CIESC Journal ›› 2025, Vol. 76 ›› Issue (3): 933-950.DOI: 10.11949/0438-1157.20240797
• Reviews and monographs • Previous Articles Next Articles
Lyusheng ZHANG1( ), Zhihong WANG1(
), Zhihong WANG1( ), Qing LIU2, Xuewen LI1, Renmin TAN1
), Qing LIU2, Xuewen LI1, Renmin TAN1
Received:2024-07-15
Revised:2024-09-15
Online:2025-03-28
Published:2025-03-25
Contact:
Zhihong WANG
张履胜1( ), 王治红1(
), 王治红1( ), 柳青2, 李雪雯1, 谭仁敏1
), 柳青2, 李雪雯1, 谭仁敏1
通讯作者:
王治红
作者简介:张履胜(2000—),男,硕士研究生,zlszls227@163.com
CLC Number:
Lyusheng ZHANG, Zhihong WANG, Qing LIU, Xuewen LI, Renmin TAN. Research progress in carbon dioxide capture using liquid-liquid phase change absorbents[J]. CIESC Journal, 2025, 76(3): 933-950.
张履胜, 王治红, 柳青, 李雪雯, 谭仁敏. 液-液相变吸收剂捕集二氧化碳研究进展[J]. 化工学报, 2025, 76(3): 933-950.
Add to citation manager EndNote|Ris|BibTeX
| 吸收剂 | 质量分数/% | 吸收温度/℃ | 再生能耗/(GJ/t CO2) | 缺点 |
|---|---|---|---|---|
| MEA | 30 | 约40 | 3.6~4.0 | 能耗高,腐蚀性强 |
| 碳酸钾溶液 | 40 | 60~80 | 2.6 | 吸收速率慢 |
| 氨水 | 8.5 | 0~10 | 2.2 | 氨气逃逸,溶液结晶 |
Table 1 Comparison of conventional CO2 absorbents[5-7, 10, 13-14]
| 吸收剂 | 质量分数/% | 吸收温度/℃ | 再生能耗/(GJ/t CO2) | 缺点 |
|---|---|---|---|---|
| MEA | 30 | 约40 | 3.6~4.0 | 能耗高,腐蚀性强 |
| 碳酸钾溶液 | 40 | 60~80 | 2.6 | 吸收速率慢 |
| 氨水 | 8.5 | 0~10 | 2.2 | 氨气逃逸,溶液结晶 |
| MEA | N-甲基二乙醇胺(MDEA) | 哌嗪(PZ) |
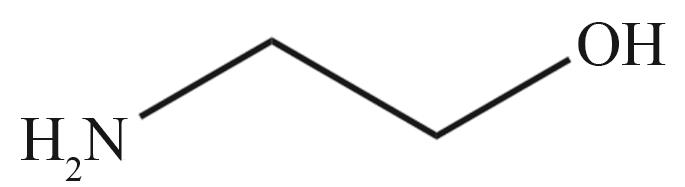 | 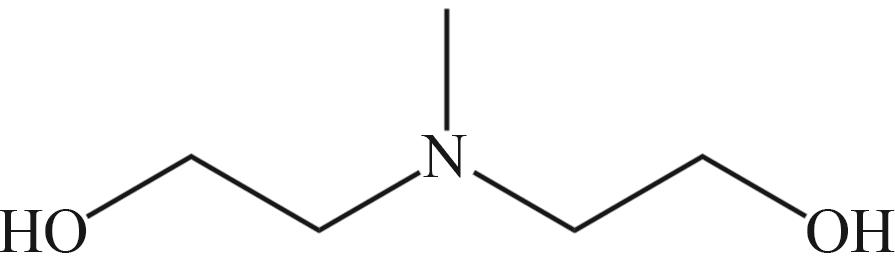 | 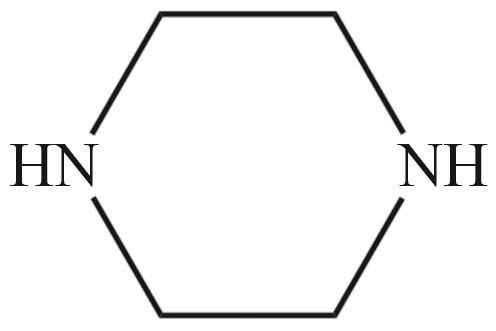 |
| 二乙醇胺(DEA) | 二甘醇胺(DGA) | 二异丙醇胺(DIPA) |
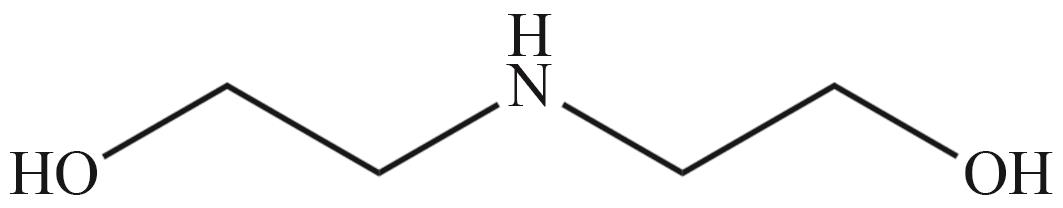 | 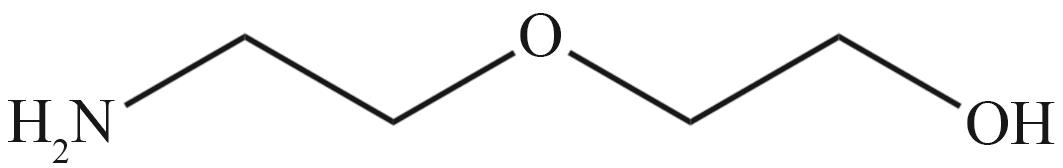 | 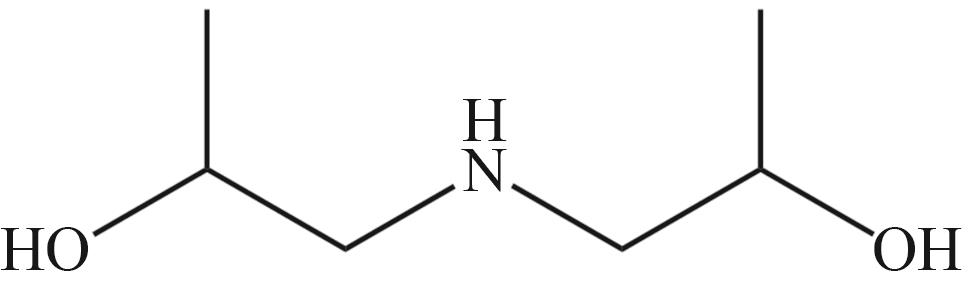 |
Fig.2 Organic amines and its structural formula commonly used for absorbing CO2
| MEA | N-甲基二乙醇胺(MDEA) | 哌嗪(PZ) |
 |  |  |
| 二乙醇胺(DEA) | 二甘醇胺(DGA) | 二异丙醇胺(DIPA) |
 |  |  |
| 吸收剂 | 再生能耗/(GJ/t CO2) | 吸收负载 | 特性/结论 | 文献 |
|---|---|---|---|---|
| DETA/DEEA/H2O | — | 1.31 mol/L | 温度对传质影响相对显著 | [ |
| DETA/DEEA/H2O | 2.14 | 4.95 mol/L | 降解和腐蚀速率低 | [ |
| S1N/DMCA/H2O | 2.30 | 1.91 mol/L | CAMD筛选组分配比 | [ |
| S1N/DMCA/H2O | 2.1 | 1.10 mol/mol | 通用模型用于两种案例 | [ |
| AMP/DMCA/MCA/H2O | — | 1.93 mol/mol | 稀溶液有较高的CO2负荷 | [ |
| DEEA/AEEA/H2O | 2.58 | 3.15 mol/mol | CO2反应和相分离 | [ |
| AEP/DPA/H2O | — | 1.08 mol/mol | 自萃取功能 | [ |
| DEEA/AEEA/H2O | 2.06 | 2.55 mol/L | 离子液体促进吸收-解吸 | [ |
Table 2 Mixed amine phase change absorbents and their relative studies
| 吸收剂 | 再生能耗/(GJ/t CO2) | 吸收负载 | 特性/结论 | 文献 |
|---|---|---|---|---|
| DETA/DEEA/H2O | — | 1.31 mol/L | 温度对传质影响相对显著 | [ |
| DETA/DEEA/H2O | 2.14 | 4.95 mol/L | 降解和腐蚀速率低 | [ |
| S1N/DMCA/H2O | 2.30 | 1.91 mol/L | CAMD筛选组分配比 | [ |
| S1N/DMCA/H2O | 2.1 | 1.10 mol/mol | 通用模型用于两种案例 | [ |
| AMP/DMCA/MCA/H2O | — | 1.93 mol/mol | 稀溶液有较高的CO2负荷 | [ |
| DEEA/AEEA/H2O | 2.58 | 3.15 mol/mol | CO2反应和相分离 | [ |
| AEP/DPA/H2O | — | 1.08 mol/mol | 自萃取功能 | [ |
| DEEA/AEEA/H2O | 2.06 | 2.55 mol/L | 离子液体促进吸收-解吸 | [ |
| 吸收剂 | 再生能耗/(GJ/t CO2) | 吸收负载 | 特性/结论 | 文献 |
|---|---|---|---|---|
| MEA/DMSO/PMDETA | 2.50 | 0.88 mol/mol | AMP增溶,腐蚀性低 | [ |
| TETA/DEEA/NMP/H2O | 1.89 | 0.94 mol/mol | 水含量影响汽化热和反应热 | [ |
| PZ/DMEA/正丁醇/H2O | 1.59 | 4.23 mol/mol | 正丁醇加快吸收速率 | [ |
| AEP/正丙醇/H2O | 2.74 | 1.26 mol/mol | AEP位阻效应有利于再生 | [ |
| MEA/正丁醇/H2O | 2.60 | 4.20 mol/L | 黏度和富相体积更小 | [ |
| MDEA/正丁醇/H2O | — | 2.48 mol/kg | 水和醇的共溶剂效应 | [ |
| MEA/AEEA/DGM/H2O | 2.69 | 2.08 mol/kg | 富相黏度低 | [ |
| DMAPA/NHD/H2O | 2.84 | 1.06 mol/mol | 富相体积占比低 | [ |
| AMP/MEA/DGM/H2O | 2.70 | 1.82 mol/kg | DGM加快了吸收速率 | [ |
| DETA/环丁砜/H2O | 2.67 | 2.21 mol/L | 环丁砜增强CO2吸收效率 | [ |
| EHA/DGM/H2O | — | 2.98 mol/kg | 吸收过程受液相传质控制 | [ |
| DAP/TMEDA/H2O | — | 3.20 mol/kg | TMEDA是良好的质子受体 | [ |
| DGA/PMDETA/EG | 2.26 | 3.11 mol/kg | 非水体系,EG调节富相黏度 | [ |
Table 3 Physical solvent phase change absorbents and their relative studies
| 吸收剂 | 再生能耗/(GJ/t CO2) | 吸收负载 | 特性/结论 | 文献 |
|---|---|---|---|---|
| MEA/DMSO/PMDETA | 2.50 | 0.88 mol/mol | AMP增溶,腐蚀性低 | [ |
| TETA/DEEA/NMP/H2O | 1.89 | 0.94 mol/mol | 水含量影响汽化热和反应热 | [ |
| PZ/DMEA/正丁醇/H2O | 1.59 | 4.23 mol/mol | 正丁醇加快吸收速率 | [ |
| AEP/正丙醇/H2O | 2.74 | 1.26 mol/mol | AEP位阻效应有利于再生 | [ |
| MEA/正丁醇/H2O | 2.60 | 4.20 mol/L | 黏度和富相体积更小 | [ |
| MDEA/正丁醇/H2O | — | 2.48 mol/kg | 水和醇的共溶剂效应 | [ |
| MEA/AEEA/DGM/H2O | 2.69 | 2.08 mol/kg | 富相黏度低 | [ |
| DMAPA/NHD/H2O | 2.84 | 1.06 mol/mol | 富相体积占比低 | [ |
