Study on the influence of adsorbent on SF6 decomposition characteristics under partial discharge[J]. Proceedings of the CSEE, 2014, 34(3): 486-494. | |
| 22 | Perdew J P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas[J]. Physical Review B, 1986, 33(12): 8822-8824. |
| 23 | Perdew J P. Erratum: Density-functional approximation for the correlation energy of the inhomogeneous electron gas[J]. Physical Review B, 1986, 34(10): 7406. |
| 24 | Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868. |
| 25 | Lu T, Chen F W. Multiwfn: a multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| 26 | Michalet X. Mean square displacement analysis of single-particle trajectories with localization error: Brownian motion in an isotropic medium[J]. Physical Review E, Statistical, Nonlinear, and Soft Matter Physics, 2010, 82(4): 041914. |
| 27 | 汤倩茜, 陈栋航, 张春杰, 等. 沸石分子筛用于挥发性有机物吸附的研究进展[J]. 材料导报, 2022, 36(S1): 74-82. |
| Tang Q X, Chen D H, Zhang C J, et al. Research progress on adsorption of volatile organic compounds by zeolite molecular sieve [J]. Materials Reports, 2022, 36(S1): 74-82. | |
| 28 | 黄乐, 郑健, 李强, 等. 正己烷在不同硅铝比HZSM-5分子筛上吸附的分子模拟研究[J]. 石油炼制与化工, 2022, 53(2): 84-92. |
| Huang L, Zheng J, Li Q, et al. Molecular simulation of n-hexane adsorption on HZSM-5 zeolites with different Si/Al ratio [J]. Petroleum Processing and Petrochemicals, 2022, 53(2): 84-92. | |
| 29 | 夏大平, 刘春兰, 陈振宏, 等. 不同煤阶煤孔隙结构与煤制生物甲烷的相互影响[J]. 煤炭转化, 2023, 46(1): 27-38. |
| Xia D P, Liu C L, Chen Z H, et al. Interaction between pore structure of different rank coals and coal-to-biomethane[J]. Coal Conversion, 2023, 46(1): 27-38. | |
| 30 | Castro-Marcano F, Mathews J P. Constitution of Illinois No. 6 Argonne premium coal: a review[J]. Energy & Fuels, 2011, 25(3): 845-853. |
| 31 | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| Cui S C, Xu H B, Peng N. Simulation analysis of two MOFs materials for O2/He adsorption separation[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. | |
| 32 | 唐宇昊, 张迎迎, 赵智伟, 等. 弱极性超微孔 Sc/In-CPM-66A 用于CH4/N2吸附分离性能[J]. 化工学报, 2024, 75(9): 3210-3220. |
| Tang Y H, Zhang Y Y, Zhao Z W, et al. Ultra-microporous Sc/In-CPM-66A with low-polar pore surfaces for efficient separation of CH4/N2 [J]. CIESC Journal, 2024, 75(9): 3210-3220. | |
| 33 | Li S, Qin Y, Tang D Z, et al. A comprehensive review of deep coalbed methane and recent developments in China[J]. International Journal of Coal Geology, 2023, 279:104369. |
| 34 | Park J W, Park Y J, Jun C H. Post-grafting of silica surfaces with pre-functionalized organosilanes: new synthetic equivalents of conventional trialkoxysilanes[J]. Chemical Communications, 2011, 47(17): 4860-4871. |
| 35 | Sun D R, Liu W J, Qiu M, et al. Introduction of a mediator for enhancing photocatalytic performance via post-synthetic metal exchange in metal-organic frameworks (MOFs)[J]. Chemical Communications, 2015, 51(11): 2056-2059. |
| 36 | Wang A N, Zhou Y J, Wang Z L, et al. Titanium incorporated with U i O - 66 ( Z r ) - t y p e metal-organic framework (MOF) for photocatalytic application[J]. RSC Advances, 2016, 6(5): 3671-3679. |
