CIESC Journal ›› 2019, Vol. 70 ›› Issue (3): 791-800.DOI: 10.11949/j.issn.0438-1157.20181067
• Reviews and monographs • Previous Articles Next Articles
Shaojuan ZENG1( ),Dawei SHANG1,Min YU1,2,Hao CHEN3,Haifeng DONG1,Xiangping ZHANG1(
),Dawei SHANG1,Min YU1,2,Hao CHEN3,Haifeng DONG1,Xiangping ZHANG1( )
)
Received:2018-09-25
Revised:2018-12-07
Online:2019-03-05
Published:2019-03-05
Contact:
Xiangping ZHANG
曾少娟1( ),尚大伟1,余敏1,2,陈昊3,董海峰1,张香平1(
),尚大伟1,余敏1,2,陈昊3,董海峰1,张香平1( )
)
通讯作者:
张香平
作者简介:<named-content content-type="corresp-name">曾少娟</named-content>(1982—),女,博士,副研究员,<email>sjzeng@ipe.ac.cn</email>|张香平(1969—),女,博士,研究员,<email>xpzhang@ipe.ac.cn</email>
基金资助:CLC Number:
Shaojuan ZENG, Dawei SHANG, Min YU, Hao CHEN, Haifeng DONG, Xiangping ZHANG. Applications and perspectives of NH3 separation and recovery with ionic liquids[J]. CIESC Journal, 2019, 70(3): 791-800.
曾少娟, 尚大伟, 余敏, 陈昊, 董海峰, 张香平. 离子液体在氨气分离回收中的应用及展望[J]. 化工学报, 2019, 70(3): 791-800.
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgxb.cip.com.cn/EN/10.11949/j.issn.0438-1157.20181067
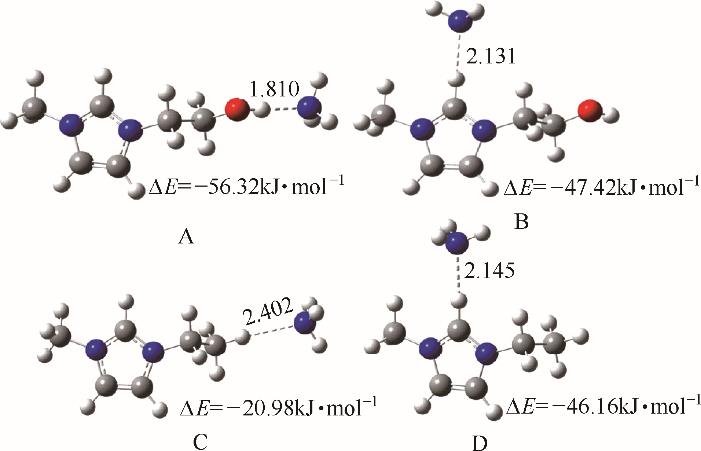
Fig.6 Optimized structures and interaction energies for [EOHmim]+-NH3 (A and B) and [Emim]+ -NH 3 (C and D) systems[38] (O, red; N, blue; H, white; C, gray) (copyright 2015 Royal Society of Chemistry)
| 离子液体种类 | 吸收温度/℃ | 压力/kPa | NH3吸收量 | 参考文献 | |
|---|---|---|---|---|---|
| /(mol NH3?(mol absorbent)-1) | /(g NH3?(g absorbent)-1) | ||||
| [Emim][BF4] | 20 | 140 | 0.274 | 0.024 | [ |
| [Emim][BF4] | 25 | 110 | 0.173 | 0.015 | [ |
| [Bmim][BF4] | 25 | 220 | 0.465 | 0.035 | [ |
| [Hmim][BF4] | 25 | 220 | 0.581 | 0.039 | [ |
| [Omim][BF4] | 25 | 120 | 0.387 | 0.025 | [ |
| [Bmim][PF6] | 25 | 174 | 0.538 | 0.032 | [ |
| [Bmim][BF4] | 25.4 | 101 | 0.112 | 0.008 | [ |
| [Emim][NTf2] | 26.4 | 101 | 0.122 | 0.005 | [ |
| [Hmim][Cl] | 24.8 | 101 | 0.252 | 0.021 | [ |
| [Emim][Ac] | 25.3 | 101 | 0.348 | 0.035 | [ |
| [Emim][EtOSO3] | 24.6 | 101 | 0.342 | 0.025 | [ |
| [Emim][SCN] | 25 | 101 | 0.188 | 0.019 | [ |
| [DMEA][Ac] | 25 | 163 | 0.905 | 0.103 | [ |
| [Bmim][BF4] | 20 | 101 | 0.449 | 0.034 | [ |
| [EOHmim][BF4] | 20 | 101 | 1.703 | 0.135 | [ |
| [Choline][NTf2] | 20 | 101 | 1.857 | 0.064 | [ |
| [MTEOA][MeOSO3] | 20 | 101 | 3.545 | 0.219 | [ |
| [MTEOA][MeOSO3] | 40 | 101 | 1.381 | 0.085 | [ |
| [EtOHmim][DCA] | 20 | 101 | 2.125 | 0.190 | [ |
| [EtOHmim][DCA] | 40 | 100 | 0.887 | 0.079 | [ |
| [EtOHmim][NTf2] | 25 | 105.5 | 0.976 | 0.041 | [ |