| AMP/MEA/DGM/H2O | 2.70 | 1.82 mol/kg | DGM加快了吸收速率 | [ |
| DETA/环丁砜/H2O | 2.67 | 2.21 mol/L | 环丁砜增强CO2吸收效率 | [ |
| EHA/DGM/H2O | — | 2.98 mol/kg | 吸收过程受液相传质控制 | [ |
| DAP/TMEDA/H2O | — | 3.20 mol/kg | TMEDA是良好的质子受体 | [ |
| DGA/PMDETA/EG | 2.26 | 3.11 mol/kg | 非水体系,EG调节富相黏度 | [ |
| 吸收剂 | 再生能耗/(GJ/t CO2) | 吸收负载 | 特性/结论 | 文献 |
|---|---|---|---|---|
| [DETAH][Tz]/正丙醇/H2O | 1.99 | 1.71 mol/mol | 降低IL的高黏度 | [ |
| [TETAH][Lys]/乙醇/H2O | — | 2.42 mol/mol | 降低黏度,强化再生 | [ |
| [TETA]Br/PMDETA/H2O | — | 2.63 mol/L | 富相体积小,胺促进吸收 | [ |
| [DETAH][Py]/DGM/H2O | — | 1.52 mol/mol | 亲水性物理溶剂利于相变 | [ |
| [TEPAH][Pro]/正丙醇/H2O | 2.49 | 2.01 mol/mol | 反应活化能低,速率常数大 | [ |
| [DMAPA][TZ]/NHD/H2O | 1.35 | 1.95 mol/L | 不挥发,不易燃,高稳定性 | [ |
| [DMAPA][TZ]/PC/H2O | 1.59 | 3.24 mol/kg | 低腐蚀性 | [ |
Table 4 Ionic liquid phase change absorbents and their relative studies
| 吸收剂 | 再生能耗/(GJ/t CO2) | 吸收负载 | 特性/结论 | 文献 |
|---|---|---|---|---|
| [DETAH][Tz]/正丙醇/H2O | 1.99 | 1.71 mol/mol | 降低IL的高黏度 | [ |
| [TETAH][Lys]/乙醇/H2O | — | 2.42 mol/mol | 降低黏度,强化再生 | [ |
| [TETA]Br/PMDETA/H2O | — | 2.63 mol/L | 富相体积小,胺促进吸收 | [ |
| [DETAH][Py]/DGM/H2O | — | 1.52 mol/mol | 亲水性物理溶剂利于相变 | [ |
| [TEPAH][Pro]/正丙醇/H2O | 2.49 | 2.01 mol/mol | 反应活化能低,速率常数大 | [ |
| [DMAPA][TZ]/NHD/H2O | 1.35 | 1.95 mol/L | 不挥发,不易燃,高稳定性 | [ |
| [DMAPA][TZ]/PC/H2O | 1.59 | 3.24 mol/kg | 低腐蚀性 | [ |
| 1 | 季东, 王健, 王可, 等. 不同CO2捕集技术的CO2耦合绿氢制甲醇工艺研究[J]. 化工学报, 2022, 73(10): 4565-4575. |
| Ji D, Wang J, Wang K, et al. Process research of methanol production by CO2 coupled green hydrogen with different CO2 capture technologies[J]. CIESC Journal, 2022, 73(10): 4565-4575. | |
| 2 | Subramanian N, Madejski P. Analysis of CO2 capture process from flue-gases in combined cycle gas turbine power plant using post-combustion capture technology[J]. Energy, 2023, 282: 128311. |
| 3 | Zhang Z Y, Zhao K, Yi P J, et al. Transition of chemical production pattern motivated by CO2 utilization: multi-dimensional evaluation and future projections[J]. Chemical Engineering Journal, 2024, 488: 150827. |
| 4 | Heidaryan E, Aghel B, Sahraie S, et al. Enhanced carbon dioxide absorption using alcohol solvents in a microreactor: comparison with MEA + water mixture[J]. Journal of the Taiwan Institute of Chemical Engineers, 2023, 151: 105105. |
| 5 | Gautam A, Mondal M K. Review of recent trends and various techniques for CO2 capture: special emphasis on biphasic amine solvents[J]. Fuel, 2023, 334: 126616. |
| 6 | 王皓, 唐思扬, 钟山, 等. MEA吸收CO2富液解吸过程中固体颗粒表面的强化作用分析[J]. 化工学报, 2023, 74(4): 1539-1548. |
| Wang H, Tang S Y, Zhong S, et al. An investigation of the enhancing effect of solid particle surface on the CO2 desorption behavior in chemical sorption process with MEA solution[J]. CIESC Journal, 2023, 74(4): 1539-1548. | |
| 7 | Gao W L, Liang S Y, Wang R J, et al. Industrial carbon dioxide capture and utilization: state of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| 8 | Zhang G Y, Liu J S, Qian J, et al. Review of research progress and stability studies of amine-based biphasic absorbents for CO2 capture[J]. Journal of Industrial and Engineering Chemistry, 2024, 134: 28-50. |
| 9 | Yang Y H, Du T, Li Y N, et al. Techno-economic assessment and exergy analysis of iron and steel plant coupled MEA- CO2 capture process[J]. Journal of Cleaner Production, 2023, 416: 137976. |
| 10 | Du J X, Yang W, Xu L L, et al. Review on post-combustion CO2 capture by amine blended solvents and aqueous ammonia[J]. Chemical Engineering Journal, 2024, 488: 150954. |
| 11 | Lu S J, Zhang J J, Lang L X, et al. Amino acids to reduce the escape of organic amines in the CO2 capture process[J]. Separation and Purification Technology, 2024, 350: 127659. |
| 12 | Aghel B, Janati S, Wongwises S, et al. Review on CO2 capture by blended amine solutions[J]. International Journal of Greenhouse Gas Control, 2022, 119: 103715. |
| 13 | Peirce S, Perfetto R, Russo M E, et al. Characterization of technical grade carbonic anhydrase as biocatalyst for CO2 capture in potassium carbonate solutions[J]. Greenhouse Gases: Science and Technology, 2018, 8(2): 279-291. |
| 14 | Bavarella S, Luqmani B, Thomas N, et al. CO2 absorption into aqueous ammonia using membrane contactors: role of solvent chemistry and pore size on solids formation for low energy solvent regeneration[J]. Separation and Purification Technology, 2022, 290: 120786. |
