CIESC Journal ›› 2019, Vol. 70 ›› Issue (3): 801-816.DOI: 10.11949/j.issn.0438-1157.20180965
• Reviews and monographs • Previous Articles Next Articles
Feng LUO1,2( ),Li LIN1,Zhenchen LI1,Wenyu LI1,Xianlin CHEN1,Sha SHA1,Tao LUO2(
),Li LIN1,Zhenchen LI1,Wenyu LI1,Xianlin CHEN1,Sha SHA1,Tao LUO2( )
)
Received:2018-08-27
Revised:2018-11-02
Online:2019-03-05
Published:2019-03-05
Contact:
Tao LUO
骆枫1,2( ),林力1,李振臣1,李文钰1,陈先林1,沙沙1,罗涛2(
),林力1,李振臣1,李文钰1,陈先林1,沙沙1,罗涛2( )
)
通讯作者:
罗涛
作者简介:<named-content content-type="corresp-name">骆枫</named-content>(1989—),男,博士,助理研究员,<email>luofenghxf@foxmail.com</email>|罗涛(1987—),男,博士,助理研究员,<email>tao.luo@scu.edu.cn</email>
CLC Number:
Feng LUO, Li LIN, Zhenchen LI, Wenyu LI, Xianlin CHEN, Sha SHA, Tao LUO. Electrochemical reactions and reactors for biomass valorisation[J]. CIESC Journal, 2019, 70(3): 801-816.
骆枫, 林力, 李振臣, 李文钰, 陈先林, 沙沙, 罗涛. 生物质的电化学转化反应及反应器[J]. 化工学报, 2019, 70(3): 801-816.
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgxb.cip.com.cn/EN/10.11949/j.issn.0438-1157.20180965
| Item | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| contents/%(mass) | 40—45 | 25—35 | 15—30 |
| monomer | D-glucose | C5 sugars (xylose) | 3 phenols |
| polymer(chain) | linear,β-1,4 glucosidic | brached | cross-linked, 3D network |
| Mw | 50—2500 | 50—400 | huge |
| crystallinity | crystalline | amorphous | amorphous |
| solubility | water[-],organics[-] | water[-] | water[-] |
| solvents | dilute H2SO4, Cu(NH3)4(OH)2 | dilute acid,base | strong base |