| [EtOHmim][PF6] | 25 | 106.8 | 0.980 | 0.062 | [ |
| [EtOHmim][BF4] | 25 | 98.9 | 0.825 | 0.066 | [ |
| [EtOHmim][SCN] | 25 | 95.3 | 0.538 | 0.050 | [ |
| [EtOHmim][DCA] | 25 | 108.5 | 0.600 | 0.054 | [ |
| [EtOHmim][NO3] | 25 | 106.6 | 0.522 | 0.047 | [ |
| [Eim][NTf2] | 40 | 105.12 | 2.73 | 0.123 | [ |
| [Mim][NTf2] | 40 | 102.71 | 2.68 | 0.128 | [ |
| [Mmim][NTf2] | 40 | 98.63 | 2.40 | 0.108 | [ |
| [Bmmim][NTf2] | 30 | 119.43 | 0.25 | 0.010 | [ |
| [Bmmim][NTf2] | 40 | 100.49 | 0.20 | 0.008 | [ |
| [Bmmim][NTf2] | 60 | 114.60 | 0.19 | 0.008 | [ |
| [Bim][SCN] | 30 | 98.82 | 2.18 | 0.202 | [ |
| [Bim][SCN] | 40 | 96.59 | 1.96 | 0.182 | [ |
| [Bim][SCN] | 60 | 102.56 | 1.56 | 0.145 | [ |
| [Bmim][SCN] | 40 | 82.92 | 0.15 | 0.013 | [ |
| [Bmim][SCN] | 60 | 79.87 | 0.09 | 0.008 | [ |
| [Bim][NO3] | 30 | 100.09 | 1.50 | 0.136 | [ |
| [Bim][NO3] | 40 | 141.53 | 1.72 | 0.156 | [ |
| [Bim][NO3] | 60 | 122.40 | 1.14 | 0.104 | [ |
| [Bmim][DCA] | 30 | 114.28 | 0.30 | 0.025 | [ |
| [Bmim][DCA] | 40 | 128.32 | 0.22 | 0.018 | [ |
| [Bmim][DCA] | 60 | 104.86 | 0.13 | 0.011 | [ |
| [Bmmim][DCA] | 30 | 115.38 | 0.25 | 0.020 | [ |
| [Bmmim][DCA] | 40 | 103.42 | 0.12 | 0.009 | [ |
| [Bmmim][DCA] | 60 | 126.48 | 0.14 | 0.011 | [ |
| [Bim][NTf2] | 40 | 101 | 2.69 | 0.113 | [ |
| [Bmim][NTf2] | 40 | 101 | 0.28 | 0.011 | [ |
| [HOOC(CH2)3mim][NTf2] | 40 | 101 | 1.54 | 0.059 | [ |
| [Bmim][Zn2Cl5] | 50 | 103.5 | 8.025 | 0.305 | [ |
| [Emim]2[Co(NCS)4] | 30 | 101 | 5.99 | 0.198 | [ |
| [Bmim]2[Co(NCS)4] | 30 | 101 | 6.03 | 0.180 | [ |
| [Hmim]2[Co(NCS)4] | 30 | 101 | 6.09 | 0.166 | [ |
| [Emim][SCN] | 30 | 101 | 0.18 | 0.018 | [ |
| [Bmim][SCN] | 30 | 101 | 0.19 | 0.016 | [ |
| [Hmim][SCN] | 30 | 101 | 0.20 | 0.015 | [ |
| [Bmim][MeSO3]/urea(1∶1) | 30 | 123.6 | — | 0.015 | [ |
| ChCl/Res/Gly (1∶3∶5) | 40 | 101 | — | 0.130 | [ |
Table 1 NH3 capacities of different ionic liquids reported in literatures
| 离子液体种类 | 吸收温度/℃ | 压力/kPa | NH3吸收量 | 参考文献 | |
|---|---|---|---|---|---|
| /(mol NH3?(mol absorbent)-1) | /(g NH3?(g absorbent)-1) | ||||
| [Emim][BF4] | 20 | 140 | 0.274 | 0.024 | [ |
| [Emim][BF4] | 25 | 110 | 0.173 | 0.015 | [ |
| [Bmim][BF4] | 25 | 220 | 0.465 | 0.035 | [ |
| [Hmim][BF4] | 25 | 220 | 0.581 | 0.039 | [ |
| [Omim][BF4] | 25 | 120 | 0.387 | 0.025 | [ |
| [Bmim][PF6] | 25 | 174 | 0.538 | 0.032 | [ |
| [Bmim][BF4] | 25.4 | 101 | 0.112 | 0.008 | [ |
| [Emim][NTf2] | 26.4 | 101 | 0.122 | 0.005 | [ |
| [Hmim][Cl] | 24.8 | 101 | 0.252 | 0.021 | [ |