| 15 | 马双忱, 陈公达, 温佳琪, 等. 氨法脱碳过程中氨逃逸规律及其抑制[J]. 化工学报, 2016, 67(5): 2064-2069. |
| Ma S C, Chen G D, Wen J Q, et al. Ammonia escape and its prevention in CO2 absorption process using ammonia solution[J]. CIESC Journal, 2016, 67(5): 2064-2069. | |
| 16 | Zhang Y, Dong L H, Feng D D, et al. Kinetic properties of solventing out crystallization of ammonium bicarbonate in a novel ammonia carbon capture system[J]. Carbon Capture Science & Technology, 2022, 5: 100077. |
| 17 | Zhan X H, Lv B H, Yang K X, et al. Dual-functionalized ionic liquid biphasic solvent for carbon dioxide capture: high-efficiency and energy saving[J]. Environmental Science & Technology, 2020, 54(10): 6281-6288. |
| 18 | Kumar S, Mondal M K. Selection of efficient absorbent for CO2 capture from gases containing low CO2 [J]. Korean Journal of Chemical Engineering, 2020, 37(2): 231-239. |
| 19 | Jing G H, Liu F, Lv B H, et al. Novel ternary absorbent: dibutylamine aqueous-organic solution for CO2 capture[J]. Energy & Fuels, 2017, 31(11): 12530-12539. |
| 20 | Cao Y D, Yang C L, Wang C, et al. Evaluation of the rapid phase change absorbents based on potassium glycinate for CO2 capture[J]. Chemical Engineering Science, 2023, 273: 118627. |
| 21 | Dreillard M, Broutin P, Briot P, et al. Application of the DMXTM CO2 capture process in steel industry[J]. Energy Procedia, 2017, 114: 2573-2589. |
| 22 | Papadopoulos A I, Tzirakis F, Tsivintzelis I, et al. Phase-change solvents and processes for postcombustion CO2 capture: a detailed review[J]. Industrial & Engineering Chemistry Research, 2019, 58(13): 5088-5111. |
| 23 | Jiang W F, Wu F, Gao G, et al. Absorption performance and reaction mechanism study on a novel anhydrous phase change absorbent for CO2 capture[J]. Chemical Engineering Journal, 2021, 420: 129897. |
| 24 | Chen W D, Chen M S, Jiang B, et al. The improvement of ionic liquids on CO2 capture with biphasic absorbents[J]. Chemical Engineering Journal, 2024, 493: 152720. |
| 25 | Longeras O, Gautier A, Ballerat-Busserolles K, et al. Tuning critical solution temperature for CO2 capture by aqueous solution of amine[J]. Journal of Molecular Liquids, 2021, 343: 117628. |
| 26 | Wang R J, Zhao H J, Wang Y C, et al. Development of biphasic solvent for CO2 capture by tailoring the polarity of amine solution[J]. Fuel, 2022, 325: 124885. |
| 27 | Wang L D, An S L, Yu S H, et al. Mass transfer characteristics of CO2 absorption into a phase-change solvent in a wetted-wall column[J]. International Journal of Greenhouse Gas Control, 2017, 64: 276-283. |
| 28 | An S L, Huang X, Li N, et al. Comprehensive performance of a diethylenetriamine/2-diethylaminoethanol biphasic absorbent for CO2 capture[J]. Fuel, 2023, 353: 129178. |
| 29 | Papadopoulos A I, Perdomo F A, Tzirakis F, et al. Molecular engineering of sustainable phase-change solvents: from digital design to scaling-up for CO2 capture[J]. Chemical Engineering Journal, 2021, 420: 127624. |
| 30 | Kazepidis P, Papadopoulos A I, Tzirakis F, et al. Optimum design of industrial post-combustion CO2 capture processes using phase-change solvents[J]. Chemical Engineering Research and Design, 2021, 175: 209-222. |
| 31 | Nessi E, Papadopoulos A I, Kazepidis P, et al. Pilot scale assessment of a novel phase-change solvent for energy efficient post-combustion CO2 capture[J]. Journal of Environmental Management, 2022, 317: 115489. |
| 32 | Mouhoubi S, Dubois L, Loldrup Fosbøl P, et al. Thermodynamic modeling of CO2 absorption in aqueous solutions of N,N-diethylethanolamine (DEEA) and N-methyl-1,3-propanediamine (MAPA) and their mixtures for carbon capture process simulation[J]. Chemical Engineering Research and Design, 2020, 158: 46-63. |
| 33 | Tzirakis F, Tsivintzelis I, Papadopoulos A I, et al. Experimental measurement and assessment of equilibrium behaviour for phase change solvents used in CO2 capture[J]. Chemical Engineering Science, 2019, 199: 20-27. |
| 34 | Tzirakis F, Tsivintzelis I, Papadopoulos A I, et al. Experimental investigation of phase change amine solutions used in CO2 capture applications: systems with dimethylcyclohexylamine (DMCA) and N-cyclohexyl-1,3-propanediamine (CHAP) or 3-methylaminopropylamine (MAPA)[J]. International Journal of Greenhouse Gas Control, 2021, 109: 103353. |