| hydrolysis | H2SO4 solutions | dilute acid,base | — |
Table 1 Characteristics of lignocellulose components
| Item | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| contents/%(mass) | 40—45 | 25—35 | 15—30 |
| monomer | D-glucose | C5 sugars (xylose) | 3 phenols |
| polymer(chain) | linear,β-1,4 glucosidic | brached | cross-linked, 3D network |
| Mw | 50—2500 | 50—400 | huge |
| crystallinity | crystalline | amorphous | amorphous |
| solubility | water[-],organics[-] | water[-] | water[-] |
| solvents | dilute H2SO4, Cu(NH3)4(OH)2 | dilute acid,base | strong base |
| hydrolysis | H2SO4 solutions | dilute acid,base | — |
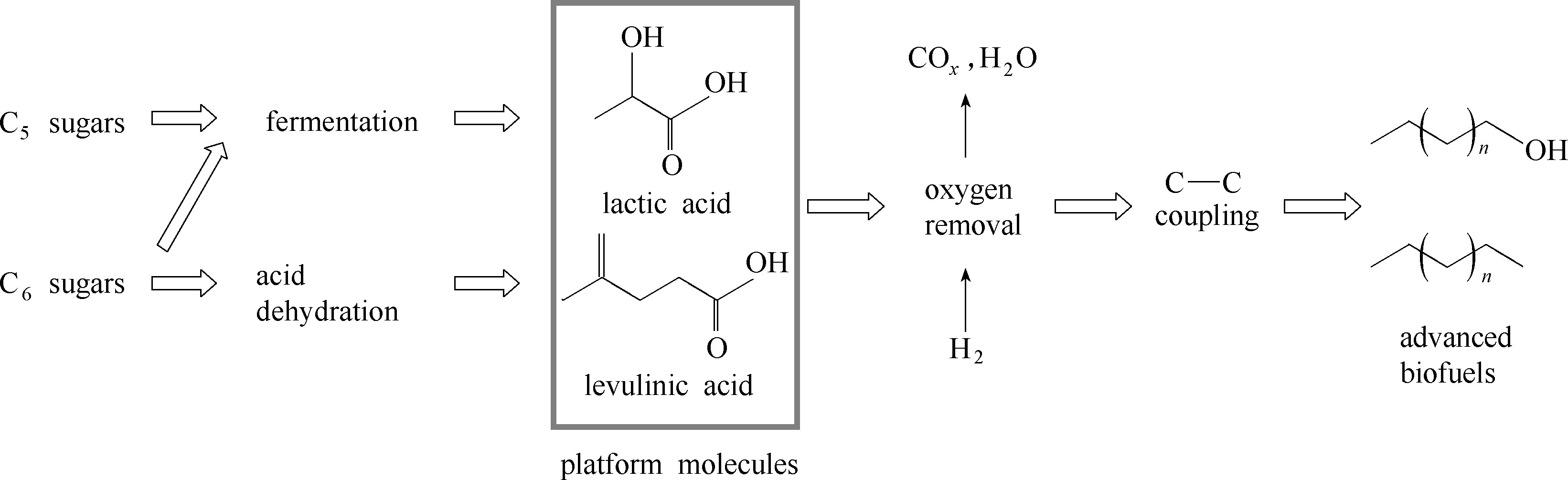
Fig.2 Organic acids, platform molecules derived from lignocellulose, stand at the crossroad of lignocellulose conversion route to advanced biofuels[34]
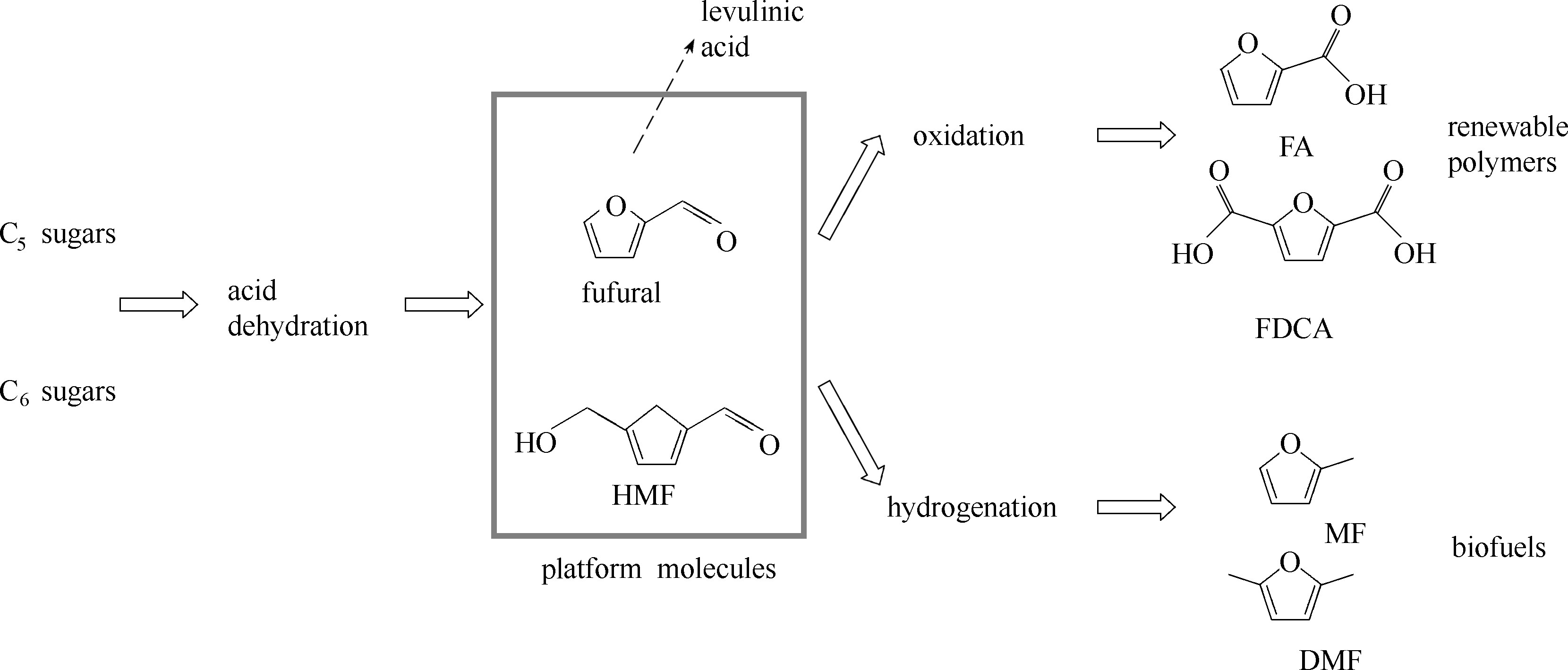
Fig.3 Fufural and 5-hydroxylmethylfufural (HMF) are representative platform molecules that can be (electrochemically) converted to monomers for renewable polymers (FA, fufuranic acid; FDCA, 2,5-furandicarboxylic acid) and biofuels (MF, 2-methylfuran; DMF, 2,5-dimethylfuran)[23]. Fufural can also be converted to levulinic acid (the dashed arrow), then undergoes another route of transformation as shown in Fig.2
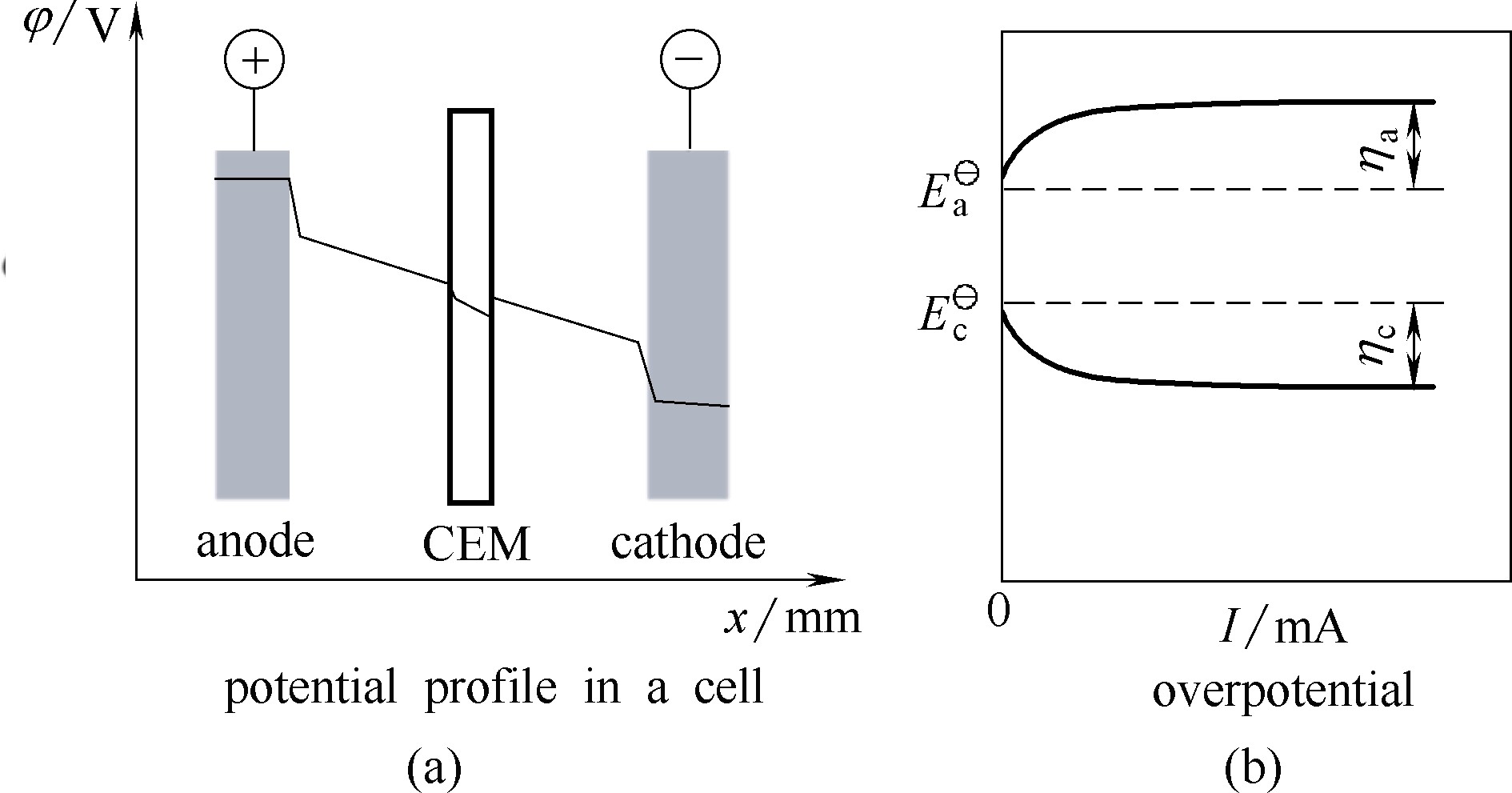
Fig.4 Potential profile in an electrolyzer for electrochemical conversion of biomass (a). CEM denotes cation exchange membrane, which is a representative separator between anolyte and catholyte[49]. Anodic overpotential (ηa) and cathodic overpential (ηc) as a function of cell current (b)[50]
