化工学报 ›› 2020, Vol. 71 ›› Issue (3): 1045-1052.DOI: 10.11949/0438-1157.20190952
收稿日期:2019-08-21
修回日期:2019-10-22
出版日期:2020-03-05
发布日期:2020-03-05
通讯作者:
焦纬洲
基金资助:
Wenqiang GAO1,2,Weizhou JIAO1( ),Youzhi LIU1
),Youzhi LIU1
Received:2019-08-21
Revised:2019-10-22
Online:2020-03-05
Published:2020-03-05
Contact:
Weizhou JIAO
摘要:
提出一种超重力环境下甲苯合成苯甲酸的新方法,对比不同臭氧化工艺合成苯甲酸的收率,研究了反应溶剂、臭氧气相浓度、过氧化氢与甲苯的摩尔比、超重力因子、液体流量对苯甲酸收率的影响规律。研究结果表明:RPB (rotating packed bed)-O3/H2O2较其他工艺具有更高的反应性能;得到优化的工艺条件是反应溶剂为乙腈、臭氧气相浓度为80 mg·L-1、过氧化氢与甲苯的摩尔比为0.15、超重力因子为40、液体流量为120 L·h-1,在优化的工艺条件下得到苯甲酸收率为45%。通过电子顺磁共振仪 (EPR)对反应过程中产生的活性自由基进行表征,结果表明,O3/H2O2体系中存在·OH。另外,用气相色谱-质谱联用仪(GC-MS)分析了中间产物,结果表明反应过程中生成的中间产物包括苯甲醇和苯甲醛。基于ERP实验和GC-MS表征结果,探索臭氧/双氧水氧化甲苯合成苯甲酸可能的反应历程。
中图分类号:
高文强, 焦纬洲, 刘有智. 超重力强化O3/H2O2氧化甲苯合成苯甲酸的研究[J]. 化工学报, 2020, 71(3): 1045-1052.
Wenqiang GAO, Weizhou JIAO, Youzhi LIU. Oxidation of toluene to benzoic acid by O3/H2O2 process enhanced usinghigh-gravity technology[J]. CIESC Journal, 2020, 71(3): 1045-1052.
| 参数 | 数值 |
|---|---|
| 填料外径 | 75 mm |
| 填料内径 | 40 mm |
| 填料轴向高度 | 75 mm |
| 比表面积 | 935.07 m2·m-3 |
| 填料密度 | 7.9 g·cm-3 |
| 孔隙率 | 0.74 |
表1 RPB反应器相关参数
Table 1 Equipment parameters of RPB
| 参数 | 数值 |
|---|---|
| 填料外径 | 75 mm |
| 填料内径 | 40 mm |
| 填料轴向高度 | 75 mm |
| 比表面积 | 935.07 m2·m-3 |
| 填料密度 | 7.9 g·cm-3 |
| 孔隙率 | 0.74 |

图3 反应溶剂对苯甲酸收率的影响以及臭氧在不同溶剂中的溶解度(超重力因子β=40; 液体流量: 100 L·h-1; 双氧水的投加量为: 0.7 ml; 臭氧气相浓度为: 80 mg·L-1; 反应时间: 60 min)
Fig.3 Effect of reaction solvents on yield of benzoic acid and solubility of ozone in different solvents
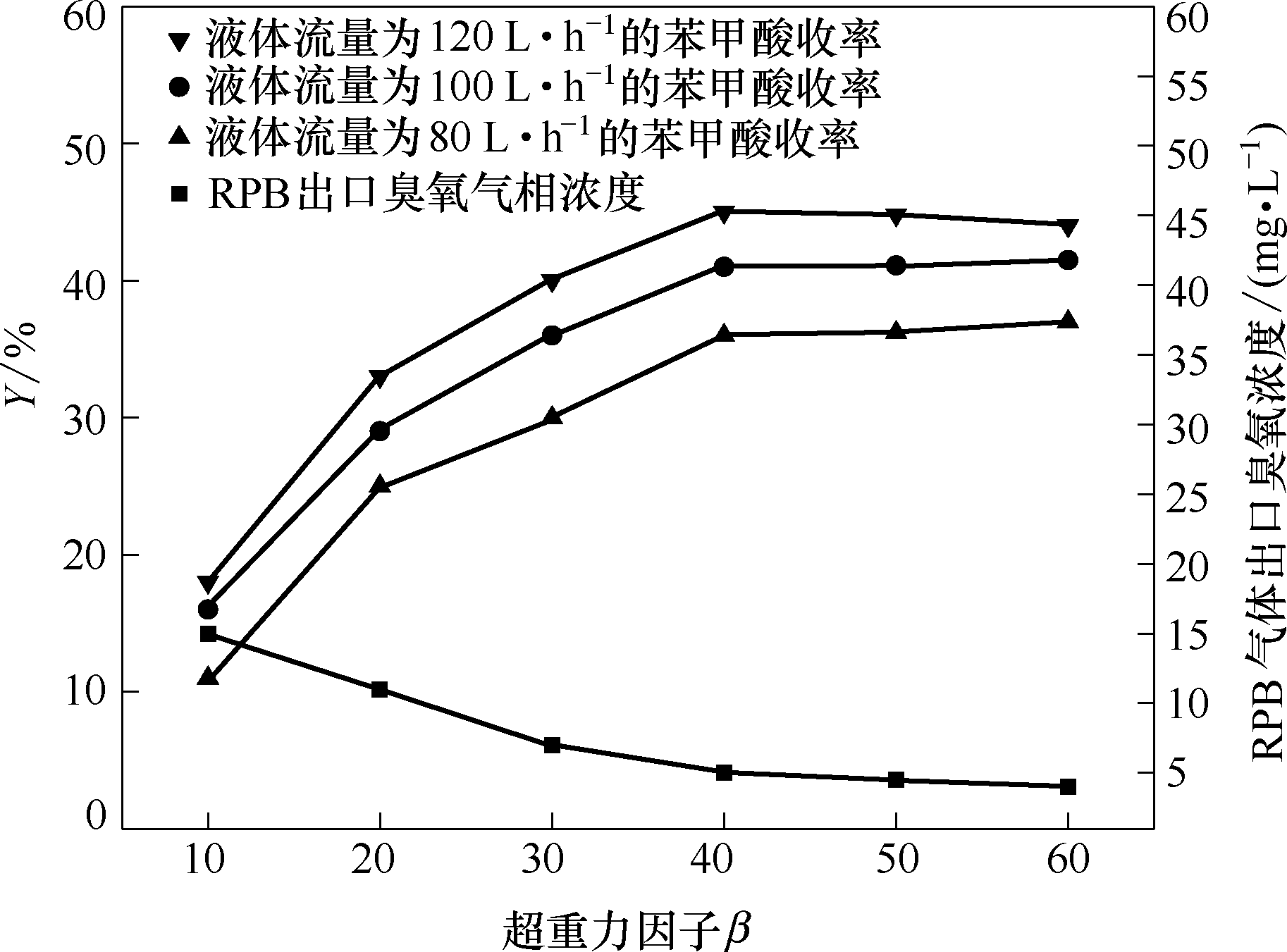
图7 超重力因子对苯甲酸收率以及RPB气体出口臭氧浓度的影响(双氧水的投加量为: 1.0 ml; 臭氧气相浓度为: 80 mg·L-1; 溶剂: 乙腈; 反应时间: 60 min)
Fig.7 Effect of high gravity factor on yield of benzoic acid and ozone concentration at gas outlet of RPB
| 序号 | 保留时间/min | 化合物 | 质谱(m/z) |
|---|---|---|---|
| 1 | 4.464~4.858 | 苯甲醛 | 106.0 |
| 2 | 5.484~5.634 | 苯甲醇 | 108.1 |
| 3 | 6.931~7.310 | 苯甲酸 | 122.0 |
表2 GC-MS分析O3/H2O2体系氧化甲苯的中间产物
Table 2 Intermediate products of O3/H2O2 system by GC-MS
| 序号 | 保留时间/min | 化合物 | 质谱(m/z) |
|---|---|---|---|
| 1 | 4.464~4.858 | 苯甲醛 | 106.0 |
| 2 | 5.484~5.634 | 苯甲醇 | 108.1 |
| 3 | 6.931~7.310 | 苯甲酸 | 122.0 |
| 1 | 杨国英,钟慧妹,程尉,等.苯甲酸的电化学合成[J].精细化工,2005,22(4):287-289. |
| Yang G Y,Zhong H M,Cheng W,et al.Electrochemical synthesis of benzoic acid[J].Fine Chemicals,2005,22(4):287-289. | |
| 2 | 王晓君,刘吉平.苯甲酸的合成工艺[J].化工进展,2011,30:603-605. |