| 35 | Kontos G, Soldatou M A, Tzimpilis E, et al. Solubility of CO2 in 2-amino-2-methyl-1-propanol (AMP) and 3-(methylamino)propylamine (MAPA): experimental investigation and modeling with the cubic-plus-association and the modified Kent-Eisenberg models[J]. Separations, 2022, 9(11): 338. |
| 36 | Dhanraj D, Biswas P. Influence of particles on amine losses during CO2 capture: a process simulation coupled aerosol dynamics model[J]. International Journal of Greenhouse Gas Control, 2020, 103: 103179. |
| 37 | Muchan P L, Kruthasoot S, Kongton T, et al. Development of a predictive model to correlate the chemical structure of amines with their oxidative degradation rate in a post-combustion amine-based CO2 capture process using multiple linear regression and machine learning regression approaches[J]. ACS Omega, 2024, 9(6): 6669-6683. |
| 38 | Bai L J, Zhao D F, Zhong X K, et al. Comprehensive technical analysis of CO2 absorption into a promising blended amine of DEEA-HMDA[J]. Chemical Engineering Science, 2023, 280: 119025. |
| 39 | Wang L D, Liu S S, Wang R J, et al. Regulating phase separation behavior of a DEEA-TETA biphasic solvent using sulfolane for energy-saving CO2 capture[J]. Environmental Science & Technology, 2019, 53(21): 12873-12881. |
| 40 | Liu F, Fang M X, Dong W F, et al. Carbon dioxide absorption in aqueous alkanolamine blends for biphasic solvents screening and evaluation[J]. Applied Energy, 2019, 233: 468-477. |
| 41 | Liu F, Fang M X, Yi N T, et al. Biphasic behaviors and regeneration energy of a 2-(diethylamino)-ethanol and 2-((2-aminoethyl)amino) ethanol blend for CO2 capture[J]. Sustainable Energy & Fuels, 2019, 3(12): 3594-3602. |
| 42 | Khan S N, Abbas F, Enujekwu F M, et al. Preparation of ionic liquids amine hybrid solvents, characterization for CO2 absorption, and kinetic performance[J]. Journal of Molecular Liquids, 2024, 394: 123725. |
| 43 | Kim J, Kim K, Lim H, et al. Structural investigation of aqueous amine solutions for CO2 capture: CO2 loading, cyclic capacity, absorption-desorption rate, and pKa [J]. Journal of Environmental Chemical Engineering, 2024, 12(3): 112664. |
| 44 | Liu F, Rochelle G T, Wang T, et al. CO2 absorption rate in biphasic solvent of aminoethylethanolamine and diethylethanolamine[J]. Chemical Engineering Journal, 2021, 404: 126503. |
| 45 | 段旭东, 文键, 王斯民. 缠绕管式换热器壳程高黏度流体流动及换热性能强化研究[J]. 高校化学工程学报, 2022, 36(5): 639-646. |
| Duan X D, Wen J, Wang S M. Enhanced flow and heat transfer performance of high viscosity fluid in shell side of spiral-winding tube heat exchangers[J]. Journal of Chemical Engineering of Chinese Universities, 2022, 36(5): 639-646. | |
| 46 | Mota-Martinez M T, Brandl P, Hallett J P, et al. Challenges and opportunities for the utilisation of ionic liquids as solvents for CO2 capture[J]. Molecular Systems Design & Engineering, 2018, 3(3): 560-571. |
| 47 | 陆诗建, 刘含笑, 吴黎明, 等. 具有自萃取功能的相变CO2吸收剂体系开发[J]. 中国电机工程学报, 2024, 44(1): 203-214. |
| Lu S J, Liu H X, Wu L M, et al. Development of CO2 capture phase change absorption system with self extraction[J]. Proceedings of the CSEE, 2024, 44(1): 203-214. | |
| 48 | Zhang Y, Ding B, Zhao D Y, et al. Effect of natural convection and diffusion on liquid-liquid phase separation behaviors of partially miscible solutions with lower critical solution temperature[J]. International Journal of Heat and Mass Transfer, 2023, 201: 123566. |
| 49 | Lu S J, Yang F, Zhang J J, et al. Experimental analysis of reaction heat of CO2 absorption of phase change absorber AEP-DPA at low partial pressure[J]. Energies, 2023, 16(4): 1867. |
| 50 | Wang D L, Liu L, Xie J P, et al. A coupling calculation method of desorption energy distribution applied to CO2 capture by chemical absorption[J]. Processes, 2024, 12(1): 187. |
| 51 | Wang S, Long Q H, Shen S F. Regulating phase change behaviors of water-lean absorbents containing potassium prolinate and 2-butoxyethanol for CO2 capture: effect of water content[J]. Separation and Purification Technology, 2022, 301: 122059. |
