CIESC Journal ›› 2025, Vol. 76 ›› Issue (6): 3041-3052.DOI: 10.11949/0438-1157.20241144
• Material science and engineering, nanotechnology • Previous Articles Next Articles
Ziyang LI( ), Peixin SHEN, Xiao'a ZHANG(
), Peixin SHEN, Xiao'a ZHANG( ), Chengzhong WANG, Ling SHI, Junying ZHANG
), Chengzhong WANG, Ling SHI, Junying ZHANG
Received:2024-10-17
Revised:2025-01-14
Online:2025-07-09
Published:2025-06-25
Contact:
Xiao'a ZHANG
李子阳( ), 申沛鑫, 张孝阿(
), 申沛鑫, 张孝阿( ), 王成忠, 史翎, 张军营
), 王成忠, 史翎, 张军营
通讯作者:
张孝阿
作者简介:李子阳(2000—),男,硕士研究生,lee13396013613@163.com
基金资助:CLC Number:
Ziyang LI, Peixin SHEN, Xiao'a ZHANG, Chengzhong WANG, Ling SHI, Junying ZHANG. Synthesis and thermal stability of α, ω-hydroxy-terminated phenyl/phenylene-containing polysiloxanes with high vinyl content[J]. CIESC Journal, 2025, 76(6): 3041-3052.
李子阳, 申沛鑫, 张孝阿, 王成忠, 史翎, 张军营. α, ω-端羟基苯基/亚苯基高乙烯基聚硅氧烷的合成及热稳定性[J]. 化工学报, 2025, 76(6): 3041-3052.
Add to citation manager EndNote|Ris|BibTeX
| 序号 | 温度/℃ | 反应 时间/h | 产率/% | η(25℃)/(Pa·s) | Mn | PDI |
|---|---|---|---|---|---|---|
| 1 | 90 | — | — | — | — | — |
| 2 | 100 | 3.5 | 62 | 8.9 | 21100 | 1.56 |
| 3 | 110 | 2 | 77 | 9.3 | 25300 | 1.64 |
| 4 | 120 | 0.5 | 90 | 10.0 | 27200 | 1.51 |
Table 1 Effect of temperature on reaction(P1-5)
| 序号 | 温度/℃ | 反应 时间/h | 产率/% | η(25℃)/(Pa·s) | Mn | PDI |
|---|---|---|---|---|---|---|
| 1 | 90 | — | — | — | — | — |
| 2 | 100 | 3.5 | 62 | 8.9 | 21100 | 1.56 |
| 3 | 110 | 2 | 77 | 9.3 | 25300 | 1.64 |
| 4 | 120 | 0.5 | 90 | 10.0 | 27200 | 1.51 |
| 序号 | TMAS/g | H2O/g | 产率/% | η/(Pa·s) | Mn | PDI |
|---|---|---|---|---|---|---|
| 1 | 0.45 | 0.25 | 78 | 13.6 | 37700 | 1.54 |
| 2 | 0.60 | 0.25 | 82 | 12.8 | 32600 | 1.66 |
| 3 | 0.75 | 0.25 | 89 | 10.7 | 25200 | 1.43 |
| 4 | 0.90 | 0.25 | 96 | 9.3 | 21400 | 1.51 |
| 5 | 0.75 | 0.10 | 95 | 14.4 | 48600 | 1.74 |
| 6 | 0.75 | 0.15 | 95 | 14.1 | 46500 | 1.36 |
| 7 | 0.75 | 0.20 | 94 | 12.6 | 31400 | 1.53 |
Table 2 Effect of catalyst and capping agent dosages on the synthesis of P1-5
| 序号 | TMAS/g | H2O/g | 产率/% | η/(Pa·s) | Mn | PDI |
|---|---|---|---|---|---|---|
| 1 | 0.45 | 0.25 | 78 | 13.6 | 37700 | 1.54 |
| 2 | 0.60 | 0.25 | 82 | 12.8 | 32600 | 1.66 |
| 3 | 0.75 | 0.25 | 89 | 10.7 | 25200 | 1.43 |
| 4 | 0.90 | 0.25 | 96 | 9.3 | 21400 | 1.51 |
| 5 | 0.75 | 0.10 | 95 | 14.4 | 48600 | 1.74 |
| 6 | 0.75 | 0.15 | 95 | 14.1 | 46500 | 1.36 |
| 7 | 0.75 | 0.20 | 94 | 12.6 | 31400 | 1.53 |
| 序号 | 温度/℃ | 反应时间/h | 产率/% | η/(Pa·s) | Mn | PDI |
|---|---|---|---|---|---|---|
| 1 | 90 | 3 | 56 | 16.6 | 69800 | 1.46 |
| 2 | 100 | 3 | 76 | 17.9 | 77700 | 1.54 |
| 3 | 110 | 3 | 95 | 20.9 | 81200 | 1.66 |
| 4 | 120 | 3 | 94 | 17.2 | 75700 | 1.64 |
| 5 | 110 | 5 | 96 | 22.1 | 92100 | 1.57 |
| 6 | 110 | 8 | 94 | 23.8 | 100300 | 1.58 |
| 7 | 110 | 10 | 95 | 24.4 | 101500 | 1.54 |
Table 3 Effect of temperature and time on the synthesis of P2
| 序号 | 温度/℃ | 反应时间/h | 产率/% | η/(Pa·s) | Mn | PDI |
|---|---|---|---|---|---|---|
| 1 | 90 | 3 | 56 | 16.6 | 69800 | 1.46 |
| 2 | 100 | 3 | 76 | 17.9 | 77700 | 1.54 |
| 3 | 110 | 3 | 95 | 20.9 | 81200 | 1.66 |
| 4 | 120 | 3 | 94 | 17.2 | 75700 | 1.64 |
| 5 | 110 | 5 | 96 | 22.1 | 92100 | 1.57 |