| [Emim][Ac] | 25.3 | 101 | 0.348 | 0.035 | [ |
| [Emim][EtOSO3] | 24.6 | 101 | 0.342 | 0.025 | [ |
| [Emim][SCN] | 25 | 101 | 0.188 | 0.019 | [ |
| [DMEA][Ac] | 25 | 163 | 0.905 | 0.103 | [ |
| [Bmim][BF4] | 20 | 101 | 0.449 | 0.034 | [ |
| [EOHmim][BF4] | 20 | 101 | 1.703 | 0.135 | [ |
| [Choline][NTf2] | 20 | 101 | 1.857 | 0.064 | [ |
| [MTEOA][MeOSO3] | 20 | 101 | 3.545 | 0.219 | [ |
| [MTEOA][MeOSO3] | 40 | 101 | 1.381 | 0.085 | [ |
| [EtOHmim][DCA] | 20 | 101 | 2.125 | 0.190 | [ |
| [EtOHmim][DCA] | 40 | 100 | 0.887 | 0.079 | [ |
| [EtOHmim][NTf2] | 25 | 105.5 | 0.976 | 0.041 | [ |
| [EtOHmim][PF6] | 25 | 106.8 | 0.980 | 0.062 | [ |
| [EtOHmim][BF4] | 25 | 98.9 | 0.825 | 0.066 | [ |
| [EtOHmim][SCN] | 25 | 95.3 | 0.538 | 0.050 | [ |
| [EtOHmim][DCA] | 25 | 108.5 | 0.600 | 0.054 | [ |
| [EtOHmim][NO3] | 25 | 106.6 | 0.522 | 0.047 | [ |
| [Eim][NTf2] | 40 | 105.12 | 2.73 | 0.123 | [ |
| [Mim][NTf2] | 40 | 102.71 | 2.68 | 0.128 | [ |
| [Mmim][NTf2] | 40 | 98.63 | 2.40 | 0.108 | [ |
| [Bmmim][NTf2] | 30 | 119.43 | 0.25 | 0.010 | [ |
| [Bmmim][NTf2] | 40 | 100.49 | 0.20 | 0.008 | [ |
| [Bmmim][NTf2] | 60 | 114.60 | 0.19 | 0.008 | [ |
| [Bim][SCN] | 30 | 98.82 | 2.18 | 0.202 | [ |
| [Bim][SCN] | 40 | 96.59 | 1.96 | 0.182 | [ |
| [Bim][SCN] | 60 | 102.56 | 1.56 | 0.145 | [ |
| [Bmim][SCN] | 40 | 82.92 | 0.15 | 0.013 | [ |
| [Bmim][SCN] | 60 | 79.87 | 0.09 | 0.008 | [ |
| [Bim][NO3] | 30 | 100.09 | 1.50 | 0.136 | [ |
| [Bim][NO3] | 40 | 141.53 | 1.72 | 0.156 | [ |
| [Bim][NO3] | 60 | 122.40 | 1.14 | 0.104 | [ |
| [Bmim][DCA] | 30 | 114.28 | 0.30 | 0.025 | [ |
| [Bmim][DCA] | 40 | 128.32 | 0.22 | 0.018 | [ |
| [Bmim][DCA] | 60 | 104.86 | 0.13 | 0.011 | [ |
| [Bmmim][DCA] | 30 | 115.38 | 0.25 | 0.020 | [ |
| [Bmmim][DCA] | 40 | 103.42 | 0.12 | 0.009 | [ |
| [Bmmim][DCA] | 60 | 126.48 | 0.14 | 0.011 | [ |
| [Bim][NTf2] | 40 | 101 | 2.69 | 0.113 | [ |
| [Bmim][NTf2] | 40 | 101 | 0.28 | 0.011 | [ |
| [HOOC(CH2)3mim][NTf2] | 40 | 101 | 1.54 | 0.059 | [ |
| [Bmim][Zn2Cl5] | 50 | 103.5 | 8.025 | 0.305 | [ |
| [Emim]2[Co(NCS)4] | 30 | 101 | 5.99 | 0.198 | [ |
| [Bmim]2[Co(NCS)4] | 30 | 101 | 6.03 | 0.180 | [ |
| [Hmim]2[Co(NCS)4] | 30 | 101 | 6.09 | 0.166 | [ |
| [Emim][SCN] | 30 | 101 | 0.18 | 0.018 | [ |
| [Bmim][SCN] | 30 | 101 | 0.19 | 0.016 | [ |
| [Hmim][SCN] | 30 | 101 | 0.20 | 0.015 | [ |
| [Bmim][MeSO3]/urea(1∶1) | 30 | 123.6 | — | 0.015 | [ |
| ChCl/Res/Gly (1∶3∶5) | 40 | 101 | — | 0.130 | [ |