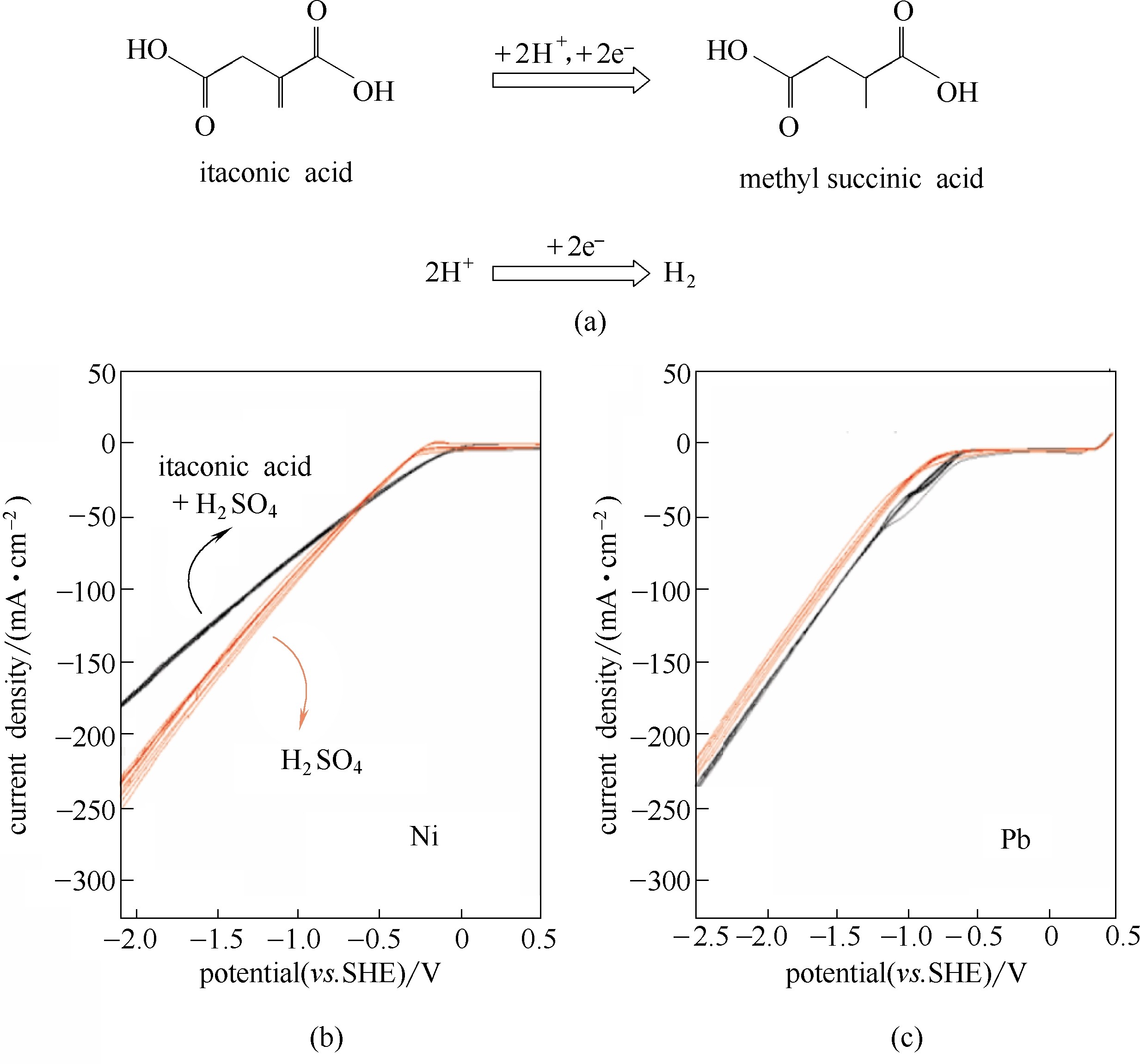
Fig.7 Electrocatalytic reduction of itaconic acid to methyl succinic acid, and competitive hydrogen evolution reaction(a); Cyclic voltagram of pure supporting electrolye (H2SO4, red curves) and itaconic acid solution (black curves) with Ni cathode(b), and with Pb cathode(c)[62]
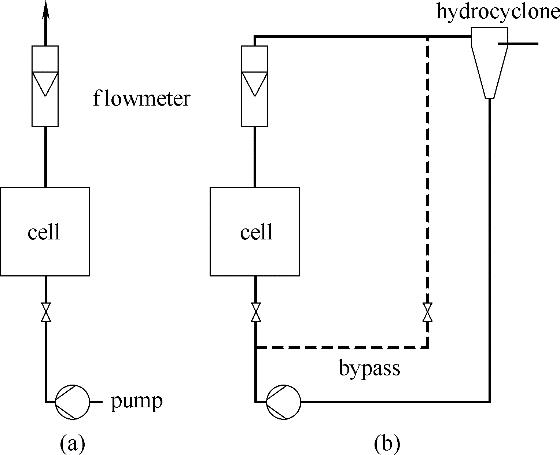
Fig.9 Single pass of electrolyte in electrochemical cell(a); multiple passes of electrolyte in cell, with a hydrocyclone as a representative separation unit(b)[58]
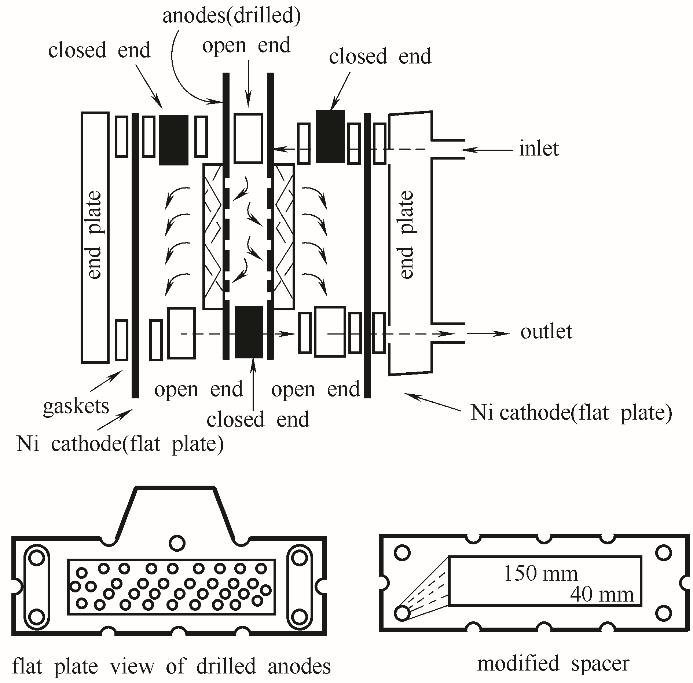
Fig.11 Schematic of a press-filter type electrochemical reactor for lignin depolymerization(The components of the reactor are all of commerical sources. The planar porous anode is drilled with holes of 3 mm diameter (lower left), allowing the electrolyte to flow through the anodes. Spacers with modified flow channels (lower right) could feed the electrolyte to the anodes in the interior of the reactor[84])
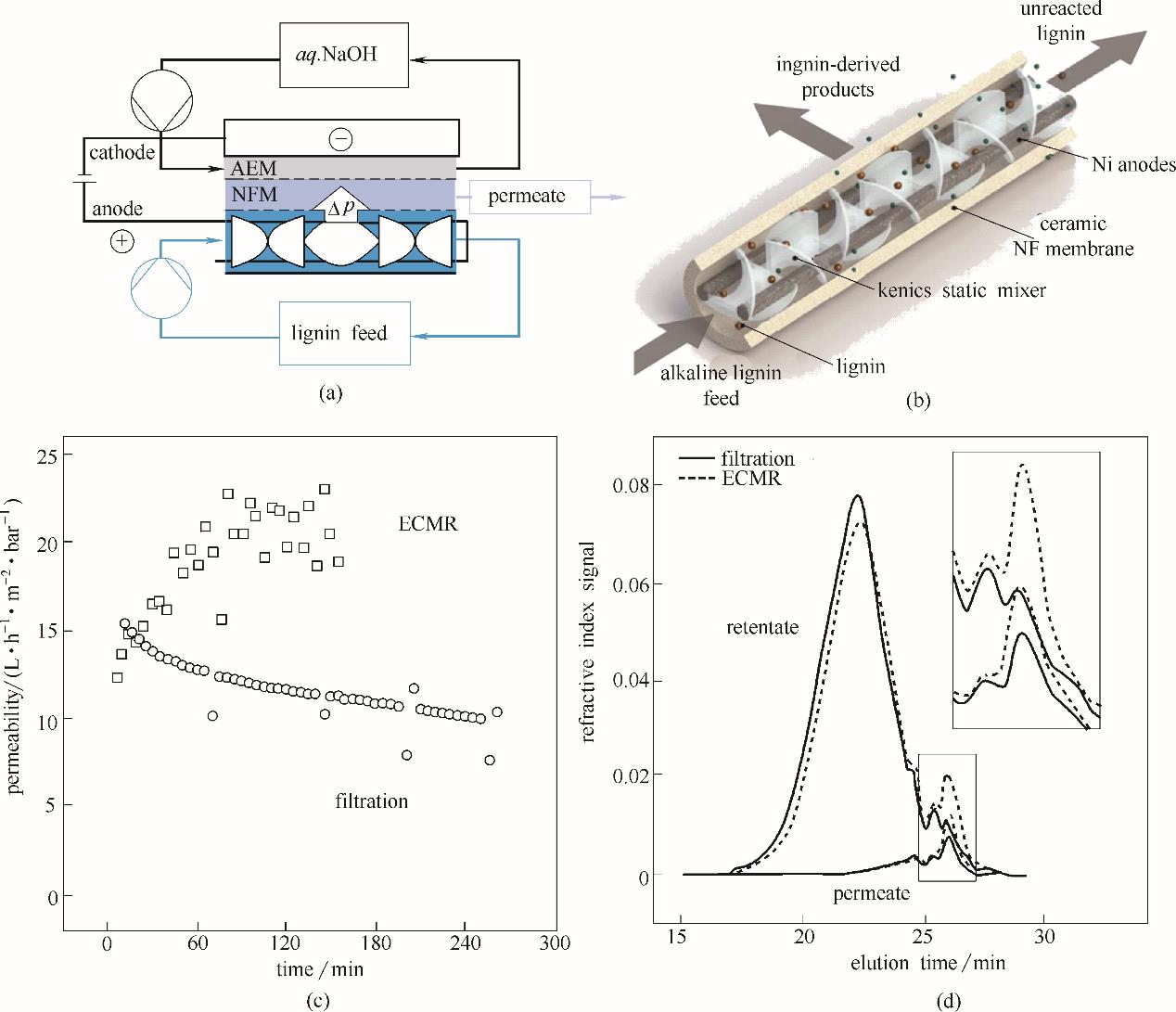
Fig.12 Flow scheme showing the electrochemical membrane reactor (ECMR) for lignin depolymerization(a)(AEM is anion exchange membrane, NFM is nanofiltration membrane); 3 D scheme of the anode compartment with static mixers right next to the Ni anodes to promote the liquid flow and in-situ product removal through the ceramic nanofiltration (NF) membrane(b); ECMR has better permeability through the NF membrane compared with post-reaction filtration of the reaction medium(c); Gel permeation chromotography shows that ECMR process has improvent in yield of small molecular components(d)[77]