| 6 | 110 | 8 | 94 | 23.8 | 100300 | 1.58 |
| 7 | 110 | 10 | 95 | 24.4 | 101500 | 1.54 |
| 序号 | 聚合物 | D4/g | D4Vi/g | D4Ph2/g | BHB/g | TMAS/g | H2O/g | η/(Pa·s) | 产率/% | Mn | PDI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P1-25 | 44.49 | 21.54 | 29.73 | — | 0.9 | 0.25 | 15.0 | 93 | 52900 | 1.71 |
| 2 | P1-45 | 29.66 | 38.77 | 29.73 | — | 0.9 | 0.25 | 14.3 | 96 | 54600 | 1.59 |
| 3 | P1-65 | 14.83 | 56.01 | 29.73 | — | 0.9 | 0.25 | 13.5 | 95 | 48500 | 1.69 |
| 4 | P1-85 | 0 | 73.24 | 29.73 | — | 0.9 | 0.25 | 11.4 | 96 | 43100 | 1.80 |
| 5 | P2-15 | 17.80 | 8.62 | — | 3.40 | 0.35 | — | 20.8 | 95 | 80600 | 1.53 |
| 6 | P2-25 | 14.83 | 8.62 | — | 5.66 | 0.35 | — | 14.4 | 97 | 64400 | 1.66 |
| 7 | P2-35 | 11.86 | 8.62 | — | 7.92 | 0.35 | — | 12.2 | 94 | 48700 | 1.60 |
| 8 | P2-45 | 8.90 | 8.62 | — | 10.18 | 0.35 | — | 13.7 | 95 | 56600 | 1.70 |
Table 4 Polysiloxanes synthesized at different feed ratios
| 序号 | 聚合物 | D4/g | D4Vi/g | D4Ph2/g | BHB/g | TMAS/g | H2O/g | η/(Pa·s) | 产率/% | Mn | PDI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P1-25 | 44.49 | 21.54 | 29.73 | — | 0.9 | 0.25 | 15.0 | 93 | 52900 | 1.71 |
| 2 | P1-45 | 29.66 | 38.77 | 29.73 | — | 0.9 | 0.25 | 14.3 | 96 | 54600 | 1.59 |
| 3 | P1-65 | 14.83 | 56.01 | 29.73 | — | 0.9 | 0.25 | 13.5 | 95 | 48500 | 1.69 |
| 4 | P1-85 | 0 | 73.24 | 29.73 | — | 0.9 | 0.25 | 11.4 | 96 | 43100 | 1.80 |
| 5 | P2-15 | 17.80 | 8.62 | — | 3.40 | 0.35 | — | 20.8 | 95 | 80600 | 1.53 |
| 6 | P2-25 | 14.83 | 8.62 | — | 5.66 | 0.35 | — | 14.4 | 97 | 64400 | 1.66 |
| 7 | P2-35 | 11.86 | 8.62 | — | 7.92 | 0.35 | — | 12.2 | 94 | 48700 | 1.60 |
| 8 | P2-45 | 8.90 | 8.62 | — | 10.18 | 0.35 | — | 13.7 | 95 | 56600 | 1.70 |
| 聚合物 | 峰位 | 硅原子位置 | δ | 聚合物 | 峰位 | 硅原子位置 | δ |
|---|---|---|---|---|---|---|---|
| P1 | A | 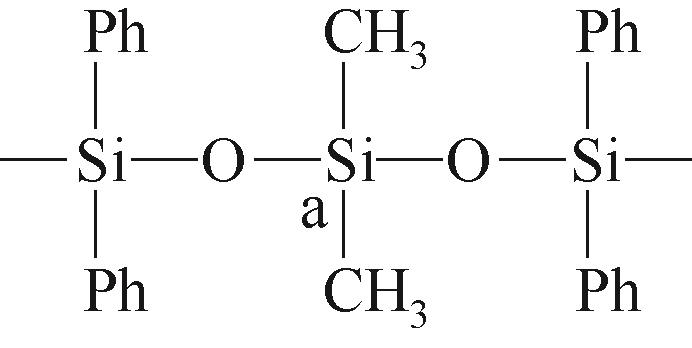 | 约-19.04 | P2 | a | 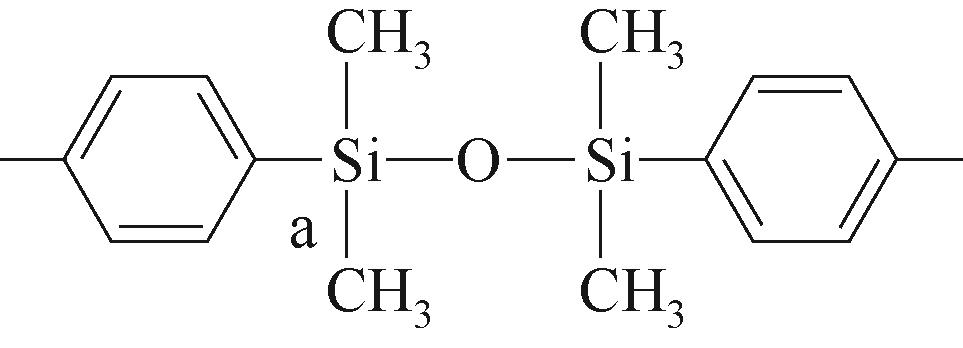 | 约-1.29 |
| b | 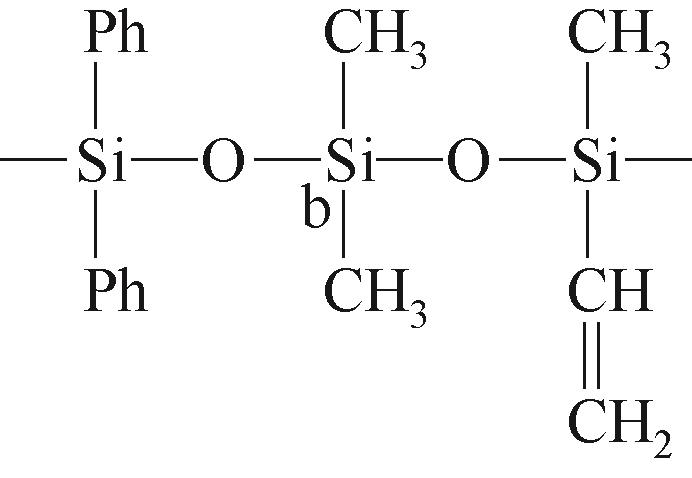 | 约-20.69 | b | 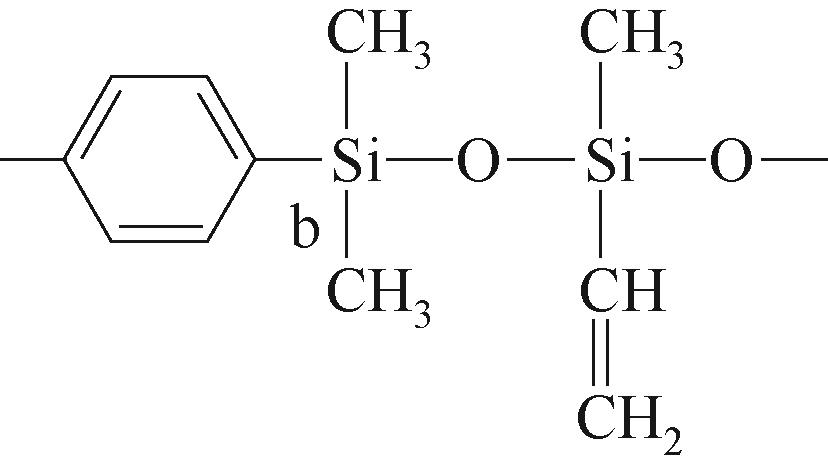 | 约-1.73 | ||
| c | 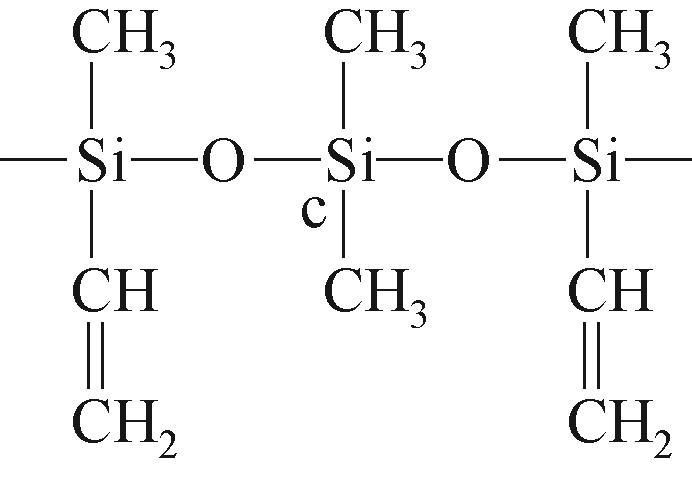 | 约-21.32 | c | 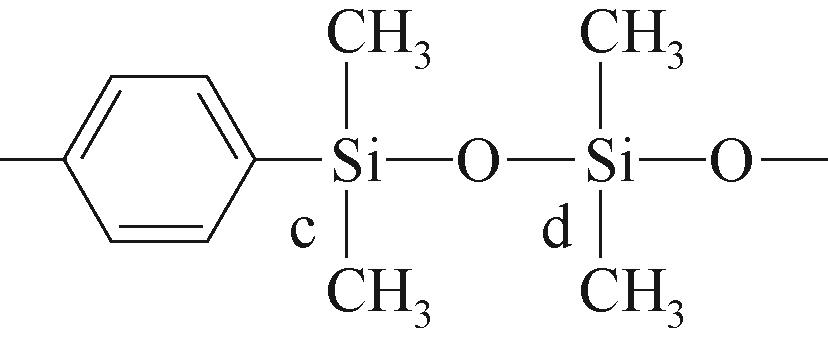 | 约-2.63 | ||
| d | 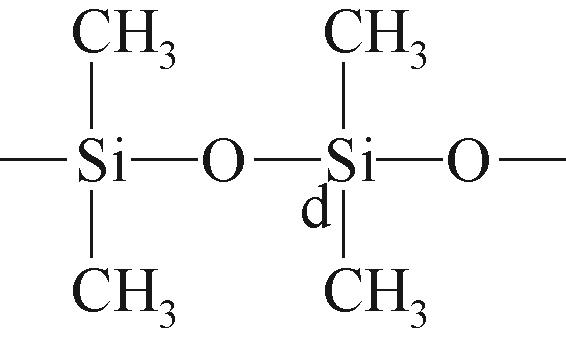 | 约-21.79 | d | 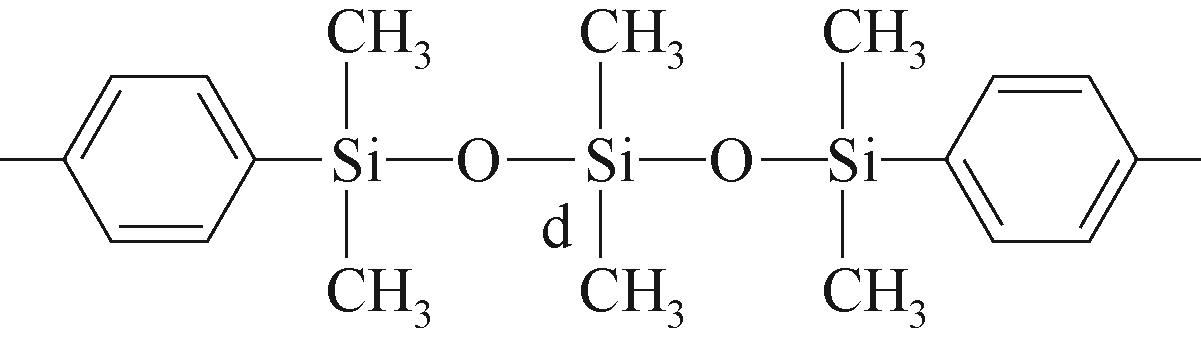 | 约-19.06 | ||
| e | 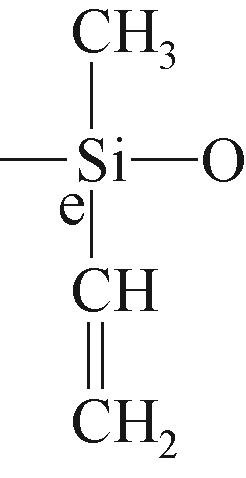 | 约-34.96 | e | 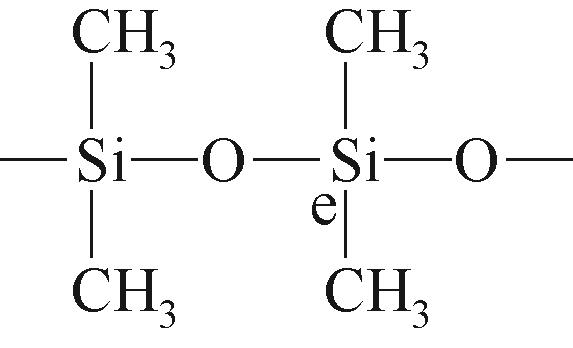 | 约-21.43 | ||
| f | 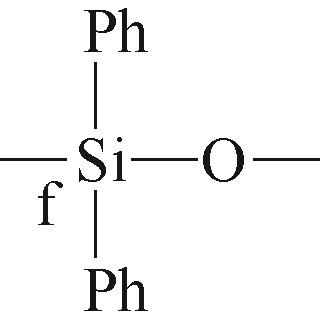 | 约-47.78 | f | 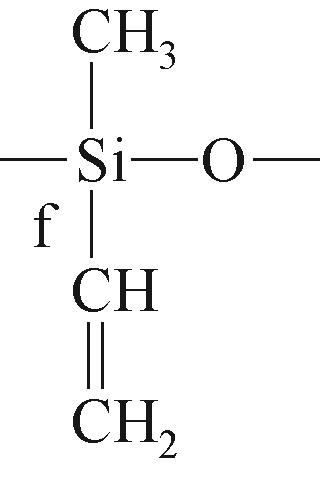 | 约-35.45 |
Table 5 Characteristic absorption peaks corresponding to different silicon atoms in P1 and P2
| 聚合物 | 峰位 | 硅原子位置 | δ | 聚合物 | 峰位 | 硅原子位置 | δ |
|---|---|---|---|---|---|---|---|
| P1 | A |  | 约-19.04 | P2 | a |  | 约-1.29 |
| b |  | 约-20.69 | b |  | 约-1.73 | ||
| c |  | 约-21.32 | c |  | 约-2.63 | ||
| d |  | 约-21.79 | d |  | 约-19.06 | ||
| e | 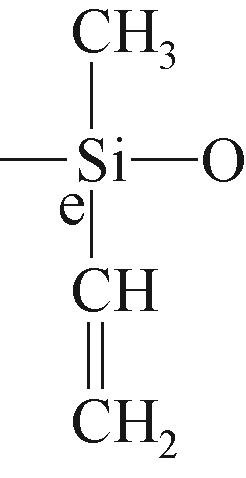 | 约-34.96 | e | 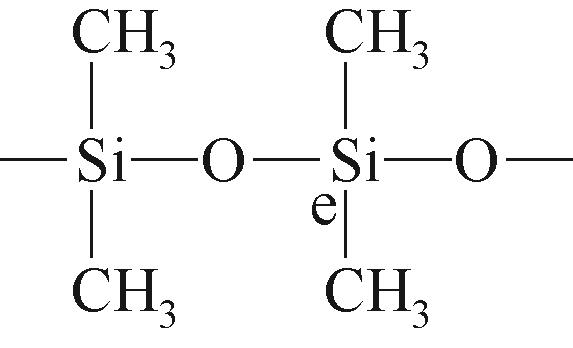 | 约-21.43 | ||
| f | 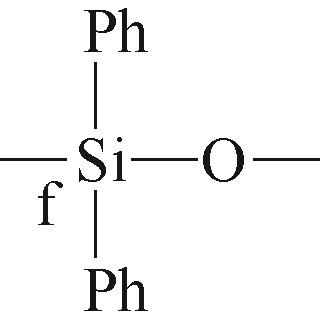 | 约-47.78 | f |  | 约-35.45 |
| 序号 | 聚合物 | 邵氏A硬度 | 拉伸强度/MPa | 断裂伸长率/% |
|---|---|---|---|---|
| 1 | P1-0 | 23±2 | 0.90 | 374 |
| 2 | P1-5 | 19±4 | 0.69 | 287 |