| 52 | Zhou X B, Li X L, Wei J W, et al. Novel nonaqueous liquid-liquid biphasic solvent for energy-efficient carbon dioxide capture with low corrosivity[J]. Environmental Science & Technology, 2020, 54(24): 16138-16146. |
| 53 | Meng F Z, Ju T Y, Han S Y, et al. Novel monoethanolamine absorption using ionic liquids as phase splitter for CO2 capture in biogas upgrading: high CH4 purity and low energy consumption[J]. Chemical Engineering Journal, 2023, 462: 142296. |
| 54 | Nasirpour N, Mohammadpourfard M, Zeinali H S. Ionic liquids: promising compounds for sustainable chemical processes and applications[J]. Chemical Engineering Research and Design, 2020, 160: 264-300. |
| 55 | Jiang W F, Li X S, Gao G, et al. Advances in applications of ionic liquids for phase change CO2 capture[J]. Chemical Engineering Journal, 2022, 445: 136767. |
| 56 | Banerjee B. [Bmim]BF4: a versatile ionic liquid for the synthesis of diverse bioactive heterocycles[J]. ChemistrySelect, 2017, 2(27): 8362-8376. |
| 57 | Jia R Q, Xu Y H, Zhang J J, et al. A novel phase change absorbent with ionic liquid as promoter for low energy-consuming CO2 capture[J]. Separation and Purification Technology, 2023, 315: 123740. |
| 58 | Chen Y Y, Zhu C Y, Fu T T, et al. Mass transfer enhancement of CO2 absorption into [Bmim][BF4] aqueous solution in microchannels by heart-shaped grooves[J]. Chemical Engineering and Processing - Process Intensification, 2021, 167: 108536. |
| 59 | Li X L, Zhou X B, Wei J W, et al. Reducing the energy penalty and corrosion of carbon dioxide capture using a novel nonaqueous monoethanolamine-based biphasic solvent[J]. Separation and Purification Technology, 2021, 265: 118481. |
| 60 | Chen Z, Yuan B L, Zhan G X, et al. Energy-efficient biphasic solvents for industrial carbon capture: role of physical solvents on CO2 absorption and phase splitting[J]. Environmental Science & Technology, 2022, 56(18): 13305-13313. |
| 61 | Jia Q Q, Ni Y F, Liu Z T, et al. Fast prediction of lipophilicity of organofluorine molecules: deep learning-derived polarity characters and experimental tests[J]. Journal of Chemical Information and Modeling, 2022, 62(20): 4928-4936. |
| 62 | Zhou X B, Jing G H, Lv B H, et al. Low-viscosity and efficient regeneration of carbon dioxide capture using a biphasic solvent regulated by 2-amino-2-methyl-1-propanol[J]. Applied Energy, 2019, 235: 379-390. |
| 63 | Lv B H, Yang K X, Zhou X B, et al. 2-Amino-2-methyl-1-propanol based non-aqueous absorbent for energy-efficient and non-corrosive carbon dioxide capture[J]. Applied Energy, 2020, 264: 114703. |
| 64 | Ye J X, Jiang C K, Chen H, et al. Novel biphasic solvent with tunable phase separation for CO2 capture: role of water content in mechanism, kinetics, and energy penalty[J]. Environmental Science & Technology, 2019, 53(8): 4470-4479. |
| 65 | Jiang C K, Chen H, Wang J L, et al. Phase splitting agent regulated biphasic solvent for efficient CO2 capture with a low heat duty[J]. Environmental Science & Technology, 2020, 54(12): 7601-7610. |
| 66 | Wang R J, Zhao H J, Qi C R, et al. Novel tertiary amine-based biphasic solvent for energy-efficient CO2 capture with low corrosivity[J]. Energy, 2022, 260: 125045. |
| 67 | Shen L, Liu F, Shen Y, et al. Novel biphasic solvent of AEP/1-propanol/H2O for CO2 capture with efficient regeneration performance and low energy consumption[J]. Separation and Purification Technology, 2021, 270: 118700. |
| 68 | Liu S, Ling H, Lv J, et al. New insights and assessment of primary alkanolamine/sulfolane biphasic solutions for post-combustion CO2 capture: absorption, desorption, phase separation, and technological process[J]. Industrial & Engineering Chemistry Research, 2019, 58(44): 20461-20471. |
| 69 | Zhang W D, Jin X H, Tu W W, et al. Development of MEA-based CO2 phase change absorbent[J]. Applied Energy, 2017, 195: 316-323. |
| 70 | Zhang W D, Jin X H, Tu W W, et al. A novel CO2 phase change absorbent: MEA/1-propanol/H2O[J]. Energy & Fuels, 2017, 31(4): 4273-4279. |
| 71 | Hu Y L, Wang Q, Hu D K, et al. Experimental study on CO2 capture by MEA/n-butanol/H2O phase change absorbent[J]. RSC Advances, 2024, 14(5): 3146-3157. |