| 1 | ShafieeS, TopalE. When will fossil fuel reserves be diminished?[J]. Energy Policy, 2009, 37(1): 181-189. |
| 2 | RagauskasA J, WilliamsC K, DavisonB H, et al. The path forward for biofuels and biomaterials[J]. Science, 2006, 311(5760): 484-489. |
| 3 | CherubiniF. The biorefinery concept: using biomass instead of oil for producing energy and chemicals[J]. Energy Conversion and Management, 2010, 51(7): 1412-1421. |
| 4 | MckendryP. Energy production from biomass (Part 1): Overview of biomass[J]. Bioresource Technology, 2002, 83(1): 37-46. |
| 5 | RuizH A, Rodriguez-JassoR M, FernandesB D, et al. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: a review[J]. Renewable & Sustainable Energy Reviews, 2013, 21: 35-51. |
| 6 | KanT, StrezovV, EvansT J. Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters[J]. Renewable & Sustainable Energy Reviews, 2016, 57: 1126-1140. |
| 7 | SansaniwalS K, PalK, RosenM A, et al. Recent advances in the development of biomass gasification technology: a comprehensive review[J]. Renewable & Sustainable Energy Reviews, 2017, 72: 363-384. |
| 8 | YangH, YanR, ChenH, et al. Characteristics of hemicellulose, cellulose and lignin pyrolysis[J]. Fuel, 2007, 86(12/13): 1781-1788. |