| 3 | P1-25 | 20±2 | 0.74 | 301 |
| 4 | P1-45 | 21±3 | 0.92 | 336 |
| 5 | P1-65 | 24±2 | 0.94 | 325 |
| 6 | P1-85 | 25±2 | 0.86 | 313 |
| 7 | P2-15 | 21±2 | 1.25 | 402 |
| 8 | P2-25 | 24±3 | 1.34 | 424 |
| 9 | P2-35 | 28±2 | 1.46 | 450 |
| 10 | P2-45 | 31±2 | 1.51 | 485 |
Table 6 Hardness, tensile strength and elongation at break of silicone rubber (cured P1 and P2)
| 序号 | 聚合物 | 邵氏A硬度 | 拉伸强度/MPa | 断裂伸长率/% |
|---|---|---|---|---|
| 1 | P1-0 | 23±2 | 0.90 | 374 |
| 2 | P1-5 | 19±4 | 0.69 | 287 |
| 3 | P1-25 | 20±2 | 0.74 | 301 |
| 4 | P1-45 | 21±3 | 0.92 | 336 |
| 5 | P1-65 | 24±2 | 0.94 | 325 |
| 6 | P1-85 | 25±2 | 0.86 | 313 |
| 7 | P2-15 | 21±2 | 1.25 | 402 |
| 8 | P2-25 | 24±3 | 1.34 | 424 |
| 9 | P2-35 | 28±2 | 1.46 | 450 |
| 10 | P2-45 | 31±2 | 1.51 | 485 |
| 序号 | 聚合物 | 气相白炭黑/份 | 邵氏A硬度 | 拉伸强度/MPa | 断裂伸长率/% |
|---|---|---|---|---|---|
| 1 | P1-25 | 20 | 24 | 1.7 | 354 |
| 2 | P1-25 | 30 | 30 | 3.2 | 327 |
| 3 | P1-25 | 40 | 36 | 4.2 | 298 |
| 4 | P2-15 | 20 | 29 | 1.9 | 432 |
| 5 | P2-15 | 30 | 31 | 3.3 | 465 |
| 6 | P2-15 | 40 | 35 | 5.2 | 541 |
Table 7 Effect of the dosage of fumed silica on the mechanical properties of silicone rubbers
| 序号 | 聚合物 | 气相白炭黑/份 | 邵氏A硬度 | 拉伸强度/MPa | 断裂伸长率/% |
|---|---|---|---|---|---|
| 1 | P1-25 | 20 | 24 | 1.7 | 354 |
| 2 | P1-25 | 30 | 30 | 3.2 | 327 |
| 3 | P1-25 | 40 | 36 | 4.2 | 298 |
| 4 | P2-15 | 20 | 29 | 1.9 | 432 |
| 5 | P2-15 | 30 | 31 | 3.3 | 465 |
| 6 | P2-15 | 40 | 35 | 5.2 | 541 |
| 序号 | 聚合物 | T5d/℃ | Tmax/℃ | 残炭率/% |
|---|---|---|---|---|
| 1 | P1-0 | 288 | 584 | 19.99 |
| 2 | P1-5 | 232 | 554 | 47.53 |
| 3 | P1-25 | 188 | 565 | 59.67 |
| 4 | P1-45 | 185 | 559 | 65.07 |
| 5 | P1-65 | 231 | 577 | 71.19 |
| 6 | P1-85 | 235 | 554 | 74.02 |
| 7 | P2-15 | 479 | 586 | 48.59 |
| 8 | P2-25 | 487 | 589 | 56.96 |
| 9 | P2-35 | 500 | 587 | 62.83 |
| 10 | P2-45 | 412 | 576 | 63.12 |
Table 8 The characteristic temperatures of the TG curves and char yield of polysiloxanes (cured P1 and P2)
| 序号 | 聚合物 | T5d/℃ | Tmax/℃ | 残炭率/% |
|---|---|---|---|---|
| 1 | P1-0 | 288 | 584 | 19.99 |
| 2 | P1-5 | 232 | 554 | 47.53 |
| 3 | P1-25 | 188 | 565 | 59.67 |
| 4 | P1-45 | 185 | 559 | 65.07 |
| 5 | P1-65 | 231 | 577 | 71.19 |
| 6 | P1-85 | 235 | 554 | 74.02 |
| 7 | P2-15 | 479 | 586 | 48.59 |
| 8 | P2-25 | 487 | 589 | 56.96 |
| 9 | P2-35 | 500 | 587 | 62.83 |
| 10 | P2-45 | 412 | 576 | 63.12 |
| 序号 | 处理温度/℃ | 交联密度/(10-5 mol·cm-3) |
|---|---|---|
| 1 | 25 | 6.03 |
| 2 | 200 | 15.59 |
| 3 | 250 | 51.18 |
| 4 | 300 | 152.36 |
| 5 | 350 | 361.02 |
| 6 | 400 | 615.56 |
Table 9 Crosslinking density of cured P1-85 after holding at different temperatures for 10 min
| 序号 | 处理温度/℃ | 交联密度/(10-5 mol·cm-3) |
|---|---|---|
| 1 | 25 | 6.03 |
| 2 | 200 | 15.59 |
| 3 | 250 | 51.18 |
| 4 | 300 | 152.36 |
| 5 | 350 | 361.02 |
| 6 | 400 | 615.56 |
| 序号 | 处理温度/℃ | 交联密度/(10-5, mol·cm-3) | |
|---|---|---|---|
| P1-25 | P2-15 | ||
| 1 | 25 | 7.24 | 6.58 |
| 2 | 200 | 11.13 | 12.55 |
| 3 | 250 | 19.21 | 21.19 |
| 4 | 300 | 23.56 | 35.12 |
| 5 | 350 | 55.97 | 78.27 |
| 6 | 400 | 91.03 | 149.77 |
Table 10 Crosslinking density of cured P1-25 and P2-15 after holding at different temperatures for 10 min
