CIESC Journal ›› 2022, Vol. 73 ›› Issue (9): 3895-3903.DOI: 10.11949/0438-1157.20220539
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Feng DU( ), Siqi YIN, Hui LUO, Wenan DENG(
), Siqi YIN, Hui LUO, Wenan DENG( ), Chuan LI, Zhenwei HUANG, Wenjing WANG
), Chuan LI, Zhenwei HUANG, Wenjing WANG
Received:2022-04-15
Revised:2022-06-30
Online:2022-10-09
Published:2022-09-05
Contact:
Wenan DENG
杜峰( ), 尹思琦, 罗辉, 邓文安(
), 尹思琦, 罗辉, 邓文安( ), 李传, 黄振薇, 王文静
), 李传, 黄振薇, 王文静
通讯作者:
邓文安
作者简介:杜峰(1972—),男,博士,副教授,Dufeng@upc.edu.cn
基金资助:CLC Number:
Feng DU, Siqi YIN, Hui LUO, Wenan DENG, Chuan LI, Zhenwei HUANG, Wenjing WANG. Study on size effect of H2 adsorption and dissociation on Mo x S y clusters[J]. CIESC Journal, 2022, 73(9): 3895-3903.
杜峰, 尹思琦, 罗辉, 邓文安, 李传, 黄振薇, 王文静. H2在Mo x S y 团簇上吸附解离的尺寸效应研究[J]. 化工学报, 2022, 73(9): 3895-3903.
Add to citation manager EndNote|Ris|BibTeX
| Energy | Mo | S | Mo7S15 | Mo12S26 | Mo18S39 | Mo25S54 | Mo33S71 |
|---|---|---|---|---|---|---|---|
| E/eV | -1855.00 | -10832.37 | -175589.75 | -304118.30 | -456185.94 | -631793.35 | -830940.07 |
| Eb/eV | — | — | 5.42 | 5.70 | 5.85 | 5.95 | 6.03 |
Table 1 Energy E of Mo x S y clusters, molybdenum atoms, sulfur atoms and binding energies Eb of Mo x S y (x=7,12,18,25,33; y=15,26,39,54,71) clusters with different sizes
| Energy | Mo | S | Mo7S15 | Mo12S26 | Mo18S39 | Mo25S54 | Mo33S71 |
|---|---|---|---|---|---|---|---|
| E/eV | -1855.00 | -10832.37 | -175589.75 | -304118.30 | -456185.94 | -631793.35 | -830940.07 |
| Eb/eV | — | — | 5.42 | 5.70 | 5.85 | 5.95 | 6.03 |
| System | E(HOMO)/eV | E(LUMO)/eV | Eg/eV |
|---|---|---|---|
| Mo7S15 | -5.539 | -5.431 | 0.108 |
| Mo12S26 | -5.409 | -5.289 | 0.120 |
| Mo18S39 | -5.469 | -5.337 | 0.132 |
| Mo25S54 | -5.612 | -5.266 | 0.346 |
| Mo33S71 | -5.814 | -5.280 | 0.534 |
Table 2 HOMO-LUMO energy gap values of Mo x S y (x=7,12,18,25,33; y=15,26,39,54,71) cluster Eg
| System | E(HOMO)/eV | E(LUMO)/eV | Eg/eV |
|---|---|---|---|
| Mo7S15 | -5.539 | -5.431 | 0.108 |
| Mo12S26 | -5.409 | -5.289 | 0.120 |
| Mo18S39 | -5.469 | -5.337 | 0.132 |
| Mo25S54 | -5.612 | -5.266 | 0.346 |
| Mo33S71 | -5.814 | -5.280 | 0.534 |