| 9 | DorrestijnE, LaarhovenL J J, ArendsI, et al. The occurrence and reactivity of phenoxyl linkages in lignin and low rank coal[J]. Journal of Analytical and Applied Pyrolysis, 2000, 54(1/2): 153-192. |
| 10 | BalatM. Biomass energy and biochemical conversion processing for fuels and chemicals[J]. Energy Sources Part A - Recovery Utilization and Environmental Effects, 2006, 28(6): 517-525. |
| 11 | ParsellT, YoheS, DegensteinJ, et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass[J]. Green Chemistry, 2015, 17(3): 1492-1499. |
| 12 | ChhedaJ N, HuberG W, DumesicJ A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals[J]. Angewandte Chemie-International Edition, 2007, 46(38): 7164-7183. |
| 13 | 江泽民. 对中国能源问题的思考[J]. 上海交通大学学报, 2008, (3): 345 - 359. |
| JiangZ M. Reflections on energy issues in China[J]. Journal of Shanghai Jiao Tong University, 2008, (3): 345 - 359. | |
| 14 | ITER. 60 years of progress[EB/OL]. [2018-10-31]. https: //. |
| 15 | YangZ G, ZhangJ L, Kintner-MeyerM C W, et al. Electrochemical energy storage for green grid[J]. Chemical Reviews, 2011, 111(5): 3577-3613. |
| 16 | CarrascoJ M, FranqueloL G, BialasiewiczJ T, et al. Power-electronic systems for the grid integration of renewable energy sources: a survey[J]. IEEE Transactions on Industrial Electronics, 2006, 53(4): 1002-1016. |
| 17 | DunnB, KamathH, TarasconJ M. Electrical energy storage for the grid: a battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 18 |
AustB, MorscherC. Negative strompreise in Deutschland[J]. Wirtschaftdienst, 2017, 97(4): 304-306. DOI: 10.1007/s10273-017-2135-0.