| 序号 | 处理温度/℃ | 交联密度/(10-5, mol·cm-3) | |
|---|---|---|---|
| P1-25 | P2-15 | ||
| 1 | 25 | 7.24 | 6.58 |
| 2 | 200 | 11.13 | 12.55 |
| 3 | 250 | 19.21 | 21.19 |
| 4 | 300 | 23.56 | 35.12 |
| 5 | 350 | 55.97 | 78.27 |
| 6 | 400 | 91.03 | 149.77 |
| 老化时间/h | 聚合物 | 质量变化率/% | 硬度变化 | 拉伸强度/MPa | 拉伸强度变化率/% | 断裂伸长率/% | 断裂伸长率变化率/% |
|---|---|---|---|---|---|---|---|
| 48 | P1-0 | -3 | +2 | 1.01 | +12 | 331 | -11 |
| P1-25 | -7 | +3 | 0.89 | +16 | 255 | -15 | |
| P1-45 | -8 | +5 | 1.12 | +16 | 283 | -16 | |
| P1-65 | -8 | +6 | 1.09 | +14 | 278 | -14 | |
| P1-85 | -4 | +15 | 1.21 | +28 | 224 | -26 | |
| P2-15 | -2 | +1 | 1.36 | +9 | 367 | -9 | |
| P2-25 | -2 | 0 | 1.45 | +8 | 378 | -11 | |
| P2-35 | -1 | +1 | 1.59 | +9 | 423 | -6 | |
| P2-45 | -1 | +1 | 1.63 | +8 | 461 | -5 | |
| 96 | P1-0 | -5 | +3 | 1.07 | +19 | 318 | -15 |
| P1-25 | -12 | +4 | 0.91 | +30 | 242 | -20 | |
| P1-45 | -15 | +5 | 1.20 | +30 | 277 | -18 | |
| P1-65 | -12 | +6 | 1.15 | +22 | 265 | -18 | |
| P1-85 | -11 | +16 | 1.31 | +52 | 214 | -32 | |
| P2-15 | -2 | +1 | 1.39 | +11 | 359 | -11 | |
| P2-25 | -2 | +1 | 1.47 | +10 | 372 | -13 | |
| P2-35 | -2 | +2 | 1.61 | +10 | 411 | -9 | |
| P2-45 | -2 | +1 | 1.66 | +10 | 456 | -6 | |
| 144 | P1-0 | -5 | +3 | 1.08 | +20 | 315 | -16 |
| P1-25 | -13 | +5 | 0.93 | +26 | 239 | -21 | |
| P1-45 | -15 | +5 | 1.21 | +32 | 276 | -18 | |
| P1-65 | -13 | +7 | 1.17 | +24 | 261 | -20 | |
| P1-85 | -15 | +17 | 1.35 | +57 | 202 | -35 | |
| P2-15 | -3 | +2 | 1.41 | +13 | 355 | -12 | |
| P2-25 | -4 | +3 | 1.52 | +13 | 370 | -13 | |
| P2-35 | -3 | +2 | 1.63 | +12 | 410 | -9 | |
| P2-45 | -3 | +2 | 1.70 | +12 | 450 | -7 |
Table 11 Mass loss and mechanical properties of P1 and P2 after thermal air aging
| 老化时间/h | 聚合物 | 质量变化率/% | 硬度变化 | 拉伸强度/MPa | 拉伸强度变化率/% | 断裂伸长率/% | 断裂伸长率变化率/% |
|---|---|---|---|---|---|---|---|
| 48 | P1-0 | -3 | +2 | 1.01 | +12 | 331 | -11 |
| P1-25 | -7 | +3 | 0.89 | +16 | 255 | -15 | |
| P1-45 | -8 | +5 | 1.12 | +16 | 283 | -16 | |
| P1-65 | -8 | +6 | 1.09 | +14 | 278 | -14 | |
| P1-85 | -4 | +15 | 1.21 | +28 | 224 | -26 | |
| P2-15 | -2 | +1 | 1.36 | +9 | 367 | -9 | |
| P2-25 | -2 | 0 | 1.45 | +8 | 378 | -11 | |
| P2-35 | -1 | +1 | 1.59 | +9 | 423 | -6 | |
| P2-45 | -1 | +1 | 1.63 | +8 | 461 | -5 | |
| 96 | P1-0 | -5 | +3 | 1.07 | +19 | 318 | -15 |
| P1-25 | -12 | +4 | 0.91 | +30 | 242 | -20 | |
| P1-45 | -15 | +5 | 1.20 | +30 | 277 | -18 | |
| P1-65 | -12 | +6 | 1.15 | +22 | 265 | -18 | |
| P1-85 | -11 | +16 | 1.31 | +52 | 214 | -32 | |
| P2-15 | -2 | +1 | 1.39 | +11 | 359 | -11 | |
| P2-25 | -2 | +1 | 1.47 | +10 | 372 | -13 | |
| P2-35 | -2 | +2 | 1.61 | +10 | 411 | -9 | |
| P2-45 | -2 | +1 | 1.66 | +10 | 456 | -6 | |
| 144 | P1-0 | -5 | +3 | 1.08 | +20 | 315 | -16 |
| P1-25 | -13 | +5 | 0.93 | +26 | 239 | -21 | |
| P1-45 | -15 | +5 | 1.21 | +32 | 276 | -18 | |
| P1-65 | -13 | +7 | 1.17 | +24 | 261 | -20 | |
| P1-85 | -15 | +17 | 1.35 | +57 | 202 | -35 | |
| P2-15 | -3 | +2 | 1.41 | +13 | 355 | -12 | |
| P2-25 | -4 | +3 | 1.52 | +13 | 370 | -13 | |
| P2-35 | -3 | +2 | 1.63 | +12 | 410 | -9 | |
| P2-45 | -3 | +2 | 1.70 | +12 | 450 | -7 |
