CIESC Journal ›› 2022, Vol. 73 ›› Issue (7): 3057-3067.DOI: 10.11949/0438-1157.20220329
• Separation engineering • Previous Articles Next Articles
Jiangwei ZHU1( ),Pengfei MA1(
),Pengfei MA1( ),Xiao DU1,Yanyan YANG2,Xiaogang HAO1(
),Xiao DU1,Yanyan YANG2,Xiaogang HAO1( ),Shanxia LUO3
),Shanxia LUO3
Received:2022-03-03
Revised:2022-05-19
Online:2022-08-01
Published:2022-07-05
Contact:
Pengfei MA,Xiaogang HAO
朱江伟1( ),马鹏飞1(
),马鹏飞1( ),杜晓1,杨言言2,郝晓刚1(
),杜晓1,杨言言2,郝晓刚1( ),罗善霞3
),罗善霞3
通讯作者:
马鹏飞,郝晓刚
作者简介:朱江伟(1997—),男,硕士研究生,基金资助:CLC Number:
Jiangwei ZHU, Pengfei MA, Xiao DU, Yanyan YANG, Xiaogang HAO, Shanxia LUO. Specific electronically controlled separation of phosphate anions based on variable valence NiFe-LDH/rGO[J]. CIESC Journal, 2022, 73(7): 3057-3067.
朱江伟, 马鹏飞, 杜晓, 杨言言, 郝晓刚, 罗善霞. 基于可变价NiFe-LDH/rGO对磷酸根离子的特异性电控分离[J]. 化工学报, 2022, 73(7): 3057-3067.
Add to citation manager EndNote|Ris|BibTeX
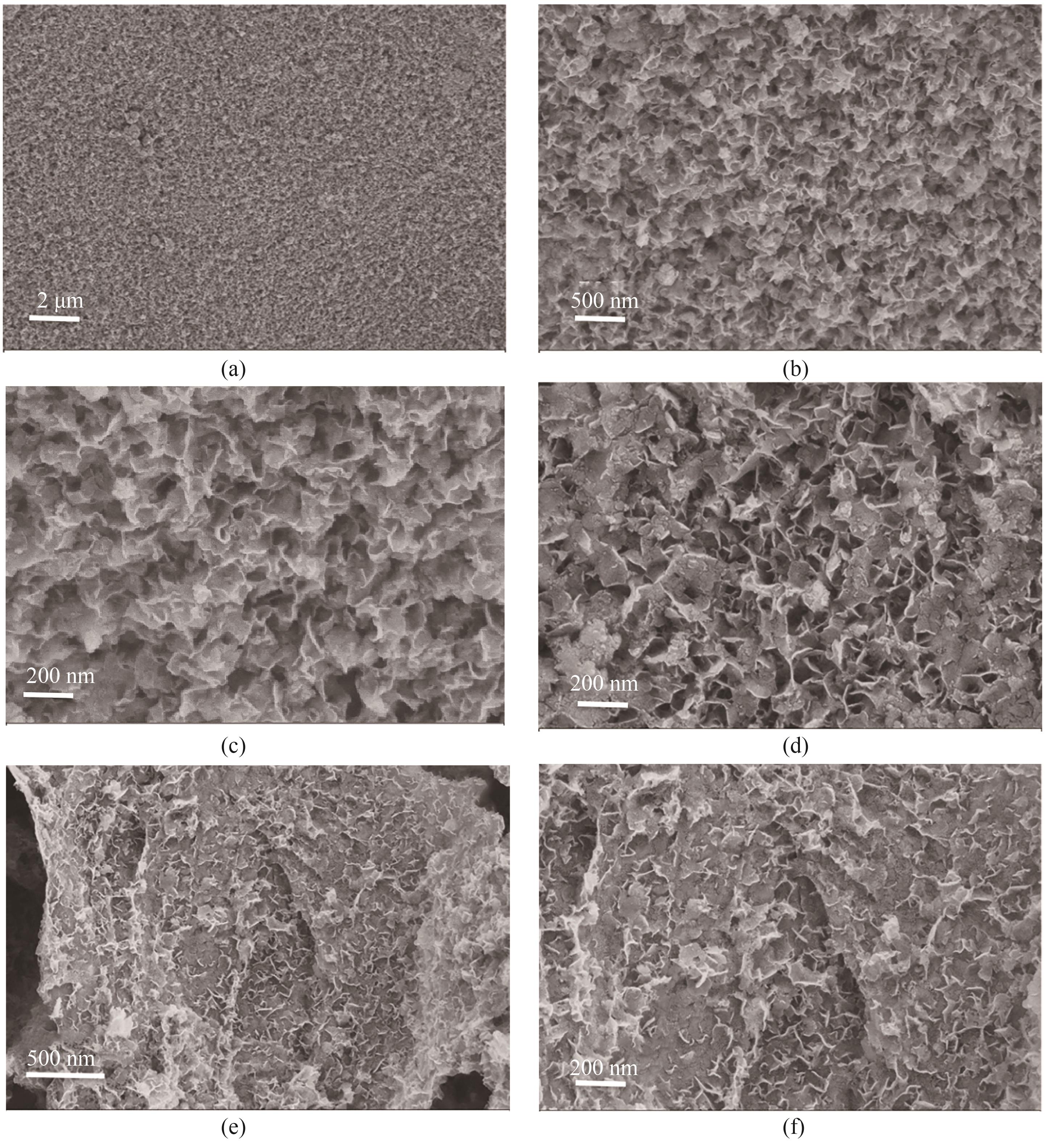
Fig.1 (a)—(c) SEM images of LDH at different magnification; (d) SEM image of LDH/GO hybrid matrials;(e), (f) SEM images of LDH/rGO hybrid matrials at different magnification
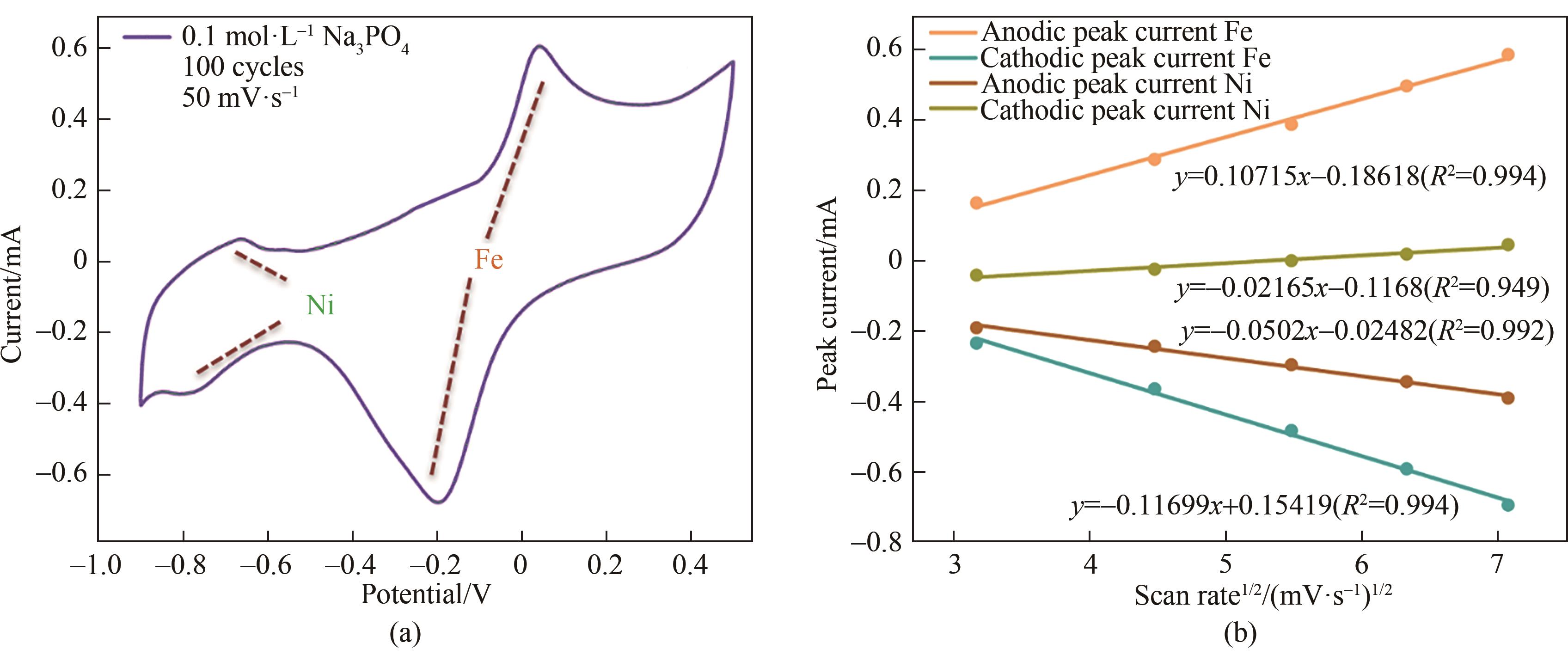
Fig.3 (a) CV curves of NiFe-LDH/rGO hybrid films in 0.1 mol·L-1 Na3PO4 solution at 50 mV·s-1; (b) The variation of anode and cathode peak current of the NiFe-LDH /rGO hybrid film with the square root of scanning speed in 0.1 mol·L-1 Na3PO4 solution
| I/mA | |||||
|---|---|---|---|---|---|
| Ni(阳极) | Ni(阴极) | Fe(阳极) | Fe(阴极) | ||
| 10 | 3.162 | 0.167 | -0.232 | -0.038 | -0.188 |
| 20 | 4.472 | 0.291 | -0.362 | -0.021 | -0.241 |
| 30 | 5.477 | 0.390 | -0.481 | 0.002 | -0.294 |
| 40 | 6.324 | 0.500 | -0.590 | 0.020 | -0.342 |
| 50 | 7.071 | 0.589 | -0.693 | 0.047 | -0.389 |
Table 1 Cathodic and anodic peak currents corresponding to different scanning rates in 0.1 mol·L-1 Na3PO4 solution
| I/mA | |||||
|---|---|---|---|---|---|
| Ni(阳极) | Ni(阴极) | Fe(阳极) | Fe(阴极) | ||
| 10 | 3.162 | 0.167 | -0.232 | -0.038 | -0.188 |
| 20 | 4.472 | 0.291 | -0.362 | -0.021 | -0.241 |
| 30 | 5.477 | 0.390 | -0.481 | 0.002 | -0.294 |
| 40 | 6.324 | 0.500 | -0.590 | 0.020 | -0.342 |