DOI URL |
| 19 | de GraaffM. Power-2-Chemicals[EB/OL]. [2018-10-31] https: //. |
| 20 | DuL, ShaoY Y, SunJ M, et al. Electrocatalytic valorisation of biomass derived chemicals[J]. Catalysis Science & Technology, 2018, 8(13): 3216-3232. |
| 21 | MitsosA, AsprionN, FloudasC A, et al. Challenges in process optimization for new feedstocks and energy sources[J]. Computers & Chemical Engineering, 2018, 113: 209-221. |
| 22 | BebelisS, BouzekK, CornellA, et al. Highlights during the development of electrochemical engineering[J]. Chemical Engineering Research & Design, 2013, 91(10): 1998-2020. |
| 23 | KwonY, SchoutenK J P, van der WaalJ C, et al. Electrocatalytic conversion of furanic compounds[J]. ACS Catalysis, 2016, 6(10): 6704-6717. |
| 24 | SimoesM, BarantonS, CoutanceauC. Electrochemical valorisation of glycerol[J]. ChemSusChem, 2012, 5(11): 2106-2124. |
| 25 | MoehleS, ZirbesM, RodrigoE, et al. Modern electrochemical aspects for the synthesis of value-added organic products[J]. Angewandte Chemie-International Edition, 2018, 57(21): 6018-6041. |
| 26 | ZirbesM, WaldvogelS R. Electro-conversion as sustainable method for the fine chemical production from the biopolymer lignin[J]. Current Opinion in Green and Sustainable Chemistry, 2018, 14: 19-25. |
| 27 | Maki-ArvelaP, SalmiT, HolmbomB, et al. Synthesis of sugars by hydrolysis of hemicelluloses—a review[J]. Chemical Reviews, 2011, 111(9): 5638-5666. |
| 28 | RinaldiR, JastrzebskiR, CloughM T, et al. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis[J]. Angewandte Chemie-International Edition, 2016, 55(29): 8164-8215. |
| 29 | ShuaiL, AmiriM T, Questell-SantiagoY M, et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization[J]. Science, 2016, 354(6310): 329-333. |
| 30 | YuJ C, BaizerM M, NobeK. Electrochemical generated acid catalysis of cellulose hydrolysis[J]. Journal of The Electrochemical Society, 1988, 135(1): 83-87. |
| 31 | MengD, LiG, LiuZ, et al. Study of depolymerization of cotton cellulose by Pb/PbO2 anode electrochemical catalysis in sulfuric acid solution[J]. Polymer Degradation and Stability, 2011, 96(7): 1173-1178. |
| 32 | WerpyT, PetersenG, AdenA, et al., Results of screening for potential candidates from sugars and synthesis gas[M]//Top Value-Added Chemicals from Biomass: Volume 1. Oak Ridge: U.S. Department of Energy, 2004. |
| 33 | HolladayJ E, WhiteJ F, BozellJ J, et al. Results of screening for potential candidates from biorefinery lignin[M]//Top Value-Added Chemicals from Biomass: Volume 2. Oak Ridge: U.S. Department of Energy, 2007. |
| 34 | Carlos Serrano-RuizJ, PinedaA, Mariana BaluA, et al. Catalytic transformations of biomass-derived acids into advanced biofuels[J]. Catalysis Today, 2012, 195(1): 162-168. |
| 35 | DengL, LiJ, LaiD M, et al. Catalytic conversion of biomass-derived carbohydrates into gamma-valerolactone without using an external H2 supply[J]. Angewandte Chemie-International Edition, 2009, 48(35): 6529-6532. |
| 36 | EppingerJ, HuangK W. Formic acid as a hydrogen energy carrier[J]. ACS Energy Letters, 2017, 2(1): 188-195. |
| 37 | VoltaChem. Formic acid, the new energy carrier[EB/OL]. [2018-10-31]. https: //. |
| 38 | BozellJ J, MoensL, ElliottD C, et al. Production of levulinic acid and use as a platform chemical for derived products[J]. Resources Conservation and Recycling, 2000, 28(3/4): 227-239. |
| 39 | HayesM H B. Biochar and biofuels for a brighter future[J]. Nature, 2006, 443(7108): 144-144. |
| 40 | NilgesP, Dos SantosT R, HarnischF, et al. Electrochemistry for biofuel generation: electrochemical conversion of levulinic acid to octane[J]. Energy & Environmental Science, 2012, 5(1): 5231-5235. |
| 41 | RosatellaA A, SimeonovS P, FradeR F M, et al. 5-Hydroxymethylfurfural (HMF) as a building block platform: biological properties, synthesis and synthetic applications[J]. Green Chemistry, 2011, 13(4): 754-793. |
| 42 | van PuttenR J, van der WaalJ C, de JongE, et al. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources[J]. Chemical Reviews, 2013, 113(3): 1499-1597. |
| 43 | PanM, ShuG, PanJ, et al. Performance comparison of 2-methylfuran and gasoline on a spark-ignition engine with cooled exhaust gas recirculation[J]. Fuel, 2014, 132: 36-43. |
| 44 | AlipourS, LiB, VaranasiS, et al. Methods for high yield production of furans from biomass sugars at mild operating conditions: US20160281120[P]. 2016-09-29. |
| 45 | RieserK P, de VriesA. BASF and Avantium intend to establish Joint Venture[EB/OL]. [2018-10-31].https: //. |
| 46 | ChengF, BrewerC E. Producing jet fuel from biomass lignin: potential pathways to alkyl benzenes and cycloalkanes[J]. Renewable & Sustainable Energy Reviews, 2017, 72: 673-722. |
| 47 | AtkinsP, PaulaJ D. Physical Chemistry[M]. 8th ed. Great Britain: Oxford University Press, 2006. |
| 48 | LapicqueF. Electrochemical reactors[M]//Chemical Engineering and Chemical Process Technology: Volume 3. United Nations Educational Scientific and Cultural Organization, and Encyclopedia of Life Support Systems (UNESCO - EOLSS), 2010: 160-191. |
| 49 | reactorsElectrochemical [EB/OL]. [2018-10-31]. https: //web.vscht.cz/~paidarm/ACHP/erasmus/ElectrochemReactors_eng.pdf. |
| 50 | 刘俊吉, 周亚平, 李松林. 物理化学[M]. 6版. 北京: 高等教育出版社, 2009. |
| LiuJ J, ZhouY P, LiS L. Physical Chemistry[M]. 6th ed. Beijing: Higher Education Press, 2009 | |