| [1] | Yang X X, Li Q G, Li Z S, et al. Preparation and characterization of room-temperature-vulcanized silicone rubber using acrylpimaric acid-modified aminopropyltriethoxysilane as a cross-linking agent[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 4964-4974. |
| [2] | Kumar V, Lee D J. Studies of nanocomposites based on carbon nanomaterials and RTV silicone rubber[J]. Journal of Applied Polymer Science, 2017, 134(4): 44407. |
| [3] | Zhou X Y, Wang G F, Wang M M, et al. A simple preparation method for superhydrophobic surface on silicon rubber and its properties[J]. Progress in Organic Coatings, 2020, 143: 105612. |
| [4] | Shimizu T, Kishi R, Kobashi K, et al. Improved thermal stability of silicone rubber nanocomposites with low filler content, achieved by well-dispersed carbon nanotubes[J]. Composites Communications, 2020, 22: 100482. |
| [5] | Wang G F, Li A L, Zhao W, et al. A review on fabrication methods and research progress of superhydrophobic silicone rubber materials[J]. Advanced Materials Interfaces, 2021, 8(1): 2001460. |
| [6] | 刘润竹, 储甜甜, 张孝阿, 等. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369. |
| Liu R Z, Chu T T, Zhang X A, et al. Synthesis and properties of phenylene-containing α,ω-hydroxy-terminated fluorosilicone polymers[J]. CIESC Journal, 2023, 74(3): 1360-1369. | |
| [7] | Jovanovic J D, Govedarica M N, Dvornic P R, et al. The thermogravimetric analysis of some polysiloxanes[J]. Polymer Degradation and Stability, 1998, 61(1): 87-93. |
| [8] | Lauter U, Kantor S W, Schmidt-Rohr K, et al. Vinyl-substituted silphenylene siloxane copolymers: novel high-temperature elastomers[J]. Macromolecules, 1999, 32(10): 3426-3431. |
| [9] | Sheng M M, Yu J C, Gong H Y, et al. Enhancing the thermal stability and mechanical properties of phenyl silicone rubbers by controlling BN addition and phenyl content[J]. Composites Communications, 2022, 35: 101340. |
| [10] | Yilgör E, Yilgör I. Silicone containing copolymers: synthesis, properties and applications[J]. Progress in Polymer Science, 2014, 39(6): 1165-1195. |
| [11] | Shit S C, Shah P. A review on silicone rubber[J]. National Academy Science Letters, 2013, 36(4): 355-365. |
| [12] | Kang D W, Yeo H G, Lee K S. Preparation and characteristics of liquid silicone rubber nanocomposite containing ultrafine magnesium ferrite powder[J]. Journal of Inorganic and Organometallic Polymers, 2004, 14(1): 73-84. |
| [13] | Huang Y H, Mu Q H, Su Z T. High and low temperature resistance of phenyl silicone rubber[J]. IOP Conference Series: Materials Science and Engineering, 2021, 1048(1): 012001. |
| [14] | Zhu H J, Dai Z L, Tu W P. Study on the preparation and performance of low gas permeability trifluoropropyl phenyl silicone rubber[J]. RSC Advances, 2017, 7(63): 39739-39747. |
| [15] | Qi M, Jia X Z, Wang G F, et al. Research on high temperature friction properties of PTFE/Fluorosilicone rubber/silicone rubber[J]. Polymer Testing, 2020, 91: 106817. |
| [16] | Gao C T, Zhang Z M, Li X D, et al. Synergistic effects in three-dimensional SnO2/TiO2/CdS multi-heterojunction structure for highly efficient photoelectrochemical hydrogen production[J]. Solar Energy Materials and Solar Cells, 2015, 141: 101-107. |