| 50 | 7.071 | 0.589 | -0.693 | 0.047 | -0.389 |
| C0/(mg·L-1) | qe(exp)/( mg·g-1) | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | qe(cal)/( mg·g-1) | R2 | k2/min-1 | qe(cal)/(mg·g-1) | R2 | ||
| 101.58 | 76.35 | 0.011 | 60.138 | 0.948 | 1.55×10-4 | 90.090 | 0.997 |
| 192.72 | 121.74 | 0.017 | 105.047 | 0.866 | 8.87×10-5 | 149.477 | 0.986 |
| 301.93 | 200.18 | 0.018 | 174.493 | 0.971 | 4.38×10-5 | 236.967 | 0.996 |
| 512.36 | 269.68 | 0.012 | 265.219 | 0.966 | 4.57×10-5 | 282.486 | 0.996 |
Table 2 Kinetic model parameters and related factors of phosphate anions induced by NiFe-LDH/rGO hybrid film at different concentrations
| C0/(mg·L-1) | qe(exp)/( mg·g-1) | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | qe(cal)/( mg·g-1) | R2 | k2/min-1 | qe(cal)/(mg·g-1) | R2 | ||
| 101.58 | 76.35 | 0.011 | 60.138 | 0.948 | 1.55×10-4 | 90.090 | 0.997 |
| 192.72 | 121.74 | 0.017 | 105.047 | 0.866 | 8.87×10-5 | 149.477 | 0.986 |
| 301.93 | 200.18 | 0.018 | 174.493 | 0.971 | 4.38×10-5 | 236.967 | 0.996 |
| 512.36 | 269.68 | 0.012 | 265.219 | 0.966 | 4.57×10-5 | 282.486 | 0.996 |

Fig.7 (a) Competitive adsorption of PO43-, SO42-, NO3-, and Cl- for NiFe-LDH/rGO hybrid films at an initial concentration of 300 mg·L-1; (b) CV curves of NiFe-LDH/rGO hybrid film in 0.30 mol·L-1 NaNO3, 0.30 mol·L-1 NaCl, 0.15 mol·L-1 Na2SO4, and 0.10 mol·L-1 Na3PO4 electrolytic solutions
| Anion | Adsorption capacity/(mg·g-1) | Separation coefficient (KD) | Relative separation factor (α) |
|---|---|---|---|
| 178.012 | 0.865 | 1 | |
| 70.871 | 0.274 | 3.163 | |
| 50.336 | 0.185 | 4.678 | |
| Cl- | 45.248 | 0.156 | 5.551 |
Table 3 Separation coefficients and relative separation factors of PO43-, SO42-, NO3-, and Cl- of the NiFe-LDH/rGO hybrid film at the initial concentration of 300 mg·L-1
| Anion | Adsorption capacity/(mg·g-1) | Separation coefficient (KD) | Relative separation factor (α) |
|---|---|---|---|
| 178.012 | 0.865 | 1 | |
| 70.871 | 0.274 | 3.163 | |
| 50.336 | 0.185 | 4.678 | |
| Cl- | 45.248 | 0.156 | 5.551 |
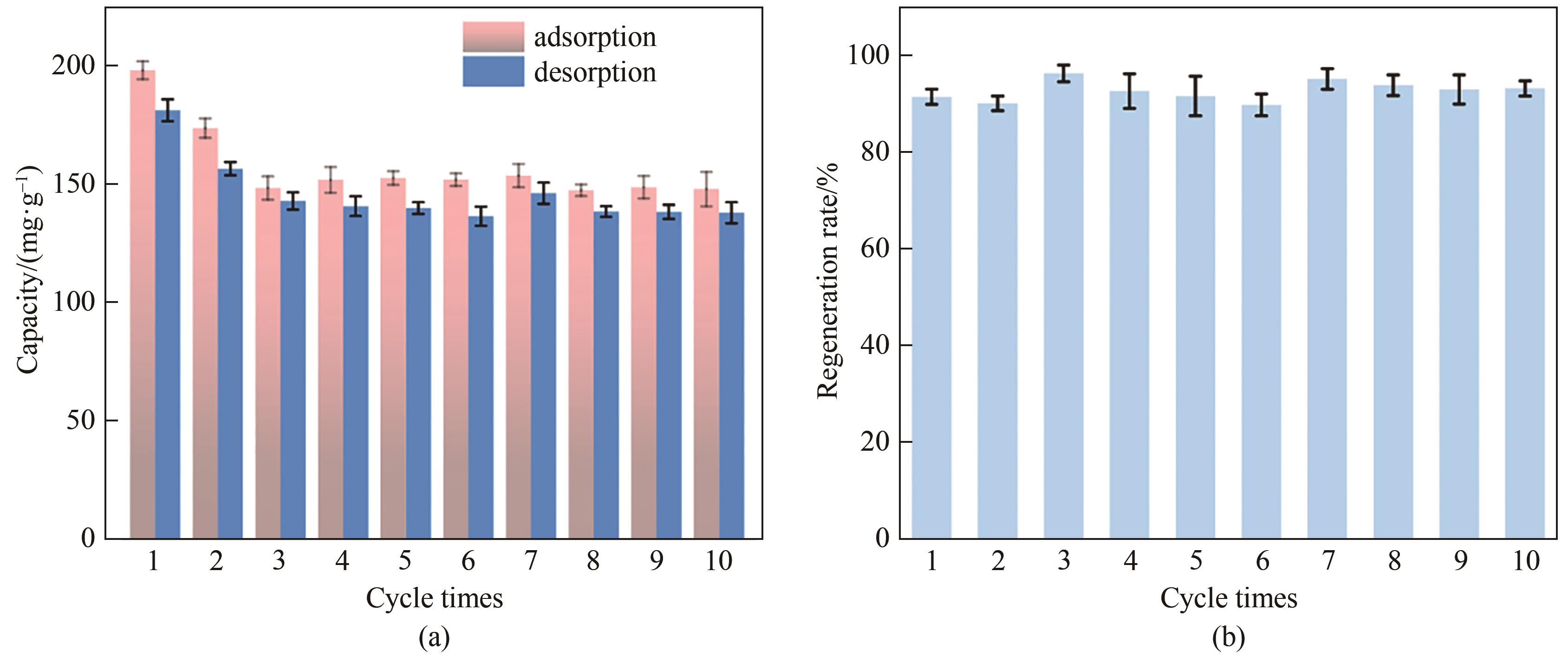