| 72 | Fang J W, Jin X H, Cui C H, et al. Evaluation of phase separation behavior of amine + organic solvent + H2O phase change absorbents[J]. Chemical Engineering Journal, 2023, 466: 143332. |
| 73 | Zhou X B, Liu C, Fan Y M, et al. Energy-efficient carbon dioxide capture using a novel low-viscous secondary amine-based nonaqueous biphasic solvent: performance, mechanism, and thermodynamics[J]. Energy, 2022, 255: 124570. |
| 74 | Hu H T, Fang M X, Liu F, et al. Novel alkanolamine-based biphasic solvent for CO2 capture with low energy consumption and phase change mechanism analysis[J]. Applied Energy, 2022, 324: 119570. |
| 75 | Wang N, Peng Z Q, Gao H X, et al. New insight and evaluation of secondary amine/N-butanol biphasic solutions for CO2 capture: equilibrium solubility, phase separation behavior, absorption rate, desorption rate, energy consumption and ion species[J]. Chemical Engineering Journal, 2022, 431: 133912. |
| 76 | Hong S M, Li T, Xiao M, et al. A low energy-consuming phase change absorbent of MAE/DGM/H2O for CO2 capture[J]. Chemical Engineering Journal, 2024, 480: 148079. |
| 77 | Li X S, Liu J, Jiang W F, et al. Low energy-consuming CO2 capture by phase change absorbents of amine/alcohol/H2O[J]. Separation and Purification Technology, 2021, 275: 119181. |
| 78 | Liu J, Li X S, Zhang Z W, et al. Promotion of CO2 capture performance using piperazine (PZ) and diethylenetriamine (DETA) bi-solvent blends[J]. Greenhouse Gases: Science and Technology, 2019, 9(2): 349-359. |
| 79 | Gao G, Jiang W F, Li X S, et al. Novel assessment of highly efficient polyamines for post-combustion CO2 capture: absorption heat, reaction rate, CO2 cyclic capacity, and phase change behavior[J]. Separation and Purification Technology, 2023, 306: 122615. |
| 80 | Jin X H, Fang J W, Ma Q, et al. Effect of amine properties on developing CO2 phase change absorbents by means of cosolvent effect[J]. Separation and Purification Technology, 2022, 289: 120630. |
| 81 | Tiwari S C, Agarwal M, Pant K K, et al. A comparative study of polyamine and piperazine as promoter for CO2 absorption performance in aqueous methyldiethanolamine blend system: 430 MW power plant data simulation and economic assessment[J]. Sustainable Chemistry for the Environment, 2023, 4: 100054. |
| 82 | Yang F S, Jin X H, Fang J W, et al. Development of CO2 phase change absorbents by means of the cosolvent effect[J]. Green Chemistry, 2018, 20(10): 2328-2336. |
| 83 | 张琪悦, 方梦祥, 周康, 等. 燃煤烟气碳捕集两相吸收剂开发及其性能研究[J]. 热力发电, 2023, 52(04): 24-33. |
| Zhang Q Y, Fang M X, Zhou K, et al. Development and property study of two-phase absorbent for carbon capture in coal-fired flue gas[J]. Thermal Power Generation, 2023, 52(4): 24-33. | |
| 84 | Zhang R, Luo X, Yang Q, et al. Impact of the inter- and intramolecular tertiary amino group on the primary amino group in the CO2 absorption process[J]. Industrial & Engineering Chemistry Research, 2016, 55(26): 7210-7217. |
| 85 | Chen Y R, Jiang W S, Luo X, et al. The study of kinetics of CO2 absorption into 3-dimethylaminopropylamine and 3-diethylaminopropylamine aqueous solution[J]. International Journal of Greenhouse Gas Control, 2018, 75: 214-223. |
| 86 | Qiu Y J, Lu H F, Zhu Y M, et al. Phase-change CO2 absorption using novel 3-dimethylaminopropylamine with primary and tertiary amino groups[J]. Industrial & Engineering Chemistry Research, 2020, 59(19): 8902-8910. |
| 87 | Yao H, Lu H F, Wu K J, et al. Kinetics and thermodynamics of CO2 absorption in aqueous 3-dimethylaminopropylamine and its blend with poly(ethylene glycol) dimethyl ether using a wetted wall column reactor[J]. Energy & Fuels, 2023, 37(12): 8352-8363. |
| 88 | Kummamuru N B, Eimer D A, Idris Z. Viscosity measurement and correlation of unloaded and CO2-loaded aqueous solutions of N-methyldiethanolamine + 2-amino-2-methyl-1-propanol[J]. Journal of Chemical & Engineering Data, 2020, 65(6): 3072-3078. |
| 89 | Liu F, Fang M X, Yi N T, et al. Research on alkanolamine-based physical-chemical solutions as biphasic solvents for CO2 capture[J]. Energy & Fuels, 2019, 33(11): 11389-11398. |
| 90 | Wang L D, Yu S H, Li Q W, et al. Performance of sulfolane/DETA hybrids for CO2 absorption: phase splitting behavior, kinetics and thermodynamics[J]. Applied Energy, 2018, 228: 568-576. |