| Wang X J,Liu J P.Synthesis of benzoic acid[J].Chemical Industry and Engineering Process,2011,30:603-605. | |
| 3 | 朱艳吉,刘雪琳,汪怀远,等.太阳能热电耦合合成苯甲酸[J].高等学校化学学报,2016,37(2):322-327. |
| Zhu Y J,Liu X L,Wang H Y,et al.Solar thermal-electrochemical synthesis of benzoic acid[J].Chemical Journal of Chinese Universities,2016,37(2):322-327. | |
| 4 | Yoshino Y,Hayashi Y,Iwahama T,et al.Catalytic oxidation of alkylbenzenes with molecular oxygen under normal pressure and temperature byN-hydroxyphthalimide combined with Co(OAc)2[J].Journal of Organic Chemistry,1997,62(20):6810-6813. |
| 5 | Hirai N,Sawatari N,Nakamura N,et al.Oxidation of substituted toluenes with molecular oxygen in the presence ofN,N',N''-trihydroxyisocyanuric acid as a key catalyst[J].Journal of Organic Chemistry,2003,68(17):6587-6590 |
| 6 | Guo C C,Liu Q,Wang X T,et al.Selective liquid phase oxidation of toluene with air[J].Applied Catalysis A: General,2005,282(1):55-59. |
| 7 | Gao J,Tong X,Li X,et al.The efficient liquid-phase oxidation of aromatic hydrocarbons by molecular oxygen in the presence of MnCO3[J].Journal of Chemical Technology and Biotechnology,2007,82(7):620-625. |
| 8 | Wang F,Xu J,Li X Q,et al.Liquid phase oxidation of toluene to benzaldehyde with molecular oxygen over copper-based heterogeneous catalysts[J].Advanced Synthesis & Catalysis,2005,347(15):1987-1992. |
| 9 | Huang G,Xiang F,Li T M,et al.Selective oxidation of toluene over the new catalyst cobalt tetra (4-hydroxyl) phenylporphyrin supported on zinc oxide[J].Catalysis Communications,2011,12(10):886-889. |
| 10 | 郭亮,焦纬洲,刘有智,等.不同臭氧组合工艺处理含硝基苯类化合物废水的实验研究[J].含能材料,2014,22(5):702-708. |
| Guo L,Jiao W Z,Liu Y Z,et al.Treatment of nitrobenzene-containing wastewater using different combined processes with ozone[J].Chinese Journal of Energetic Materials,2014,22(5):702-708. | |
| 11 | Park C G,Choi E S,Jeon H W,et al.Effect of nitrate on the degradation of bisphenol A by UV/H2O2 and ozone/H2O2 oxidation in aqueous solution[J].Desalination and Water Treatment,2014,52:1-8. |
| 12 | Van-Aken P,Van-Eyck K,Degrèeve J,et al.COD and AOX removal and biodegradability assessment for fenton and O3/UV oxidation processes: a case study from a graphical industry wastewater[J].Ozone: Science & Engineering,2013,35(1):16-21. |