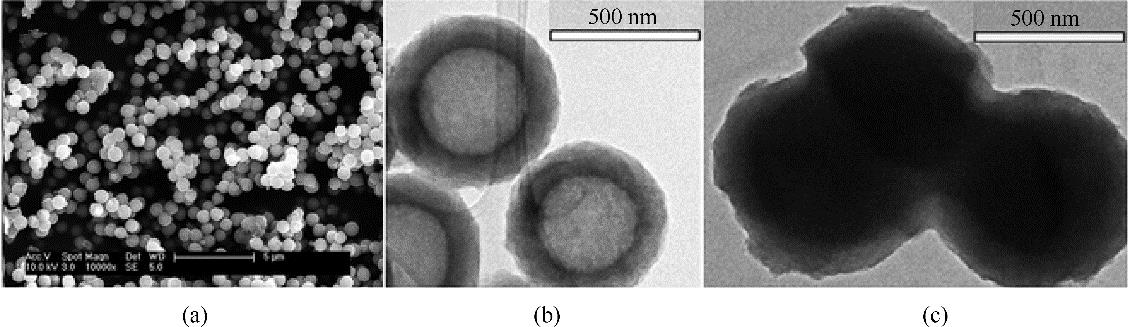
Fig.9 SEM images of carbon submicrocapsules (a), TEM images of carbon submicrocapsules (b) and [EtOHmim][BF4]-based ENILs (c) [49] (copyright 2016 Royal Society of Chemistry)
| 1 | WarnerJ X, DickersonR R, WeiZ, et al. Increased atmospheric ammonia over the world s major agricultural areas detected from space[J]. Geophys. Res. Lett., 2017, 44(6): 2875-2884. |
| 2 | ErismanJ W, BleekerA, GallowayJ, et al. Reduced nitrogen in ecology and the environment[J]. Environ. Pollut., 2007, 150(1): 140-149. |
| 3 | SuttonM A, ErismanJ W, DentenerF, et al. Ammonia in the environment: from ancient times to the present[J]. Environ. Pollut., 2008, 156(3): 583-604. |
| 4 | SchaferD, XiaJ Z, VogtM, et al. Experimental investigation of the solubility of ammonia in methanol[J].J. Chem. Eng. Data, 2007, 52(5): 1653-1659. |
| 5 | WangL, HuangX, YuY, et al. Eliminating ammonia emissions during rare earth separation through control of equilibrium acidity in a HEH(EHP)-Cl system[J]. Green Chem., 2013, 15(7): 1889-1894. |
| 6 | 王芬, 周敏. 焦炉煤气中氨的回收[J]. 洁净煤技术, 2009, (4): 108-111. |
| WangF, ZhouM. Recovery of ammonia from coking product[J]. Clean Coal Technol., 2009,( 4): 108-111. | |
| 7 | 李强. 氨回收系统存在问题及改进措施[J]. 大氮肥, 2011, (6): 418-419. |
| LiQ. Problems in ammonia recovery system and improvement measures[J]. Large Scale Nitrogenous Fertilizer Industry, 2011, (6): 418-419. | |
| 8 | 林璐璐. 氨回收工艺方案的选择[J]. 广东化工, 2016, 43(334): 239-243. |
| LinL L. Process project selection for ammonia recovery[J]. Guangdong Chemical Industry, 2016, 43(334): 239-243. | |
| 9 | LeiZ G, DaiC N, ChenB H. Gas solubility in ionic liquids[J]. Chem. Rev., 2014, 114(2): 1289-1326. |
| 10 | ZengS J, ZhangX P, BaiL, et al. Ionic liquid-based CO2 capture systems: structure, interaction and process[J]. Chem. Rev., 2017, 117(14): 9625-9673. |
| 11 | ZhangX P, ZhangX C, DongH F, et al. Carbon capture with ionic liquids: overview and progress[J]. Energ. Environ. Sci., 2012, 5(5): 6668-6681. |
| 12 | 陈晏杰, 姚月华, 张香平, 等. 基于离子液体的合成氨驰放气中氨回收工艺模拟计算[J]. 过程工程学报, 2011, 11(4): 644-651. |
| ChenY J, YaoY H, ZhangX P, et al. Simulation and optimization of ammonia recovery with ionic liquid from purge gas in ammonia synthesis plant[J]. Chin. J. Process Eng., 2011, 11(4): 644-651. | |
| 13 | ZengS J, GaoH, ZhangX, et al. Efficient and reversible capture of SO2 by pyridinium-based ionic liquids[J]. Chem. Eng. J., 2014, 251: 248-256. |