| [1] | Lyusheng ZHANG, Zhihong WANG, Qing LIU, Xuewen LI, Renmin TAN. Research progress in carbon dioxide capture using liquid-liquid phase change absorbents [J]. CIESC Journal, 2025, 76(3): 933-950. |
| [2] | Yinjie ZHOU, Sibei JI, Songyang HE, Xu JI, Ge HE. Machine learning-assisted high-throughput screening approach for CO2 separation from CO2-rich natural gas using metal-organic frameworks [J]. CIESC Journal, 2025, 76(3): 1093-1101. |
| [3] | Fang XU, Rui ZHANG, Da CUI, Qing WANG. Study of pyrolysis reaction mechanism of lignin revealed by ReaxFF-MD simulation [J]. CIESC Journal, 2025, 76(3): 1253-1263. |
| [4] | Qi ZHANG, Rui ZHANG, Tao ZHENG, Xin CAO, Zhichang LIU, Haiyan LIU, Chunming XU, Rong ZHANG, Xianghai MENG. Revealing CO2 capture by a novel dual-cation protic ionic liquid using molecular simulation [J]. CIESC Journal, 2025, 76(2): 797-811. |
| [5] | Siwen ZHANG, Haiming GU, Shanhui ZHAO. Molecular mechanism study on chemical looping gasification of cellulose over iron oxide nanocluster [J]. CIESC Journal, 2025, 76(1): 363-373. |
| [6] | Angran ZHAO, Yongqiang HAN, Zhipeng WANG, Pengfei LI, Yawei XU, Huiling TONG. Experimental study on simultaneous desulfurization and denitrification of red mud at low temperature [J]. CIESC Journal, 2024, 75(S1): 276-282. |
| [7] | Xinyue WANG, Xiaohu XU, Haiyang ZHANG, Chunhua YIN. Study on encapsulation and properties vitamin A acetate/cyclodextrin [J]. CIESC Journal, 2024, 75(S1): 321-328. |
| [8] | Wenbo ZHOU, Jiangwei YIN, Dan ZHANG, Yue YANG, Jiahao YU, Bingchao ZHAO. Experimental study on evaporation of aqueous NaCl solution droplet heating by thermal irradiation [J]. CIESC Journal, 2024, 75(S1): 85-94. |
| [9] | Yong YANG, Zixuan ZU, Yukun LI, Dongliang WANG, Zongliang FAN, Huairong ZHOU. Numerical simulation of CO2 absorption by alkali liquor in T-junction cylindrical microchannels [J]. CIESC Journal, 2024, 75(S1): 135-142. |
| [10] | Zheming WU, Biyun ZHANG, Renchao ZHENG. Engineering of nitrilase enantioselectivity for efficient synthesis of brivaracetam [J]. CIESC Journal, 2024, 75(7): 2633-2643. |
| [11] | Guangyao ZHAO, Minglei YANG, Feng QIAN. Variance reduction sampling strategy-based stochastic reconstruction method [J]. CIESC Journal, 2024, 75(5): 1939-1950. |
| [12] | Hansong QIN, Guoliang LI, Hao YAN, Xiang FENG, Yibin LIU, Xiaobo CHEN, Chaohe YANG. Theoretical study on the adsorption and diffusion behavior of methyl oleate catalytic cracking in hierarchical ZSM-5 zeolite [J]. CIESC Journal, 2024, 75(5): 1870-1881. |
| [13] | Dongfei LIU, Fan ZHANG, Zheng LIU, Diannan LU. A review of machine learning potentials and their applications to molecular simulation [J]. CIESC Journal, 2024, 75(4): 1241-1255. |
| [14] | Zheng ZHANG, Wuqiong WANG, Yajing ZHANG, Kangjun WANG, Yuanhui JI. Research progress in theoretical calculation of pharmaceutical formulation design [J]. CIESC Journal, 2024, 75(4): 1429-1438. |
| [15] | Kang ZHOU, Jianxin WANG, Hai YU, Chaoliang WEI, Fengqi FAN, Xinhao CHE, Lei ZHANG. Foam rupture properties of mineral base oils based on molecular dynamics simulation [J]. CIESC Journal, 2024, 75(4): 1668-1678. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||