| Species | Corners | Edges | |||||
|---|---|---|---|---|---|---|---|
| r(H—H)/nm | r(H—S)/nm | Eads/(kJ/mol) | r(H—H)/nm | r(H—Mo)/nm | r(H—S)/nm | Eads/(kJ/mol) | |
| Mo7S15H2 | 0.0772 | 0.292 | -48.58 | 0.0773 | 0.220 | 0.271 | -64.25 |
| Mo12S26H2 | 0.0749 | 0.359 | -14.62 | 0.0769 | 0.228 | 0.273 | -34.60 |
| Mo18S39H2 | 0.0748 | 0.362 | -14.26 | 0.0763 | 0.231 | 0.275 | -34.14 |
| Mo25S54H2 | 0.0748 | 0.365 | -7.13 | 0.0750 | 0.311 | 0.297 | -7.20 |
| Mo33S71H2 | 0.0748 | 0.370 | -5.59 | 0.0748 | 0.332 | 0.319 | -6.82 |
Table 3 Adsorption structure parameters and adsorption energy (Eads) of hydrogen at the edges and corners of clusters with different sizes
| Species | Corners | Edges | |||||
|---|---|---|---|---|---|---|---|
| r(H—H)/nm | r(H—S)/nm | Eads/(kJ/mol) | r(H—H)/nm | r(H—Mo)/nm | r(H—S)/nm | Eads/(kJ/mol) | |
| Mo7S15H2 | 0.0772 | 0.292 | -48.58 | 0.0773 | 0.220 | 0.271 | -64.25 |
| Mo12S26H2 | 0.0749 | 0.359 | -14.62 | 0.0769 | 0.228 | 0.273 | -34.60 |
| Mo18S39H2 | 0.0748 | 0.362 | -14.26 | 0.0763 | 0.231 | 0.275 | -34.14 |
| Mo25S54H2 | 0.0748 | 0.365 | -7.13 | 0.0750 | 0.311 | 0.297 | -7.20 |
| Mo33S71H2 | 0.0748 | 0.370 | -5.59 | 0.0748 | 0.332 | 0.319 | -6.82 |

Fig.2 PDOS of Mo 4d, S 3p and H 1s orbitals at edge sites of Mo x S y clusters with different sizes:(a) Mo7S15 clusters; (b) Mo12S26 clusters; (c) Mo18S39 clusters; (d) Mo25S54 clusters; (e) Mo33S71 clusters
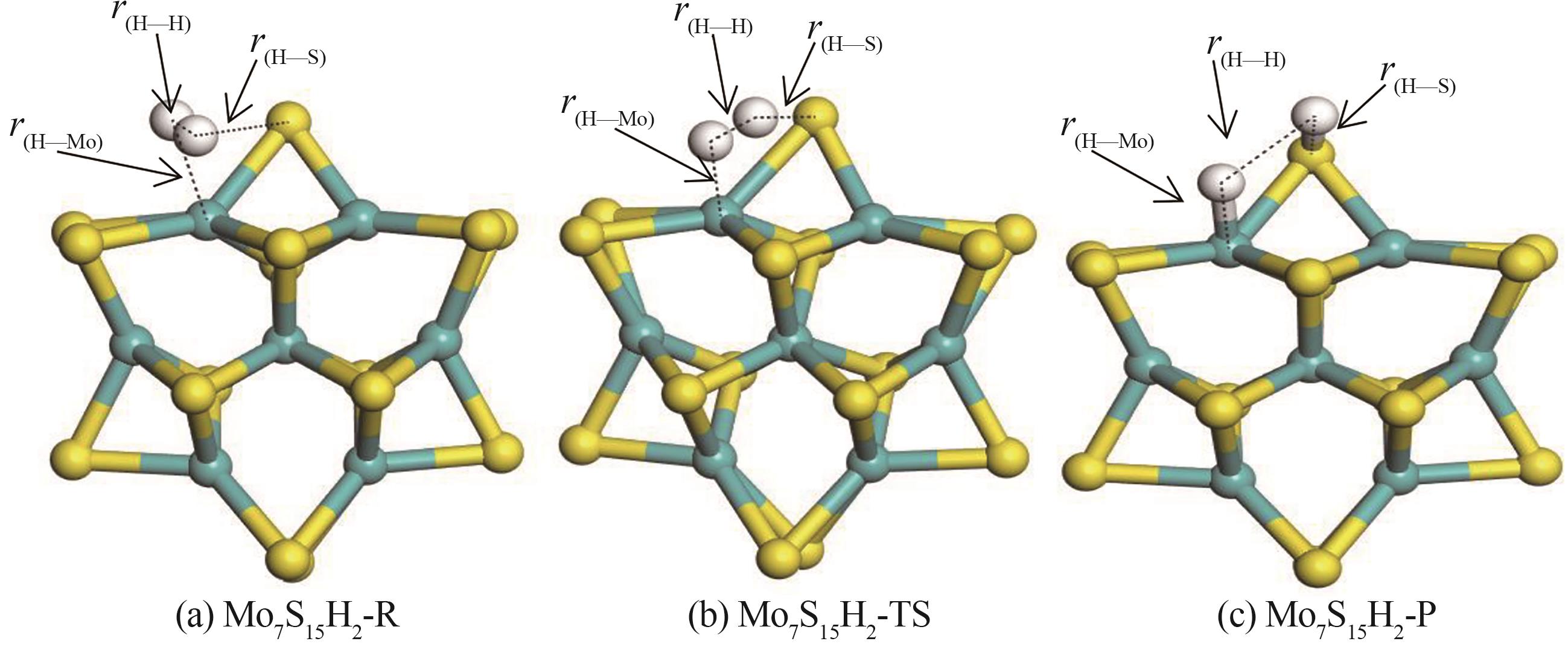
Fig.3 The optimized configuration of initial state (Mo7S15H2-R), transition state (Mo7S15H2-TS) and final state (Mo7S15H2-P) of H2 dissociation on Mo7S15 clusters
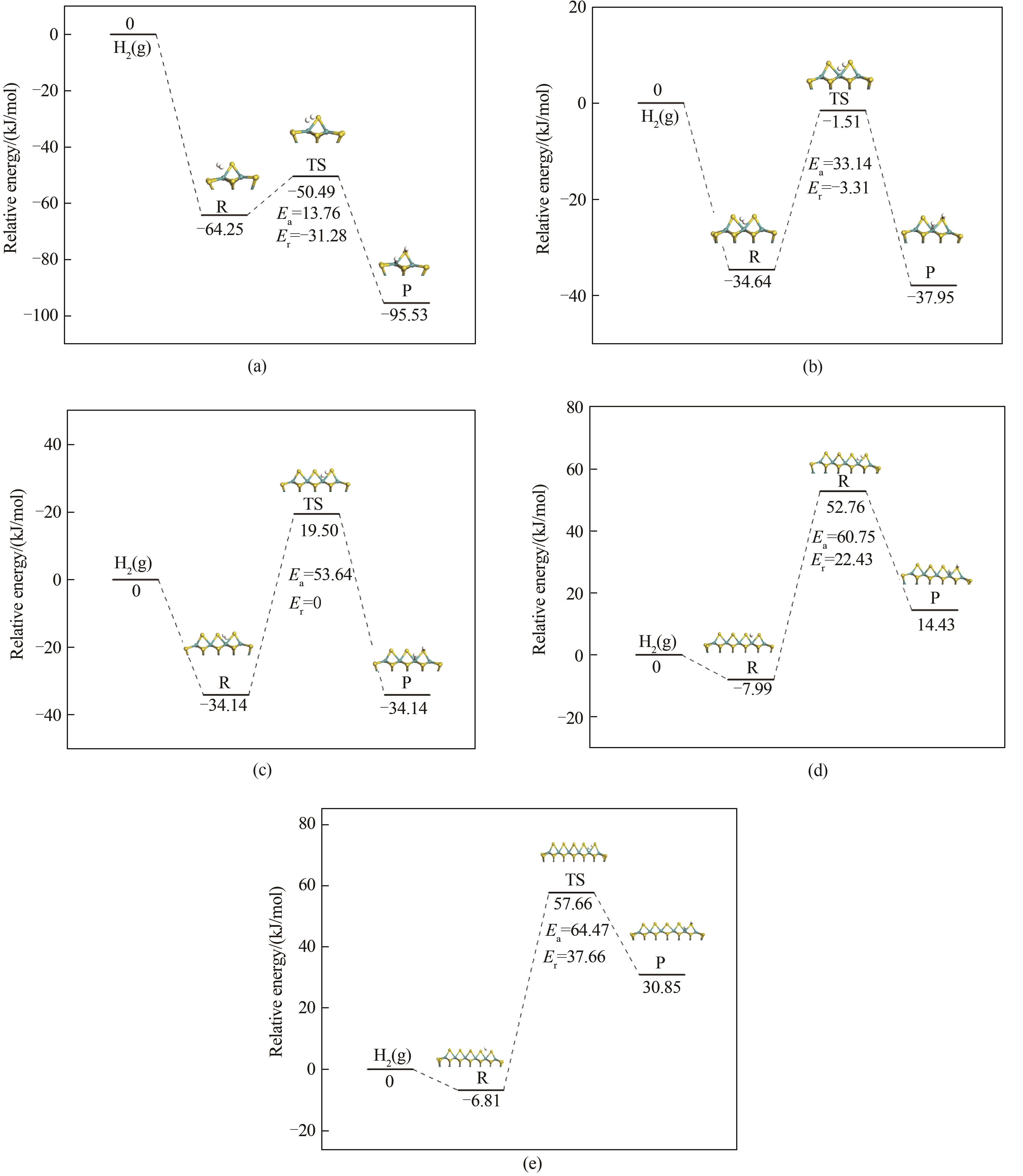
Fig.4 Adsorption and dissociation process of hydrogen molecules on Mo x S y (x=7,12,18,25,33; y=15,26,39,54,71) clusters:(a) Mo7S15 clusters; (b) Mo12S26 clusters; (c) Mo18S39 clusters; (d) Mo25S54 clusters; (e) Mo33S71 clusters
| Species | r(H—H) /nm | r(H—Mo)/nm | r(H—S) /nm | Ea/ (kJ/mol) |
|---|---|---|---|---|
| Mo7S15H2-R | 0.0773 | 0.2200 | 0.2710 | 13.76 |
| Mo7S15H2-TS | 0.1084 | 0.1824 | 0.2553 | |
| Mo7S15H2-P | 0.2241 | 0.1693 | 0.1362 | |
| Mo12S26H2-R | 0.0769 | 0.2280 | 0.2730 | 33.14 |