| [17] | Tariq Nazir M, Phung B T, Hoffman M, et al. Micro-AlN/nano-SiO2 co-filled silicone rubber composites with high thermal stability and excellent dielectric properties[J]. Materials Letters, 2017, 209: 421-424. |
| [18] | Pakaya F, Ardhyananta H, Wicaksono S T. Mechanical properties and thermal stability of epoxy/RTV silicone rubber[J]. IPTEK the Journal for Technology and Science, 2017, 28(1): 7. |
| [19] | Ochi M, Takemiya K, Kiyohara O, et al. Effect of the addition of aramid-silicone block copolymer on phase structure and toughness of cured epoxy resins modified with silicone[J]. Polymer, 1998, 39(3): 725-731. |
| [20] | Dong F Y, Lu H, Feng S Y, et al. Preparation and characterization of silicone rubber through the reaction between γ-chloropropyl and amino groups with siloxane polyamidoamine dendrimers as cross-linkers[J]. Polymers for Advanced Technologies, 2018, 29(2): 934-940. |
| [21] | Zhu L, Chen X, Shi R R, et al. Tetraphenylphenyl-modified damping additives for silicone rubber: experimental and molecular simulation investigation[J]. Materials & Design, 2021, 202: 109551. |
| [22] | Hayashida K, Tsuge S, Ohtani H. Flame retardant mechanism of polydimethylsiloxane material containing platinum compound studied by analytical pyrolysis techniques and alkaline hydrolysis gas chromatography[J]. Polymer, 2003, 44(19): 5611-5616. |
| [23] | Han R J, Quan X D, Shao Y R, et al. Tribological properties of phenyl-silicone rubber composites with nano-CeO2 and graphene underthermal-oxidative aging[J]. Applied Nanoscience, 2020, 10(7): 2129-2138. |
| [24] | Zhou W J, Yang H, Zhou J. The thermal degradation of bisphenol A polycarbonate containing methylphenyl–silicone additive[J]. Journal of Analytical and Applied Pyrolysis, 2007, 78(2): 413-418. |
| [25] | Zhou W J, Yang H. Flame retarding mechanism of polycarbonate containing methylphenyl-silicone[J]. Thermochimica Acta, 2007, 452(1): 43-48. |
| [26] | Zlatanic A, Radojcic D, Wan X M, et al. Monitoring of the course of the silanolate-initiated polymerization of cyclic siloxanes. A mechanism for the copolymerization of dimethyl and diphenyl monomers[J]. Macromolecules, 2018, 51(3): 895-905. |
| [27] | Li Z X, Bai L, Zheng J P. Effect of π–π interaction between carbon nanotubes and phenyl groups on the thermal stability of silicone rubber[J]. Journal of Thermal Analysis and Calorimetry, 2018, 131(3): 2503-2512. |
| [28] | He C, Li B Q, Ren Y, et al. How the crosslinking agent influences the thermal stability of RTV phenyl silicone rubber[J]. Materials, 2018, 12(1): 88. |
| [29] | Zhu H D, Kantor S W, MacKnight W J. Thermally stable silphenylene vinyl siloxane elastomers and their blends[J]. Macromolecules, 1998, 31(3): 850-856. |
| [30] | Fuqua S A, Silverstein R M. Phenylene-perfluoralkylene silicones[J]. Journal of Applied Polymer Science, 1964, 8(4): 1729-1735. |
| [31] | Ma X T, Zhang J A, Ma X Y, et al. Tetrafunctional vinyl polysilsesquioxane and its covalently cross-linked vinyl liquid silicone rubber for resistance to high temperature oxidation combustion and ablative behavior[J]. Corrosion Science, 2023, 221: 111315. |