| 51 | AgarJ N, BowdenF P. The kinetics of electrode reactions (I and II)[J]. Proceedings of the Royal Society of London Series A-Mathematical and Physical Sciences, 1938, 169(A937): 0206-0234. |
| 52 | WalshF C. The kinetics of electrode-reactions (2): Mass-transfer and mixed control[J]. Transactions of the Institute of Metal Finishing, 1992, 70: 95-99. |
| 53 | RodunerE. Selected fundamentals of catalysis and electrocatalysis in energy conversion reactions—a tutorial[J]. Catalysis Today, 2018, 309: 263-268. |
| 54 | BagotskyV S. Fundamentals of Electrochemistry[M]. 2nd ed. John Wiley & Sons, Inc., 2005. |
| 55 | WalshF, ReadeG. Design and performance of electrochemical reactors for efficient synthesis and environmental treatment (1): Electrode gemoetry and figures of merit[J]. Analyst, 1994, 119(5): 791-796. |
| 56 | PletcherD, GreenR A, BrownR C D. Flow electrolysis cells for the synthetic organic chemistry laboratory[J]. Chemical Reviews, 2018, 118(9): 4573-4591. |
| 57 | ParkK, PintauroP N, BaizerM M, et al. Current efficiencies and regeneration of poisoned Raney-Nickel in the electrohydrogenation of glucose to sorbitol[J]. Journal of Applied Electrochemistry, 1986, 16(6): 941-946. |
| 58 | PintauroP N, JohnsonD K, ParkK, et al. The paired electrochemical synthesis of sorbitol and gluconic acid in undivided flow cels(1)[J]. Journal of Applied Electrochemistry, 1984, 14(2): 209-220. |
| 59 | Garcia-MateosF J, Cordero-LanzacT, BerenguerR, et al. Lignin-derived Pt supported carbon (submicron)fiber electrocatalysts for alcohol electro-oxidation[J]. Applied Catalysis B-Environmental, 2017, 211: 18-30. |
| 60 | DingY, ChenM W. Nanoporous metals for catalytic and optical applications[J]. MRS Bulletin, 2009, 34(8): 569-576. |
| 61 | KwonY. Biomass electrochemistry: from cellulose to sorbitol[D]. Leiden University, 2013. |
| 62 | HolzhaeuserF J, ArtzJ, PalkovitsS, et al. Electrocatalytic upgrading of itaconic acid to methylsuccinic acid using fermentation broth as a substrate solution[J]. Green Chemistry, 2017, 19(10): 2390-2397. |
| 63 | ZhangZ, SongJ, HanB. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids[J]. Chemical Reviews, 2017, 117(10): 6834-6880. |
| 64 | Di MarinoD, StoeckmannD, KriescherS, et al. Electrochemical depolymerisation of lignin in a deep eutectic solvent[J]. Green Chemistry, 2016, 18(22): 6021-6028. |
| 65 | DierT K F, RauberD, DurneataD, et al. Sustainable electrochemical depolymerization of lignin in reusable ionic liquids[J]. Scientific Reports, 2017, 7(1): 5041-5052. |
| 66 | ReichertE, WintringerR, VolmerD A, et al. Electro-catalytic oxidative cleavage of lignin in a protic ionic liquid[J]. Physical Chemistry Chemical Physics, 2012, 14(15): 5214-5221. |
| 67 | HossainM M, AldousL. Ionic liquids for lignin processing: dissolution, isolation, and conversion[J]. Australian Journal of Chemistry, 2012, 65(11): 1465-1477. |
| 68 | 瞿广飞, 赵茜, 李军燕, 等. 以离子液体和超临界CO2为介质的生物质电化学液化方法及装置: 105441107 A [P]. 2016-03-30. |
| QuG F, ZhaoQ, LiJ Y, et al. Methods and set-ups for the electrochemical liquification of biomass with ionic liquids and super-critical CO2 as reaction medium: 105441107 A [P]. 2016-03-30. | |
| 69 | TianM, WenJ, MacdonaldD, et al. A novel approach for lignin modification and degradation[J]. Electrochemistry Communications, 2010, 12(4): 527-530. |
| 70 | ShaoD, LiangJ, CuiX, et al. Electrochemical oxidation of lignin by two typical electrodes: Ti/Sb-SnO2 and Ti/PbO2[J]. Chemical Engineering Journal, 2014, 244: 288-295. |
| 71 | ZhuH, WangL, ChenY, et al. Electrochemical depolymerization of lignin into renewable aromatic compounds in a non-diaphragm electrolytic cell[J]. RSC Advances, 2014, 4(56): 29917-29924. |
| 72 | ZhuH, ChenY, QinT, et al. Lignin depolymerization via an integrated approach of anode oxidation and electro-generated H2O2 oxidation[J]. RSC Advances, 2014, 4(12): 6232-6238. |
| 73 | LiuM, WenY, QiJ, et al. Fine chemicals prepared by bamboo lignin degradation through electrocatalytic redox between Cu cathode and Pb/PbO2 anode in alkali solution[J]. Chemistry Select, 2017, 2(17): 4956-4962. |
| 74 | LuoT, AbduS, WesslingM. Selectivity of ion exchange membranes: a review[J]. Journal of Membrane Science, 2018, 555: 429-454. |
| 75 | StiefelS, SchmitzA, PetersJ, et al. An integrated electrochemical process to convert lignin to value-added products under mild conditions[J]. Green Chemistry, 2016, 18(18): 4999-5007. |
| 76 | StiefelS, MarksC, SchmidtT, et al. Overcoming lignin heterogeneity: reliably characterizing the cleavage of technical lignin[J]. Green Chemistry, 2016, 18(2): 531-540. |
| 77 | StiefelS, LoelsbergJ, KipshagenL, et al. Controlled depolymerization of lignin in an electrochemical membrane reactor[J]. Electrochemistry Communications, 2015, 61: 49-52. |
| 78 | Di MarinoD, AnikoV, StoccoA, et al. Emulsion electro-oxidation of kraft lignin[J]. Green Chemistry, 2017, 19(20): 4778-4784. |
| 79 | KimH J, LeeJ, GreenS K, et al. Selective glycerol oxidation by electrocatalytic dehydrogenation[J]. ChemSusChem, 2014, 7(4): 1051-1056. |
| 80 |
SekarN, RamasamyR P. Electrochemical impedance spectroscopy for microbial fuel cell characterization[J]. Journal of Microbial and Biochemical Technology, 2013, S6: 004. DOI: 10.4172/1948-5948.S6-004.