| Mo12S26H2-TS | 0.1035 | 0.1828 | 0.1593 | |
| Mo12S26H2-P | 0.2310 | 0.1690 | 0.1364 | |
| Mo18S39H2-R | 0.0763 | 0.2310 | 0.2750 | 53.64 |
| Mo18S39H2-TS | 0.1102 | 0.1809 | 0.1517 | |
| Mo18S39H2-P | 0.2252 | 0.1685 | 0.1362 | |
| Mo25S54H2-R | 0.0750 | 0.3110 | 0.2970 | 60.75 |
| Mo25S54H2-TS | 0.1096 | 0.1807 | 0.1531 | |
| Mo25S54H2-P | 0.2252 | 0.1688 | 0.1361 | |
| Mo33S71H2-R | 0.0748 | 0.3320 | 0.3190 | 64.47 |
| Mo33S71H2-TS | 0.1045 | 0.1827 | 0.1576 | |
| Mo33S71H2-P | 0.2292 | 0.1690 | 0.1364 |
Table 4 Dissociation barriers Ea of hydrogen on Mo x S y (x=7,12,18,25,33; y=15,26,39,54,71) clusters and configuration parameters of initial state(R), transition states(TS) and final state(P)
| Species | r(H—H) /nm | r(H—Mo)/nm | r(H—S) /nm | Ea/ (kJ/mol) |
|---|---|---|---|---|
| Mo7S15H2-R | 0.0773 | 0.2200 | 0.2710 | 13.76 |
| Mo7S15H2-TS | 0.1084 | 0.1824 | 0.2553 | |
| Mo7S15H2-P | 0.2241 | 0.1693 | 0.1362 | |
| Mo12S26H2-R | 0.0769 | 0.2280 | 0.2730 | 33.14 |
| Mo12S26H2-TS | 0.1035 | 0.1828 | 0.1593 | |
| Mo12S26H2-P | 0.2310 | 0.1690 | 0.1364 | |
| Mo18S39H2-R | 0.0763 | 0.2310 | 0.2750 | 53.64 |
| Mo18S39H2-TS | 0.1102 | 0.1809 | 0.1517 | |
| Mo18S39H2-P | 0.2252 | 0.1685 | 0.1362 | |
| Mo25S54H2-R | 0.0750 | 0.3110 | 0.2970 | 60.75 |
| Mo25S54H2-TS | 0.1096 | 0.1807 | 0.1531 | |
| Mo25S54H2-P | 0.2252 | 0.1688 | 0.1361 | |
| Mo33S71H2-R | 0.0748 | 0.3320 | 0.3190 | 64.47 |
| Mo33S71H2-TS | 0.1045 | 0.1827 | 0.1576 | |
| Mo33S71H2-P | 0.2292 | 0.1690 | 0.1364 |
| 31 | Bacaud R. Dispersed phase catalysis: past and future. Celebrating one century of industrial development[J]. Fuel, 2014, 117: 624-632. |
| 32 | Kim K D, Lee Y K. Active phase of dispersed MoS2 catalysts for slurry phase hydrocracking of vacuum residue[J]. Journal of Catalysis, 2019, 369: 111-121. |
| 1 | Energy Information Administration U.S.. Annual energy outlook 2020 with projections to 2050[R]. U. S. Department of Energy: Washington, DC, 2020. |
| 2 | 刘美, 刘金东, 张树广, 等. 悬浮床重油加氢裂化技术进展[J]. 应用化工, 2017, 46(12): 2435-2440. |
| Liu M, Liu J D, Zhang S G, et al. Advances of heavy oil hydrocracking in suspended bed[J]. Applied Chemical Industry, 2017, 46(12): 2435-2440. | |