| 91 | Wang L D, Zhang Y F, Wang R J, et al. Advanced monoethanolamine absorption using sulfolane as a phase splitter for CO2 capture[J]. Environmental Science & Technology, 2018, 52(24): 14556-14563. |
| 92 | Lv J, Liu S, Ling H, et al. Development of a promising biphasic absorbent for postcombustion CO2 capture: sulfolane +2-(methylamino)ethanol + H2O[J]. Industrial & Engineering Chemistry Research, 2020, 59(32): 14496-14506. |
| 93 | Fu K, Zheng M Z, Fu D. Low partial pressure CO2 capture in packed tower by EHA+Diglyme water-lean absorbent[J]. Energy, 2023, 266: 126530. |
| 94 | Liu A H, Zheng Y J, Ma G T, et al. Dual-functionalized amines as single-component water-lean CO2 absorbents with low-viscosity and high-capacity: the synergistic effect of alkoxy and silyl groups[J]. Separation and Purification Technology, 2024, 333: 125922. |
| 95 | Liu J S, Qian J, He Y. Water-lean triethylenetetramine/N, N-diethylethanolamine/n-propanol biphasic solvents: phase-separation performance and mechanism for CO2 capture[J]. Separation and Purification Technology, 2022, 289: 120740. |
| 96 | Gu L N, Hou X Y, Jin L J, et al. Phase change study of a new two-phase absorbent based on DAP[J]. The Journal of Physical Chemistry. B, 2024, 128(7): 1737-1747. |
| 97 | Fu K, Zheng M Z, Wang H J, et al. Effect of water content on the characteristics of CO2 capture processes in absorbents of 2-ethylhexan-1-amine + diglyme[J]. Energy, 2022, 244: 122656. |
| 98 | Wu K X, Zhou X B, Wu X M, et al. Understanding the corrosion behavior of carbon steel in amino-functionalized ionic liquids for CO2 capture assisted by weight loss and electrochemical techniques[J]. International Journal of Greenhouse Gas Control, 2019, 83: 216-227. |
| 99 | He S, Qiu Y P, Sun Y J, et al. Corrosion behavior of AISI 1020 steel in MEA and [Bmim]BF4 mixed solution containing saturated CO2 [J]. International Journal of Greenhouse Gas Control, 2020, 94: 102931. |
| 100 | Li X Y, Wang Y H, Lu H F, et al. Phase splitting rules of the primary/secondary amine-tertiary amine systems: experimental rapid screening and corrected quasi-activity coefficient model[J]. Industrial & Engineering Chemistry Research, 2022, 61(23): 7709-7717. |
| 101 | Shen Z W, Tang S Y, Lu H F, et al. Effect of alcohols on CO2 absorption, phase splitting, and desorption behaviors of biphasic anhydrous system: the primary amine-tertiary amine of DGA-PMDETA[J]. Chemical Engineering Science, 2023, 281: 119083. |
| 102 | 杨灿, 孙雪琦, 尚明华, 等. 相变离子液体体系吸收分离CO2的研究现状及展望[J]. 化工学报, 2023, 74(04): 1419-1432. |
| Yang C, Sun X Q, Shang M H, et al. Research status and prospect of CO2 absorption and separation by phase-change ionic liquid systems[J]. CIESC Journal, 2023, 74(4): 1419-1432. | |
| 103 | Huang Q S, Jing G H, Zhou X B, et al. A novel biphasic solvent of amino-functionalized ionic liquid for CO2 capture: high efficiency and regenerability[J]. Journal of CO2 Utilization, 2018, 25: 22-30. |
| 104 | Zhou H C, Xu X, Chen X C, et al. Novel ionic liquids phase change solvents for CO2 capture[J]. International Journal of Greenhouse Gas Control, 2020, 98: 103068. |
| 105 | Han W F, Yue W H, Yuan M H, et al. A functionalized ionic liquid phase change absorbent ([DETAH][Py]-DEDM-water) for reversible carbon dioxide capture[J]. Journal of Molecular Liquids, 2023, 392: 123480. |
| 106 | Song T, Chen W Q, Zhang Y C, et al. Mass transfer and reaction mechanism of CO2 capture into a novel amino acid ionic liquid phase-change absorbent[J]. Separation and Purification Technology, 2024, 330: 125538. |
| 107 | Mao J M, Yun Y B, Li M, et al. Dual-functionalized ionic liquid biphasic solvents with aqueous-lean for industrial carbon capture: energy-saving and high efficiency[J]. Separation and Purification Technology, 2023, 315: 123722. |
| 108 | Mao J M, Li C, Yun Y B, et al. Biphasic solvents based on dual-functionalized ionic liquid for enhanced post-combustion CO2 capture and corrosion inhibition during the absorption process[J]. Chemical Engineering Journal, 2024, 481: 148691. |
| 109 | Yagihara K, Fukushima K, Ohno H, et al. Assessing economic trade-off for advances in amine-based post-combustion capture technology[J]. International Journal of Greenhouse Gas Control, 2024, 132: 104068. |