| 14 | HuangK, CaiD N, ChenY, et al. Thermodynamic validation of 1-alkyl-3-methylimidazolium carboxylates as task-specific ionic liquids for H2S absorption[J]. AIChE J., 2013, 59(6): 2227-2235. |
| 15 | LuoX Y, GuoY, DingF, et al. Significant improvements in CO2 capture by pyridine-containing anion-functionalized ionic liquids through multiple-site cooperative interactions[J]. Angew Chem. Int. Edit., 2014, 53(27): 7053-7057. |
| 16 | ShiW, MaginnE J. Molecular simulation of ammonia absorption in the ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EmimTf2N)[J]. AIChE J., 2009, 55(9): 2414-2421. |
| 17 | LiG H, ZhouQ, ZhangX P, et al. Solubilities of ammonia in basic imidazolium ionic liquids[J]. Fluid Phase Equilibr., 2010, 297(1): 34-39. |
| 18 | HuangW J, SunG M, ZhengD X, et al. Vapor-liquid equilibrium measurements of NH3 + H2O + ionic liquid (DmimCl, DmimBF4, and DmimDMP) systems[J].J. Chem. Eng. Data, 2013, 58(5): 1354-1360. |
| 19 | YokozekiA, ShiflettM B. Ammonia solubilities in room-temperature ionic liquids[J]. Ind. Eng. Chem. Res., 2007, 46(5): 1605-1610. |
| 20 | YokozekiA, ShiflettM B. Vapor-liquid equilibria of ammonia plus ionic liquid mixtures[J]. Appl. Energ., 2007, 84(12): 1258-1273. |
| 21 | AkiS, MelleinB R, SaurerE M, et al. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids[J]. J. Phys. Chem. B, 2004, 108(52): 20355-20365. |
| 22 | HuangX, MargulisC J, LiY, et al. Why is the partial molar volume of CO2 so small when dissolved in a room temperature ionic liquid? Structure and dynamics of CO2 dissolved in [Bmim][PF6][J]. J. Am. Chem. Soc., 2005, 127(50): 17842-17851. |
| 23 | YunusN M, MutalibM I A, ManZ, et al. Solubility of CO2 in pyridinium based ionic liquids[J]. Chem. Eng. J., 2012, 189: 94-100. |
| 24 | KazarianS G, BriscoeB J, WeltonT. Combining ionic liquids and supercritical fluids: in situ ATR-IR study of CO2 dissolved in two ionic liquids at high pressures[J]. Chem. Commun., 2000, 20: 2047-2048. |
| 25 | DongK, ZhangS J, WangD X, et al. Hydrogen bonds in imidazolium ionic liquids[J]. J. Phys. Chem. A, 2006, 110(31): 9775-9782. |
| 26 | CrowhurstL, MawdsleyP R, Perez-ArlandisJ M, et al. Solvent-solute interactions in ionic liquids[J]. Phys. Chem. Chem. Phys., 2003, 5(13): 2790-2794. |
| 27 | CadenaC, AnthonyJ L, ShahJ K, et al. Why is CO2 so soluble in imidazolium-based ionic liquids?[J]. J. Am. Chem. Soc., 2004, 126(16): 5300-5308. |
| 28 | AnthonyJ L, AndersonJ L, MaginnE J, et al. Anion effects on gas solubility in ionic liquids[J]. J. Phys. Chem. B, 2005, 109(13): 6366-6374. |