| 3 | Angeles M J, Leyva C, Ancheyta J, et al. A review of experimental procedures for heavy oil hydrocracking with dispersed catalyst[J]. Catalysis Today, 2014, 220: 274-294. |
| 4 | Al-Attas T A, Ali S A, Zahir M H, et al. Recent advances in heavy oil upgrading using dispersed catalysts[J]. Energy & Fuels, 2019, 33(9): 7917-7949. |
| 5 | Nguyen M T, Nguyen N T, Cho J, et al. A review on the oil-soluble dispersed catalyst for slurry-phase hydrocracking of heavy oil[J]. Journal of Industrial & Engineering Chemistry, 2016,43(25): 1-12. |
| 6 | Varakin A N, Mozhaev A V, Pimerzin A A, et al. Toward HYD/DEC selectivity control in hydrodeoxygenation over supported and unsupported Co(Ni)-MoS2 catalysts. A key to effective dual-bed catalyst reactor for co-hydroprocessing of diesel and vegetable oil[J]. Catalysis Today, 2020, 357: 556-564. |
| 7 | Vutolkina A, Glotov A, Baygildin I, et al. Ni-Mo sulfide nanosized catalysts from water-soluble precursors for hydrogenation of aromatics under water gas shift conditions[J]. Pure and Applied Chemistry 2020, 92: 949-966. |
| 8 | 屈丹龙, 李毅. 含油污泥高值转化过程Mo基负载催化剂的研究[J]. 应用化工, 2021, 50(2): 383-387. |
| Qu D L, Li Y. The preparation of Mo based catalysts for high value catalytic pyrolysis of oily sludge[J]. Applied Chemical Industry, 2021,50(2): 383-387. | |
| 9 | Cai Z P, Ma Y D, Zhang J Y, et al. Tunable ionic liquids as oil-soluble precursors of dispersed catalysts for suspended-bed hydrocracking of heavy residues[J]. Fuel, 2022, 313: 122-130. |
| 10 | 戴咏川, 赵德智. 石油化学基础[M]. 北京: 中国石化出版社, 2009: 248. |
| Dai Y C, Zhao D Z. Fundamentals of Petrochemical[M]. Beijing: China Petrochemical Press, 2009: 248. | |
| 11 | Zhou X D, Ma F Y, Wu H, et al. The effects of Fe2O3 and MoS2 on the catalytic activation pathway of hydrogen sources during direct coal liquefaction[J]. Energy, 2021, 234: 121-129. |
| 12 | Travert A, Nakamura H, van Santen R A, et al. Hydrogen activation on Mo-based sulfide catalysts, a periodic DFT study[J]. Journal of the American Chemical Society, 2002, 124(24): 7084-7095. |