Fig.8 The adsorption and desorption capacity (a) and regeneration rate (b) of NiFe-LDH /rGO hybrid film for phosphate ions in 300 mg·L-1 sodium phosphate and sodium nitrate solutions, respectively
| 1 | 袁伟皓, 王华, 曾一川, 等. 大型通江湖泊藻类增殖驱动要素的时空分异特征[J]. 环境工程, 2021, 39(10): 64-71, 128. |
| Yuan W H, Wang H, Zeng Y C, et al. Spatiotemporal variation of driving factors of algal proliferation in a large river-connected lake[J]. Environmental Engineering, 2021, 39(10): 64-71, 128. | |
| 2 | Wildemeersch M, Tang S H, Ermolieva T, et al. Containing the risk of phosphorus pollution in agricultural watersheds[J]. Sustainability, 2022, 14(3): 1717. |
| 3 | Garnache C, Swinton S, Herriges J, et al. Solving the phosphorus pollution puzzle: synthesis and directions for future research[J]. American Journal of Agricultural Economics, 2016, 98: 1334-1359. |
| 4 | 秦伯强. 浅水湖泊湖沼学与太湖富营养化控制研究[J]. 湖泊科学, 2020, 32(5): 1229-1243. |
| Qin B Q. Shallow lake limnology and control of eutrophication in Lake Taihu[J]. Journal of Lake Sciences, 2020, 32(5): 1229-1243. | |
| 5 | Sun Y, Feng X L, Zheng W S. Nanoscale lanthanum carbonate hybridized with polyacrylic resin for enhanced phosphate removal from secondary effluent[J]. Journal of Chemical & Engineering Data, 2020, 65(9): 4512-4522. |
| 6 | Wu B L, Lo I M C. Surface functional group engineering of CeO2 particles for enhanced phosphate adsorption[J]. Environmental Science & Technology, 2020, 54(7): 4601-4608. |
| 7 | Prashantha Kumar T K M, Mandlimath T R, Sangeetha P, et al. Nanoscale materials as sorbents for nitrate and phosphate removal from water[J]. Environmental Chemistry Letters, 2018, 16(2): 389-400. |
| 8 | Morimoto K, Anraku S, Hoshino J, et al. Surface complexation reactions of inorganic anions on hydrotalcite-like compounds[J]. Journal of Colloid and Interface Science, 2012, 384: 99-104. |
| 9 | Li N, Tian Y, Zhao J H, et al. Ultrafast selective capture of phosphorus from sewage by 3D Fe3O4@ZnO via weak magnetic field enhanced adsorption[J]. Chemical Engineering Journal, 2018, 341: 289-297. |