| 29 | BhargavaB L, BalasubramanianS. Probing anion-carbon dioxide interactions in room temperature ionic liquids: gas phase cluster calculations[J]. Chem. Phys. Lett., 2007, 444(4/5/6): 242-246. |
| 30 | SekiT, GrunwaldtJ D, BaikerA. In situ attenuated total reflection infrared spectroscopy of imidazolium-based room-temperature ionic liquids under “supercritical” CO2[J]. J. Phys. Chem. B, 2009, 113(1): 114-122. |
| 31 | PalomarJ, Gonzalez-MiquelM, BediaJ, et al. Task-specific ionic liquids for efficient ammonia absorption[J]. Sep. Purif. Technol., 2011, 82: 43-52. |
| 32 | RuizE, FerroV R, de RivaJ, et al. Evaluation of ionic liquids as absorbents for ammonia absorption refrigeration cycles using COSMO-based process simulations[J]. Appl. Energ., 2014, 123: 281-291. |
| 33 | BediaJ, PalomarJ, Gonzalez-MiquelM, et al. Screening ionic liquids as suitable ammonia absorbents on the basis of thermodynamic and kinetic analysis[J]. Sep. Purif. Technol., 2012, 95: 188-195. |
| 34 | ZhangX, DongH F, HuangY, et al. Experimental study on gas holdup and bubble behavior in carbon capture systems with ionic liquid[J]. Chem. Eng. J., 2012, 209: 607-615. |
| 35 | HolbreyJ D, ReichertW M, SwatloskiR P, et al. Efficient, halide free synthesis of new, low cost ionic liquids: 1,3-dialkylimidazolium salts containing methyl- and ethyl-sulfate anions[J]. Green Chem., 2002, 4(5): 407-413. |
| 36 | YokozekiA, ShiflettM B. Vapor-liquid equilibria of ammonia + ionic liquid mixtures[J]. Appl. Energ., 2007, 84(12): 1258-1273. |
| 37 | IarikovD D, HacarliogluP, OyamaS T. Supported room temperature ionic liquid membranes for CO2/CH4 separation[J]. Chem. Eng. J., 2011, 166(1): 401-406. |
| 38 | LiZ J, ZhangX P, DongH F, et al. Efficient absorption of ammonia with hydroxyl-functionalized ionic liquids[J]. RSC Adv., 2015, 5(99): 81362-81370. |
| 39 | ShangD W, BaiL, ZengS J, et al. Enhanced NH3 capture by imidazolium-based protic ionic liquids with different anions and cation substituents[J]. J. Chem. Technol. Biotechnol., 2018, 93(5): 1228-1236. |
| 40 | ShangD W, ZhangX P, ZengS J, et al. Protic ionic liquid BimNTf2 with strong hydrogen bond donating ability for highly efficient ammonia absorption[J]. Green Chem., 2017, 19(4): 937-945. |
| 41 | SherwoodT K. Solubilities of sulfur dioxide and ammonia in water [J]. Industrial and Engineering Chemistry, 1925, 17: 745-747. |