| 13 | Kadiev K M, Maximov A L, Kadieva M K. The effect of MoS2 active site dispersion on suppression of polycondensation reactions during heavy oil hydroconversion[J]. Catalysts, 2021, 11(6): 676-693. |
| 14 | Zheng A D, Wang D, Wang L, et al. Highly efficient MoS2 nanocatalysts for slurry-phase hydrogenation of unconventional feedstocks into fuels[J]. Energy & Fuels, 2021, 35(3): 2590-2601. |
| 15 | 梁瑜, 赵彤, 赵斌彬, 等. WO3对Pt/α-Al2O3催化萘深度加氢的促进作用[J]. 化工学报, 2021, 72(11): 5643-5652. |
| Liang Y, Zhao T, Zhao B B, et al. Promotion of WO3 species on Pt/α-Al2O3 for the deep hydrogenation of naphthalene[J]. CIESC Journal, 2021, 72(11): 5643-5652 | |
| 16 | Ding S J, Peng S Z, Yan Z J, et al. Charge effects on quinoline hydrodenitrogenation catalyzed by Ni-Mo-S active sites—a theoretical study by DFT calculation[J]. Petroleum Science, 2022, 19(1): 339-344. |
| 17 | 魏淑贤, 李阳, 葛少辉,等. MoS2催化剂活性位形成及甲硫醇脱硫机理的研究[J].高校化学工程学报, 2018, 32(4): 956-962. |
| Wei S X, Li Y, Ge S H, et al. Study on active site formation of MoS2 catalysts and their desulfurization mechanism of CH3SH[J]. Journal of Chemical Engineering of Chinese Universities, 2018, 32(4): 956-962. | |
| 18 | Delley B. An all-electron numerical method for solving the local density functional for polyatomic molecules[J]. The Journal of Chemical Physics, 1990, 92(1): 508-517. |
| 19 | Delley B. Fast calculation of electrostatics in crystals and large molecules[J]. The Journal of Physical Chemistry, 1996, 100(15): 6107-6110. |
| 20 | Delley B. From molecules to solids with the DMol3 approach [J]. Journal of Chemical Physics, 2000, 113(18): 7756-7764. |
| 21 | Perdew J P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas[J]. Physical Review. B, 1986, 33(12): 8822-8824. |
| 22 | Delley B. Efficient and accurate expansion methods for molecules in local density models[J]. The Journal of Chemical Physics, 1982, 76(4): 1949-1960. |
| 23 | Perdew J P, Wang Y. Accurate and simple analytic representation of the electron-gas correlation energy[J]. Physical Review B, 1992, 45(23): 13244-13249. |
| 24 | Moses P G, Hinnemann B, Topsøe H, et al. The hydrogenation and direct desulfurization reaction pathway in thiophene hydrodesulfurization over MoS2 catalysts at realistic conditions: a density functional study[J]. Journal of Catalysis, 2007, 248(2): 188-203. |