| 10 | Wu B, Wan J, Zhang Y, et al. Selective phosphate removal from water and wastewater using sorption: process fundamentals and removal mechanisms[J]. Environmental Science & Technology, 2020, 54: 50-66. |
| 11 | Niu J J, Yan W, Du J, et al. An electrically switched ion exchange film with molecular coupling synergistically-driven ability for recovery of Ag+ ions from wastewater[J]. Chemical Engineering Journal, 2020, 389: 12449-12457. |
| 12 | 郝晓刚, 郭金霞, 张忠林, 等. 电沉积铁氰化镍薄膜的电控离子交换性能[J]. 化工学报, 2005, 56(12): 2380-2386. |
| Hao X G, Guo J X, Zhang Z L, et al. Electrochemically switched ion exchange properties of electrodeposited nickel hexacyanoferrate thin films[J]. Journal of Chemical Industry and Engineering (China), 2005, 56 (12): 2380-2386. | |
| 13 | 马旭莉, 张权, 杜晓, 等. α-ZrP/PANI电控离子交换膜对Pb2+的选择性分离[J]. 稀有金属材料与工程, 2016, 45(8): 2139-2145. |
| Ma X L, Zhang Q, Du X, et al. Selective separation to Pb2+ of electrochemically switched ion exchange film of α-ZrP/PANI[J]. Rare Metal Materials and Engineering, 2016, 45(8): 2139-2145. | |
| 14 | Du X, Guan G Q, Li X M, et al. A novel electroactive λ-MnO2/PPy/PSS core-shell nanorod coated electrode for selective recovery of lithium ions at low concentration[J]. Journal of Materials Chemistry A, 2016, 4(36): 13989-13996. |
| 15 | Hong S P, Yoon H, Lee J, et al. Selective phosphate removal using layered double hydroxide/reduced graphene oxide (LDH/rGO) composite electrode in capacitive deionization[J]. Journal of Colloid and Interface Science, 2020, 564: 1-7. |
| 16 | Rahman S, Navarathna C M, Krishna Das N, et al. High capacity aqueous phosphate reclamation using Fe/Mg-layered double hydroxide (LDH) dispersed on biochar[J]. Journal of Colloid and Interface Science, 2021, 597: 182-195. |
| 17 | 来天艺, 王纪康, 李天, 等. 光电解水产活性氢/氧耦合加氢/氧化过程用水滑石基纳米材料[J]. 化工学报, 2020, 71(10): 4327-4349. |
| Lai T Y, Wang J K, Li T, et al. Photoelectrochemical water splitting into active hydrogen/oxygen species coupling with hydrogenation/oxidation process using layered double hydroxides-based nanocatalysts[J]. CIESC Journal, 2020, 71(10): 4327-4349. | |