| [32] | Che B, Wang A, Zhou C, et al. A novel family of silicone prepolymers containing p-silphenylene unit[J]. Acta Polymerica Sinica, 1997: 235-239. |
| [33] | Delebecq E, Hamdani-Devarennes S, Raeke J, et al. High residue contents indebted by platinum and silica synergistic action during the pyrolysis of silicone formulations[J]. ACS Applied Materials & Interfaces, 2011, 3(3): 869-880. |
| [34] | Zhang W Z, Hu S K, Li H X, et al. Epoxidized vinyl silicone rubber-based flexible ablative material with low linear ablation rate[J]. Composites Communications, 2023, 40: 101606. |
| [35] | Jia M Q, Wu C B, Li W, et al. Synthesis and characterization of a silicone resin with silphenylene units in Si—O—Si backbones[J]. Journal of Applied Polymer Science, 2009, 114(2): 971-977. |
| [36] | Uehara H, Saitoh M, Morita R, et al. In situ NMR measurement of novel silicone elastomer obtained by cross-linking of silicones having phenylene backbone and hyperbranched molecular architectures[J]. Macromolecules, 2014, 47(3): 888-896. |
| [37] | Wu C B, Jin Y H, Li W, et al. Synthesis and characterization of a silicone resin with silphenylene units in Si—O—Si backbones II[J]. High Performance Polymers, 2010, 22(8): 959-973. |
| [38] | Grassie N, Beattie S R. The thermal degradation of polysiloxanes Part 7: Mechanism of degradation of poly(tetramethyl-p-silphenylene siloxane) and copolymers with dimethylsiloxane[J]. Polymer Degradation and Stability, 1984, 8(3): 177-193. |
| [39] | Zhu L, Cheng X, Su W L, et al. Molecular insights into sequence distributions and conformation-dependent properties of high-phenyl polysiloxanes[J]. Polymers, 2019, 11(12): 1989. |
| [40] | Hamdani S, Longuet C, Perrin D, et al. Flame retardancy of silicone-based materials[J]. Polymer Degradation and Stability, 2009, 94(4): 465-495. |
| [41] | Liu T, Sun C, Ma F G. Study on the synthesis and thermal degradation of vinylphenylpolysilsesquioxane[J]. Journal of Analytical and Applied Pyrolysis, 2018, 130: 249-255. |
| [42] | Indulekha K, Thomas D, Supriya N, et al. Inherently flame retardant vinyl bearing hyperbranched polysiloxanes having improved thermal stability—ceramization and analysis of associated thermal properties[J]. Polymer Degradation and Stability, 2018, 147: 12-24. |
| [43] | Camino G, Lomakin S M, Lazzari M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects[J]. Polymer, 2001, 42(6): 2395-2402. |
| [44] | Sun J T, Huang Y D, Cao H L, et al. Effects of ambient-temperature curing agents on the thermal stability of poly(methylphenylsiloxane)[J]. Polymer Degradation and Stability, 2004, 85(1): 725-731. |
| [45] | Yang Z Z, Han S, Zhang R, et al. Effects of silphenylene units on the thermal stability of silicone resins[J]. Polymer Degradation and Stability, 2011, 96(12): 2145-2151. |
| [1] | Wenhao SUN, Jun TIAN, Kun ZHANG, Na LIU, Baosen CAO, Xiaoqiang LIANG. New development of novel separators with high thermal stability for lithium-ion batteries [J]. CIESC Journal, 2025, 76(6): 2524-2543. |