| 25 | Schweiger H, Raybaud P, Kresse G, et al. Shape and edge sites modifications of MoS2 catalytic nanoparticles induced by working conditions: a theoretical study[J]. Journal of Catalysis, 2002, 207(1): 76-87. |
| 26 | Joo P H, Cheng J L, Yang K S. Size effects and odd-even effects in MoS2 nanosheets: first-principles studies[J]. Physical Chemistry Chemical Physics, 2017, 19(44): 29927-29933. |
| 27 | Mcbride K L, Head J D. DFT investigation of MoS2 nanoclusters used as desulfurization catalysts[J]. International Journal of Quantum Chemistry, 2009, 109(15): 3570-3582. |
| 28 | Govind N, Petersen M, Fitzgerald G, et al. A generalized synchronous transit method for transition state location[J]. Computational Materials Science, 2003, 28(2): 250-258 |
| 29 | Raybaud P, Hafner J, Kresse G, et al. Ab initio study of the H2-H2S/MoS2 gas-solid interface: the nature of the catalytically active sites[J]. Journal of Catalysis, 2000, 189(1): 129-146. |
| 30 | Wang W, Zhao X G, Li H F, et al. DFT study of H2 dissociation on Mo x S y clusters[J]. China Petroleum Processing & Petrochemical Technology, 2015, 17(1): 16-23. |
| [1] | Xiaqi YU, Ge FENG, Jinyan ZHAO, Jiayuan LI, Shengwei DENG, Jingnan ZHENG, Wenwen LI, Yaqiu WANG, Lan SHEN, Xu LIU, Weiwei XU, Jianguo WANG, Shibin WANG, Zihao YAO, Chengli MAO. A first-principles study of the interaction between TDI-TMP-T313 and AP [J]. CIESC Journal, 2022, 73(8): 3511-3517. |
| [2] | Jihao ZHAO, Weiqiang TANG, Xiaofei XU, Shuangliang ZHAO, Jionghao HE. Adsorption energy of bonding agent on nano-filler in polymer composites [J]. CIESC Journal, 2022, 73(7): 3174-3181. |
| [3] | Xiaokun HE, Yuan XUE, Ran ZUO. Quantum chemistry study on gas reaction path in InN MOCVD growth [J]. CIESC Journal, 2022, 73(12): 5638-5647. |
| [4] | Xiaosong LUO, Jinbao HUANG, Mei ZHOU, Xin MU, Weiwei XU, Lei WU. Theoretical study on the mechanism of hydrolysis/alcoholysis/ammonolysis of butanediol terephthalate dimer [J]. CIESC Journal, 2022, 73(11): 4859-4871. |