| 13 | Pengphol S,Uthaibutra J,Arquero O,et al.Oxidative degradation and detoxification of chlorpyrifos by ultrasonic and ozone treatments[J].Journal of Agricultural Science,2012,4(8):164. |
| 14 | Silva G H R,Daniel L A,Bruning H,et al.Anaerobic effluent disinfection using ozone: byproducts formation[J].Bioresource Technology,2010,101:6981-6986. |
| 15 | Al-Momani F,Shawaqfah M,Shawaqfeh A,et al.Impact of Fenton and ozone on oxidation of wastewater containing nitroaromatic compounds[J].Journal of Environmental Sciences,2008,20(6):675-682. |
| 16 | Burns J R,Ramshaw C.Process intensification: visual study of liquid maldistribution in rotating packed beds[J].Chemical Engineering Science,1996,51:1347-1352. |
| 17 | Jiao W Z,Liu Y Z,Qi G S.Gas pressure drop and mass transfer characteristics in a cross-flow rotating packed bed with porous plate packing[J].Industrial Engineering Chemistry Research,2010,49:3732-3740. |
| 18 | Zeng Z Q,Zou H K,Li X,et al.Degradation of phenol by ozone in the presence of Fenton reagent in a rotating packed bed[J].Chemical Engineering Journal,2013,229:404-411. |
| 19 | Qiao J J,Luo S,Yang P Z,et al.Degradation of nitrobenzene-containing wastewater by ozone/persulfate oxidation process in a rotating packed[J].Journal of the Taiwan Institute of Chemical Engineers,2019,99:1-8. |
| 20 | Luo Y,Chu G W,Zou H K,et al.Characteristics of a two-stage counter-current rotating packed bed for continuous distillation[J].Chemical Engineering and Processing,2012,52:55-62. |
| 21 | Chu G W,Fei J,Cai Y,et al.Removal of SO2 with sodium sulfite solution in a rotating packed bed[J].Industrial & Engineering Chemistry Research,2018,57:2329-2335. |
| 22 | Li Y M,Ji J B,Yu Y L,et al.Hydrodynamic behavior in a rotating zigzag bed[J].Chinese Journal of Chemical Engineering,2010,18(1):34-38. |
| 23 | Jiao W Z,Luo S,He Z,et al.Applications of high gravity technologies for wastewater treatment: a review[J].Chemical Engineering Journal,2017,313:912-927. |
| 24 | Buxton G V,Greenstock C L,Helman W P,et al.Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O-) in aqueous solution[J].Journal of Physical and Chemical Reference Data,1988,17(2):513-886. |
| 25 | Yang P Z,Luo S,Liu H Y,et al.Aqueous ozone decomposition kinetics in a rotating packed bed[J].Journal of the Taiwan Institute of Chemical Engineers,2019,96:11-17. |