| 18 | Liu C, Zhang M Y, Pan G, et al. Phosphate capture by ultrathin MgAl layered double hydroxide nanoparticles[J]. Applied Clay Science, 2019, 177: 82-90. |
| 19 | Tian M, Liu C F, Neale Z G, et al. Chemically bonding NiFe-LDH nanosheets on rGO for superior lithium-ion capacitors[J]. ACS Applied Materials & Interfaces, 2019, 11(39): 35977-35986. |
| 20 | Son Y R, Park S J. Influence of carboxymethyl cellulose content on structures and electrochemical behaviors of reduced graphene oxide films[J]. Electrochimica Acta, 2020, 330: 135219. |
| 21 | Shinde D B, Vlassiouk I V, Talipov M R, et al. Exclusively proton conductive membranes based on reduced graphene oxide polymer composites[J]. ACS Nano, 2019, 13(11): 13136-13143. |
| 22 | 杨言言, 李永国, 祝小雯, 等. 电活性镍钴双金属氧化物高选择性去除/回收水中磷酸盐离子[J]. 无机材料学报, 2021, 36(3): 292-298. |
| Yang Y Y, Li Y G, Zhu X W, et al. Potential induced reversible removal/recovery of phosphate anions with high selectivity using an electroactive NiCo-layered double oxide film[J]. Journal of Inorganic Materials, 2021, 36(3): 292-298. | |
| 23 | Forticaux A, Dang L N, Liang H F, et al. Controlled synthesis of layered double hydroxide nanoplates driven by screw dislocations[J]. Nano Letters, 2015, 15(5): 3403-3409. |
| 24 | Ghani M, Ghoreishi S M, Azamati M. Magnesium-aluminum-layered double hydroxide-graphene oxide composite mixed-matrix membrane for the thin-film microextraction of diclofenac in biological fluids[J]. Journal of Chromatography A, 2018, 1575: 11-17. |
| 25 | He H M, Kang H L, Ma S L, et al. High adsorption selectivity of ZnAl layered double hydroxides and the calcined materials toward phosphate[J]. Journal of Colloid and Interface Science, 2010, 343: 225-231. |
| 26 | Abo El-Reesh G Y, Farghali A A, Taha M, et al. Novel synthesis of Ni/Fe layered double hydroxides using urea and glycerol and their enhanced adsorption behavior for Cr(Ⅵ) removal[J]. Scientific Reports, 2020, 10: 587. |
| 27 | Chen J, Fan X L, Ji X, et al. Intercalation of Bi nanoparticles into graphite results in an ultra-fast and ultra-stable anode material for sodium-ion batteries[J]. Energy & Environmental Science, 2018, 11(5): 1218-1225. |