| [5] | Xiang GONG, Linsen LI, Zhao JIANG. Employing PdCo/SiO2 catalyst in high activity dehydrogenation reaction of heterocyclic H2 storage carrier [J]. CIESC Journal, 2022, 73(10): 4448-4460. |
| [6] | Xianhui ZHU, Fu WANG, Jiecheng XIA, Jinliang YUAN. Density functional theory investigation on the NH3 and CO2 absorption by functional ionic liquids [J]. CIESC Journal, 2022, 73(10): 4324-4334. |
| [7] | Shenggui MA, Bowen TIAN, Yuwei ZHOU, Lin CHEN, Xia JIANG, Tao GAO. DFT study of adsorption of H2S on N-doped Stone-Wales defected graphene [J]. CIESC Journal, 2021, 72(9): 4496-4503. |
| [8] | ZHANG Fangfang, HAN Min, ZHAO Juan, LING Lixia, ZHANG Riguang, WANG Baojun. DFT study on reduction of NO over Pd atom anchored on single-vacancy graphene [J]. CIESC Journal, 2021, 72(3): 1382-1391. |
| [9] | TANG Weiqiang, XIE Peng, XU Xiaofei, ZHAO Shuangliang. Development and applications of reaction density functional theory [J]. CIESC Journal, 2021, 72(2): 633-652. |
| [10] | GE Bingqing, YIN Yixuan, WANG Yaxi, ZHANG Hongwei, YUAN Pei. Study of solvent effect on the dissolution, size, structure and catalytic hydrogenation of nitrile butadiene rubber [J]. CIESC Journal, 2021, 72(1): 543-554. |
| [11] | Jiaxin LIU, Yu XU, Er HUA. Structure and hydrogen bonding study on acylamino acid protic ionic liquids composed of 2-N-ethylhexylethylenediaminim cation with acylalanineate anions [J]. CIESC Journal, 2020, 71(S1): 15-22. |
| [12] | Ling DI, Fang CHEN, Rongrong FU, Chen YANG, Yang XING, Xiaoning WANG. Mechanism research of organic pesticides detection by rich electronic LMOF [J]. CIESC Journal, 2020, 71(8): 3830-3838. |
| [13] | Wei SUN, Ran ZUO. Study on adsorption and diffusion of MMAl on AlN(0001)-Al surface covered with NH2/H [J]. CIESC Journal, 2020, 71(7): 3213-3219. |
| [14] | Meng GONG, Yang FANG, Wei CHEN, Yingquan CHEN, Qiang LU, Haiping YANG, Hanping CHEN. Effect of cellulose composition on amino acids pyrolysis [J]. CIESC Journal, 2020, 71(5): 2312-2319. |
| [15] | Xiaorong ZHU, Yafei LI. Theoretical study on electrocatalytic nitrogen fixation performance of two-dimensional AuP2 [J]. CIESC Journal, 2020, 71(10): 4820-4825. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||