| 110 | Guo H, Li C X, Shi X Q, et al. Nonaqueous amine-based absorbents for energy efficient CO2 capture[J]. Applied Energy, 2019, 239: 725-734. |
| 111 | Yang J Y, Yu W, Wang T, et al. Process simulations of the direct non-aqueous gas stripping process for CO2 desorption[J]. Industrial & Engineering Chemistry Research, 2020, 59(15): 7121-7129. |
| 112 | Wilfong W C, Ji T, Bao Z H, et al. Big data analysis and technical review of regeneration for carbon capture processes[J]. Energy & Fuels, 2023, 37(16): 11497-11531. |
| 113 | Mantripragada H C, Zhai H B, Rubin E S. Boundary Dam or Petra Nova-Which is a better model for CCS energy supply?[J]. International Journal of Greenhouse Gas Control, 2019, 82: 59-68. |
| 114 | He S, Zheng Y W. CO2 capture cost reduction potential of the coal-fired power plants under high penetration of renewable power in China[J]. Energies, 2024, 17(9): 2050. |
| [1] | Junbing XIAO, Xiangyu ZHONG, Jiandi REN, Fangfang ZHONG, Changhui LIU, Chuankun JIA. Research on the heat storage properties of stearic acid phase change materials enhanced by bio-carbon materials [J]. CIESC Journal, 2025, 76(3): 1312-1322. |
| [2] | Xiankai ZHANG, Boyu WANG, Yali GUO, Shengqiang SHEN. Calculation and analysis of thermal performance of horizontal circular tube falling film evaporative condenser [J]. CIESC Journal, 2025, 76(3): 995-1005. |
| [3] | Yiming ZHANG, Peng YANG, Xianbing JI, Jixing REN, Lei ZHANG, Zheng MIAO. Thermal performance of multi-loop flat loop heat pipes [J]. CIESC Journal, 2025, 76(3): 1018-1028. |
| [4] | Junbing XIAO, Bo ZOU, Jiandi REN, Changhui LIU, Chuankun JIA. Research on heat storage performance of chloride composite molten salt based on phase diagram analysis [J]. CIESC Journal, 2025, 76(3): 963-974. |
| [5] | Jiayi YAO, Donghui ZHANG, Zhongli TANG, Wenbin LI. Research on carbon capture by pressure swing adsorption based on two-stage dual reflux [J]. CIESC Journal, 2025, 76(2): 744-754. |
| [6] | Jinning YANG, Weifan WANG, Dong XU, Yi LIU, Xiaohan WENG, Ye YUAN, Zhi WANG. Progress in the scale-up research of membrane technologies for industrial flue gas carbon capture [J]. CIESC Journal, 2025, 76(2): 504-518. |
| [7] | Qi ZHANG, Rui ZHANG, Tao ZHENG, Xin CAO, Zhichang LIU, Haiyan LIU, Chunming XU, Rong ZHANG, Xianghai MENG. Revealing CO2 capture by a novel dual-cation protic ionic liquid using molecular simulation [J]. CIESC Journal, 2025, 76(2): 797-811. |
| [8] | Angran ZHAO, Yongqiang HAN, Zhipeng WANG, Pengfei LI, Yawei XU, Huiling TONG. Experimental study on simultaneous desulfurization and denitrification of red mud at low temperature [J]. CIESC Journal, 2024, 75(S1): 276-282. |
| [9] | Zhangzhou WANG, Tianqi TANG, Jiajun XIA, Yurong HE. Battery thermal management performance simulation based on composite phase change material [J]. CIESC Journal, 2024, 75(S1): 329-338. |
| [10] | Siyu QIN, Yijia LIU, Jiacheng YANG, Wei TONG, Liwen JIN, Xiangzhao MENG. Characteristics of gas-liquid two-phase heat transfer in a confined vapor chamber [J]. CIESC Journal, 2024, 75(S1): 47-55. |
| [11] | Wenbo ZHOU, Jiangwei YIN, Dan ZHANG, Yue YANG, Jiahao YU, Bingchao ZHAO. Experimental study on evaporation of aqueous NaCl solution droplet heating by thermal irradiation [J]. CIESC Journal, 2024, 75(S1): 85-94. |
| [12] | Dehui DU, Wei FENG, Jianghui ZHANG, Yanlong XIANG, Gaopan QIAO, Wei LI. Prediction model of flow boiling heat transfer in microfinned hydrophobic composite enhanced tube [J]. CIESC Journal, 2024, 75(S1): 95-107. |
| [13] | Yong YANG, Zixuan ZU, Yukun LI, Dongliang WANG, Zongliang FAN, Huairong ZHOU. Numerical simulation of CO2 absorption by alkali liquor in T-junction cylindrical microchannels [J]. CIESC Journal, 2024, 75(S1): 135-142. |
| [14] | Ziliang ZHU, Shuang WANG, Yu'ang JIANG, Mei LIN, Qiuwang WANG. Solid-liquid phase change algorithm with Euler-Lagrange iteration [J]. CIESC Journal, 2024, 75(8): 2763-2776. |
| [15] | Jiuzhe QU, Peng YANG, Xufei YANG, Wei ZHANG, Bo YU, Dongliang SUN, Xiaodong WANG. Experimental study on flow boiling in silicon-based microchannels with micropillar cluster arrays [J]. CIESC Journal, 2024, 75(8): 2840-2851. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||