| 28 | Hao X G, Yan T, Wang Z D, et al. Unipolar pulse electrodeposition of nickel hexacyanoferrate thin films with controllable structure on platinum substrates[J]. Thin Solid Films, 2012, 520(7): 2438-2448. |
| 29 | Youmbi B S, Pélisson C H, Denicourt-Nowicki A, et al. Impact of the charge transfer process on the Fe2+/Fe3+ distribution at Fe3O4 magnetic surface induced by deposited Pd clusters[J]. Surface Science, 2021, 712: 121879. |
| 30 | Wan J, Wu B L, Lo I M C. Development of Fe0/Fe3O4 composites with tunable properties facilitated by Fe2+ for phosphate removal from river water[J]. Chemical Engineering Journal, 2020, 388: 124242. |
| 31 | Ji W W, Niu J J, Zhang W, et al. An electroactive ion exchange hybrid film with collaboratively-driven ability for electrochemically-mediated selective extraction of chloride ions[J]. Chemical Engineering Journal, 2022, 427: 130807. |
| 32 | Zhao G Q, Li C F, Wu X, et al. Reduced graphene oxide modified NiFe-calcinated layered double hydroxides for enhanced photocatalytic removal of methylene blue[J]. Applied Surface Science, 2018, 434: 251-259. |
| 33 | Xu W S, Zheng W J, Wang F J, et al. Using iron ion-loaded aminated polyacrylonitrile fiber to efficiently remove wastewater phosphate[J]. Chemical Engineering Journal, 2021, 403: 126349. |
| [1] | He JIANG, Junfei YUAN, Lin WANG, Guyu XING. Experimental study on the effect of flow sharing cavity structure on phase change flow characteristics in microchannels [J]. CIESC Journal, 2023, 74(S1): 235-244. |
| [2] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [3] | Yuyuan ZHENG, Zhiwei GE, Xiangyu HAN, Liang WANG, Haisheng CHEN. Progress and prospect of medium and high temperature thermochemical energy storage of calcium-based materials [J]. CIESC Journal, 2023, 74(8): 3171-3192. |
| [4] | Lingding MENG, Ruqing CHONG, Feixue SUN, Zihui MENG, Wenfang LIU. Immobilization of carbonic anhydrase on modified polyethylene membrane and silica [J]. CIESC Journal, 2023, 74(8): 3472-3484. |