| [2] | Jiayuan FAN, Wenhui ZENG, Zhichao REN, Wentao ZHANG, Shuang LYU. Preparation and heat transfer enhancement of phase change slurry with multi-phase change temperature [J]. CIESC Journal, 2025, 76(4): 1863-1874. |
| [3] | Xiangshang CHEN, Zhenjie MA, Xihua REN, Yue JIA, Xiaolong LYU, Huayan CHEN. Preparation and mass transfer efficiency of three-dimensional network extraction membrane [J]. CIESC Journal, 2023, 74(3): 1126-1133. |
| [4] | Xueting ZHANG, Jijiang HU, Jing ZHAO, Bogeng LI. Preparation of high molecular weight polypropylene glycol in microchannel reactor [J]. CIESC Journal, 2023, 74(3): 1343-1351. |
| [5] | Runzhu LIU, Tiantian CHU, Xiaoa ZHANG, Chengzhong WANG, Junying ZHANG. Synthesis and properties of phenylene-containing α,ω-hydroxy-terminated fluorosilicone polymers [J]. CIESC Journal, 2023, 74(3): 1360-1369. |
| [6] | GAO Jianchen, ZHAO Bingchen, HE Feng, LI Tingxian. Preparation and investigation of the thermal charging and discharging of modified magnesium nitrate hexahydrate composite phase change material [J]. CIESC Journal, 2021, 72(6): 3328-3337. |
| [7] | WEI Xiaolan, XIE Pei, WANG Weilong, LU Jianfeng, DING Jing. Calculation of phase diagram and thermal stability of molten salt for ternary chloride systems containing calcium [J]. CIESC Journal, 2021, 72(6): 3074-3083. |
| [8] | XIN Mudi, XING Enhui. Researches on trimethylphosphine and metal oxide modification on ZSM-5 and their influence on catalytic cracking [J]. CIESC Journal, 2021, 72(5): 2657-2668. |
| [9] | ZHANG Rui, SHAO Qi, ZHANG Huayu, JIN Zelong, ZHANG Xiaoliang. Fabrication of boron-doped hybrid silica membranes for pervaporation desalination [J]. CIESC Journal, 2021, 72(4): 2317-2327. |
| [10] | Wenbo ZHANG, Ziye LING, Xiaoming FANG, Zhengguo ZHANG. Preparation and thermal properties research of a novel magnesium chloride hexahydrate-magnesium nitrate hexahydrate/graphite phase carbon nitride composite phase change material [J]. CIESC Journal, 2021, 72(12): 6399-6406. |
| [11] | Xiaolan WEI, Pei XIE, Xuechuan ZHANG, Weilong WANG, Jianfeng LU, Jing DING. Research on preparation and thermodynamic properties of chloride molten salt materials [J]. CIESC Journal, 2020, 71(5): 2423-2431. |
| [12] | YANG Runnong,YU Lin,ZHAO Xiangyun,YANG Xiaobo,GAO Zihan,FU Guangying,JIANG Jiuxing,LIAN Weilin,LIU Wuyuan,FAN Qun. Phi zeolite synthesized by template-free method for selective catalytic reduction of NO [J]. CIESC Journal, 2020, 71(12): 5578-5588. |
| [13] | Jianxin MAO, Ziqing YUAN, Hongxiao YANG, Renxian ZHOU. Effects of Sm addition on reactivity and thermal stability of Pt/SBA-15 for catalytic complete oxidation of benzene [J]. CIESC Journal, 2020, 71(1): 306-313. |
| [14] | Baicen ZHAO, Jing DING, Xiaolan WEI, Bin LIU, Jianfeng LU, Weilong WANG. Design and thermal stability study of LiNO3-NaNO3-KNO3 ternary molten salt system [J]. CIESC Journal, 2019, 70(6): 2083-2091. |
| [15] | WU Dongling, LI Tingxian, HE Feng, WANG Ruzhu. Preparation and performance of modified sodium acetate trihydrate composite phase change material for thermal energy storage [J]. CIESC Journal, 2018, 69(7): 2860-2868. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||