DOI URL |
| 81 | 赵博. 电化学方法在生物质催化转化中的应用研究[D]. 合肥: 中国科学技术大学, 2015. |
| ZhaoB. Studies on the catalytic conversion of biomass with electrochemical methods[D]. Hefei: University of Science and Technology of China, 2015. | |
| 82 | SchmittD, RegenbrechtC, HartmerM, et al. Highly selective generation of vanillin by anodic degradation of lignin: a combined approach of electrochemistry and product isolation by adsorption[J]. Beilstein Journal of Organic Chemistry, 2015, 11: 473-480. |
| 83 | HarrisonK W, MartinG D, RamsdenT G, et al. The wind-to-hydrogen project: operational experience, testingperformance, and systems integration[R/OL].Colorado: U.S. Department of Energy, 2009. https: //. |
| 84 | SmithC Z, UtleyJ H P, HammondJ K. Electro-organic reactions. Part 60[1]. The electro-oxidative conversion at laboratory scale of a lignosulfonate into vanillin in an FM01 filter press flow reactor: preparative and mechanistic aspects[J]. Journal of Applied Electrochemistry, 2011, 41(4): 363-375. |
| 85 | Ponce De LeonC, HusseyW, FrazaoF, et al. The 3D printing of a polymeric electrochemical cell body and its characterisation[J]. Chemical Engineering Transactions, 2014, 41(Special Issue): 1-6. |
| 86 | LölsbergJ, StarckO, StiefelS, et al. 3D-printed electrodes with improved mass transport properties[J]. ChemElectroChem, 2017, 4(12): 3309-3313. |
| 87 |
ZirbesM, SchmittD, BeiserN, et al. Anodic degradation of lignin at active transition metal-based alloys and performance-enhanced anodes[J]. ChemElectroChem, 2018. DOI: 10.1002/celc.201801218.
DOI URL |
| 88 | de GraaffM. VoltaChem demonstrates continuous electrochemical FDCA production[EB/OL]. [2018-10-31].https: //, 2017. |
| 89 | 李振环, 曹磊, 苏坤梅, 等. 一种次氯酸钠电催化5-HMF制备FDCA的方法: 108130554A [P]. 2018-06-08. |
| LiZ H, CaoL, SuK M, et al. A method for the electrochemical conversion of 5-HMF to FDCA with sodium hypochlorite as mediator: 108130554A [P]. 2018-06-08. |
| [1] | Jiali ZHENG, Zhihui LI, Xinqiang ZHAO, Yanji WANG. Kinetics of ionic liquid catalyzed synthesis of 2-cyanofuran [J]. CIESC Journal, 2023, 74(9): 3708-3715. |
| [2] | Jiaqi CHEN, Wanyu ZHAO, Ruichong YAO, Daolin HOU, Sheying DONG. Synthesis of pistachio shell-based carbon dots and their corrosion inhibition behavior on Q235 carbon steel [J]. CIESC Journal, 2023, 74(8): 3446-3456. |
| [3] | Yuyuan ZHENG, Zhiwei GE, Xiangyu HAN, Liang WANG, Haisheng CHEN. Progress and prospect of medium and high temperature thermochemical energy storage of calcium-based materials [J]. CIESC Journal, 2023, 74(8): 3171-3192. |
| [4] | Wentao WU, Liangyong CHU, Lingjie ZHANG, Weimin TAN, Liming SHEN, Ningzhong BAO. High-efficient preparation of cardanol-based self-healing microcapsules [J]. CIESC Journal, 2023, 74(7): 3103-3115. |
| [5] | Maolin DONG, Lidong CHEN, Liulian HUANG, Weibing WU, Hongqi DAI, Huiyang BIAN. Research progress in preparation of lignonanocellulose by acid hydrotropes and their functional applications [J]. CIESC Journal, 2023, 74(6): 2281-2295. |
| [6] | Zhenghao YANG, Zhen HE, Yulong CHANG, Ziheng JIN, Xia JIANG. Research progress in downer fluidized bed reactor for biomass fast pyrolysis [J]. CIESC Journal, 2023, 74(6): 2249-2263. |
| [7] | Xiaowen ZHOU, Jie DU, Zhanguo ZHANG, Guangwen XU. Study on the methane-pulsing reduction characteristics of Fe2O3-Al2O3 oxygen carrier [J]. CIESC Journal, 2023, 74(6): 2611-2623. |
| [8] | Lanhe ZHANG, Qingyi LAI, Tiezheng WANG, Xiaozhuo GUAN, Mingshuang ZHANG, Xin CHENG, Xiaohui XU, Yanping JIA. Effect of H2O2 on nitrogen removal and sludge properties in SBR [J]. CIESC Journal, 2023, 74(5): 2186-2196. |
| [9] | Zedong WANG, Zhiping SHI, Liyan LIU. Numerical simulation and optimization of acoustic streaming considering inhomogeneous bubble cloud dissipation in rectangular reactor [J]. CIESC Journal, 2023, 74(5): 1965-1973. |
| [10] | Jianhua ZHANG, Mengmeng CHEN, Yawen SUN, Yongzhen PENG. Efficient nitrogen and phosphorus removal from domestic wastewater via simultaneous partial nitritation and phosphorus removal combined Anammox [J]. CIESC Journal, 2023, 74(5): 2147-2156. |
| [11] | Zefeng GE, Yuqing WU, Mingxun ZENG, Zhenting ZHA, Yuna MA, Zenghui HOU, Huiyan ZHANG. Effect of ash chemical components on biomass gasification properties [J]. CIESC Journal, 2023, 74(5): 2136-2146. |
| [12] | Lingxin ZU, Rongting HU, Xin LI, Yudao CHEN, Guanglin CHEN. Carbon release products and denitrification bioavailability from chemical components of woody biomass [J]. CIESC Journal, 2023, 74(3): 1332-1342. |
| [13] | Haiqin LIU, Bowen LI, Zhe LING, Liang LIU, Juan YU, Yimin FAN, Qiang YONG. Facile preparation and properties of chemically modified galactomannan films via mild hydroxy-alkyne click reaction [J]. CIESC Journal, 2023, 74(3): 1370-1378. |
| [14] | Jiawei FU, Shuaishuai CHEN, Kailun FANG, Xin JIANG. Advantage of microreactor on the synthesis of high-activity Cu-Mn catalyst by co-precipitation [J]. CIESC Journal, 2023, 74(2): 776-783. |
| [15] | Jieyuan ZHENG, Xianwei ZHANG, Jintao WAN, Hong FAN. Synthesis and curing kinetic analysis of eugenol-based siloxane epoxy resin [J]. CIESC Journal, 2023, 74(2): 924-932. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||