| 42 | ChenW, LiangS Q, GuoY X, et al. Investigation on vapor-liquid equilibria for binary systems of metal ion-containing ionic liquid bmim Zn2Cl5/NH3 by experiment and modified UNIFAC model[J]. Fluid Phase Equilibr., 2013, 360: 1-6. |
| 43 | KohlerF, PoppS, KleferH, et al. Supported ionic liquid phase (SILP) materials for removal of hazardous gas compounds - efficient and irreversible NH3 adsorption[J]. Green Chem., 2014, 16(7): 3560-3568. |
| 44 | ZengS J, LiuL, ShangD W, et al. Efficient and reversible absorption of ammonia by cobalt ionic liquids through Lewis acid-base and cooperative hydrogen bond interactions[J]. Green Chem., 2018, 20(9): 2075-2083. |
| 45 | WangJ L, ZengS J, HuoF, et al. Metal chloride anion-based ionic liquids for efficient separation of NH3[J]. J. Clean Prod., 2019, 206: 661-669. |
| 46 | CaoX Z, SongT Y, WangX Q. Inorganic Chemistry[M]. 2nd ed. Beijing: Higher Education Press, 1994: 898-899. |
| 47 | AkhmetshinaA I, PetukhovA N, MecherguiA, et al. Evaluation of methanesulfonate-based deep eutectic solvent for ammonia sorption[J]. J. Chem. Eng. Data, 2018, 63(6): 1896-1904. |
| 48 | LiY H, AliM C, YangQ W, et al. Hybrid deep eutectic solvents with flexible hydrogen-bonded supramolecular networks for highly efficient uptake of NH3[J]. ChemSusChem, 2017, 10(17): 3368-3377. |
| 49 | LemusJ, BediaJ, MoyaC, et al. Ammonia capture from the gas phase by encapsulated ionic liquids (ENILs)[J]. RSC Adv., 2016, 6(66): 61650-61660. |
| 50 | PalomarJ, LemusJ, Alonso-MoralesN, et al. Encapsulated ionic liquids (ENILs): from continuous to discrete liquid phase[J]. Chem. Comm., 2012, 48(80): 10046-10048. |
| 51 | RuckartK N, ZhangY C, ReichertW M, et al. Sorption of ammonia in mesoporous-silica ionic liquid composites[J]. Ind. Eng. Chem. Res., 2016, 55(47): 12191-12204. |
| [1] | Qi WANG, Bin ZHANG, Xiaoxin ZHANG, Hujian WU, Haitao ZHAN, Tao WANG. Synthesis of isoxepac and 2-ethylanthraquinone catalyzed by chloroaluminate-triethylamine ionic liquid/P2O5 [J]. CIESC Journal, 2023, 74(S1): 245-249. |
| [2] | Congqi HUANG, Yimei WU, Jianye CHEN, Shuangquan SHAO. Simulation study of thermal management system of alkaline water electrolysis device for hydrogen production [J]. CIESC Journal, 2023, 74(S1): 320-328. |
| [3] | Zhenghao JIN, Lijie FENG, Shuhong LI. Energy and exergy analysis of a solution cross-type absorption-resorption heat pump using NH3/H2O as working fluid [J]. CIESC Journal, 2023, 74(S1): 53-63. |