| [5] | Yali HU, Junyong HU, Suxia MA, Yukun SUN, Xueyi TAN, Jiaxin HUANG, Fengyuan YANG. Development of novel working fluid and study on electrochemical characteristics of reverse electrodialysis heat engine [J]. CIESC Journal, 2023, 74(8): 3513-3521. |
| [6] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| [7] | Jiali GE, Tuxiang GUAN, Xinmin QIU, Jian WU, Liming SHEN, Ningzhong BAO. Synthesis of FeF3 nanoparticles covered by vertical porous carbon for high performance Li-ion battery cathode [J]. CIESC Journal, 2023, 74(7): 3058-3067. |
| [8] | Yuanhao QU, Wenyi DENG, Xiaodan XIE, Yaxin SU. Study on electro-osmotic dewatering of sludge assisted by activated carbon/graphite [J]. CIESC Journal, 2023, 74(7): 3038-3050. |
| [9] | Tan ZHANG, Guang LIU, Jinping LI, Yuhan SUN. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts [J]. CIESC Journal, 2023, 74(6): 2264-2280. |
| [10] | Bin CAI, Xiaolin ZHANG, Qian LUO, Jiangtao DANG, Liyuan ZUO, Xinmei LIU. Research progress of conductive thin film materials [J]. CIESC Journal, 2023, 74(6): 2308-2321. |
| [11] | Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics [J]. CIESC Journal, 2023, 74(6): 2589-2598. |
| [12] | Wenchao XU, Zhigao SUN, Cuimin LI, Juan LI, Haifeng HUANG. Effect of surfactant E-1310 on the formation of HCFC-141b hydrate under static conditions [J]. CIESC Journal, 2023, 74(5): 2179-2185. |
| [13] | Xu GUO, Yongzheng ZHANG, Houbing XIA, Na YANG, Zhenzhen ZHU, Jingyao QI. Research progress in the removal of water pollutants by carbon-based materials via electrooxidation [J]. CIESC Journal, 2023, 74(5): 1862-1874. |
| [14] | Hao GU, Fujian ZHANG, Zhen LIU, Wenxuan ZHOU, Peng ZHANG, Zhongqiang ZHANG. Desalination performance and mechanism of porous graphene membrane in temporal dimension under mechanical-electrical coupling [J]. CIESC Journal, 2023, 74(5): 2067-2074. |
| [15] | Zheng ZHANG, Yongping HE, Haidong SUN, Rongzi ZHANG, Zhengping SUN, Jinlan CHEN, Yixuan ZHENG, Xiao DU, Xiaogang HAO. Electrochemically switched ion exchange device with serpentine flow field for selective extraction of lithium [J]. CIESC Journal, 2023, 74(5): 2022-2033. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||