| [4] | Zehao MI, Er HUA. DFT and COSMO-RS theoretical analysis of SO2 absorption by polyamines type ionic liquids [J]. CIESC Journal, 2023, 74(9): 3681-3696. |
| [5] | Minghao SONG, Fei ZHAO, Shuqing LIU, Guoxuan LI, Sheng YANG, Zhigang LEI. Multi-scale simulation and study of volatile phenols removal from simulated oil by ionic liquids [J]. CIESC Journal, 2023, 74(9): 3654-3664. |
| [6] | Shaoqi YANG, Shuheng ZHAO, Lungang CHEN, Chenguang WANG, Jianjun HU, Qing ZHOU, Longlong MA. Hydrodeoxygenation of lignin-derived compounds to alkanes in Raney Ni-protic ionic liquid system [J]. CIESC Journal, 2023, 74(9): 3697-3707. |
| [7] | Ruimin CHE, Wenqiu ZHENG, Xiaoyu WANG, Xin LI, Feng XU. Research progress on homogeneous processing of cellulose in ionic liquids [J]. CIESC Journal, 2023, 74(9): 3615-3627. |
| [8] | Yaxin ZHAO, Xueqin ZHANG, Rongzhu WANG, Guo SUN, Shanjing YAO, Dongqiang LIN. Removal of monoclonal antibody aggregates with ion exchange chromatography by flow-through mode [J]. CIESC Journal, 2023, 74(9): 3879-3887. |
| [9] | Meisi CHEN, Weida CHEN, Xinyao LI, Shangyu LI, Youting WU, Feng ZHANG, Zhibing ZHANG. Advances in silicon-based ionic liquid microparticle enhanced gas capture and conversion [J]. CIESC Journal, 2023, 74(9): 3628-3639. |
| [10] | Lizhi WANG, Qiancheng HANG, Yeling ZHENG, Yan DING, Jiaji CHEN, Qing YE, Jinlong LI. Separation of methyl propionate + methanol azeotrope using ionic liquid entrainers [J]. CIESC Journal, 2023, 74(9): 3731-3741. |
| [11] | Jie CHEN, Yongsheng LIN, Kai XIAO, Chen YANG, Ting QIU. Study on catalytic synthesis of sec-butanol by tunable choline-based basic ionic liquids [J]. CIESC Journal, 2023, 74(9): 3716-3730. |
| [12] | Jiayi ZHANG, Jiali HE, Jiangpeng XIE, Jian WANG, Yu ZHAO, Dongqiang ZHANG. Research progress of pervaporation technology for N-methylpyrrolidone recovery in lithium battery production [J]. CIESC Journal, 2023, 74(8): 3203-3215. |
| [13] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [14] | Xingzhi HU, Haoyan ZHANG, Jingkun ZHUANG, Yuqing FAN, Kaiyin ZHANG, Jun XIANG. Preparation and microwave absorption properties of carbon nanofibers embedded with ultra-small CeO2 nanoparticles [J]. CIESC Journal, 2023, 74(8): 3584-3596. |
| [15] | Lei XING, Chunyu MIAO, Minghu JIANG, Lixin ZHAO, Xinya LI. Optimal design and performance analysis of downhole micro gas-liquid hydrocyclone [J]. CIESC Journal, 2023, 74(8): 3394-3406. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||