| 26 | Dong Y M,He K,Yin L,et al.Catalytic degradation of nitrobenzene and aniline in presence of ozone by magnesia from natural mineral[J].Catalysis Letters,2007,119:222-227. |
| 27 | Lin C C,Chao C Y,Liu M Y,et al.Feasibility of ozone absorption by H2O2 solution in rotating packed bes[J].Journal of Hazardous Materials,2009,167(1):1014-1020. |
| 28 | Lin C C,Su Y R.Performance of rotating packed beds in removing ozone from gaseous streams[J].Separation and Purification Technology,2008,61(3):311-316. |
| 29 | Rajan S,Kumar M,Kaistha N,et al.Limiting gas liquid flows and mass transfer in a novel rotating packed bed (HiGee)[J].Industrial & Engineering Chemistry Research,2011,50:986-997. |
| 30 | Zhao L,Ma J,Sun Z Z,et al.Mechanism of heterogeneous catalytic ozonation of nitrobenene in aqueous solution with modified ceramic honedycomb[J].Applied Catalysis B: Environmental,2009,89:326-334. |
| 31 | Stefan M I,Mack J,Bolton J R.Degradation pathways during the treatment of methyl-tert-butyl ether by the UV/H2O2 process[J].Environmental Science & Technology,2000,34:650-658. |
| 32 | Safarzadeh-Amiri A.O3/H2O2 treatment of methyl-tert-butyl ether (MTBE) in contaminated waters[J].Water Research,2001,35:3706-3714. |
| 33 | Bataineh H,Pestovsky O,Bakac A.Iron(Ⅱ) catalysis in oxidation of hydrocarbons with ozone in acetonitrile[J].ACS Catalysis,2015,5:1629-1637. |
| [1] | 李彬, 徐正虎, 姜爽, 张天永. 双氧水催化氧化法清洁高效合成促进剂CBS[J]. 化工学报, 2023, 74(7): 2919-2925. |
| [2] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| [3] | 王辰, 史秀锋, 武鲜凤, 魏方佳, 张昊虹, 车寅, 吴旭. 氧化还原法制备Mn3O4催化剂及其甲苯催化氧化性能与机理研究[J]. 化工学报, 2023, 74(6): 2447-2457. |
| [4] | 王姝焱, 张瑞阳, 刘润, 刘凯, 周莹. Mn(BO2)2/BNO界面结构调控增强催化臭氧分解性能研究[J]. 化工学报, 2022, 73(7): 3193-3201. |
| [5] | 张逸伟, 唐海荣, 何勇, 朱燕群, 王智化. 臭氧低温氧化烟气脱硝过程中的氮平衡试验研究[J]. 化工学报, 2022, 73(4): 1732-1742. |
| [6] | 钱庆玲, 朱晴, 杨正金, 徐铜文. 微孔Noria聚合物用于二甲苯异构体吸附分离研究[J]. 化工学报, 2022, 73(12): 5438-5448. |
| [7] | 石秀娟, 梁文俊, 尹国彬, 王金柱. 低温等离子体协同Mn基催化剂降解氯苯研究[J]. 化工学报, 2022, 73(10): 4472-4483. |
| [8] | 黄莉婷, 韩昫身, 金艳, 马强, 于建国. 煤化工反渗透浓水的高效降解菌株筛选、鉴定及应用研究[J]. 化工学报, 2021, 72(9): 4881-4891. |
| [9] | 徐健元, 吴艳阳, 徐菊美, 彭阳峰. 2 kPa下均三甲苯-偏三甲苯与均三甲苯-邻甲乙苯体系二元汽液相平衡数据研究及精馏模拟[J]. 化工学报, 2021, 72(9): 4504-4510. |
| [10] | 叶凯, 刘香华, 姜月, 于颖, 赵亚飞, 庄烨, 郑进保, 陈秉辉. 低温等离子体协同CeO2/13X催化降解甲苯[J]. 化工学报, 2021, 72(7): 3706-3715. |
| [11] | 张天永, 杨坤龙, 崔现宝, 李彬, 宋禹昕, 姜爽. 橡胶促进剂NS的绿色合成工艺与应用研究进展[J]. 化工学报, 2021, 72(2): 876-885. |
| [12] | 吕全明, 孙伟振, 赵玲. 连四甲苯液相氧化过程热力学分析及动力学模拟[J]. 化工学报, 2021, 72(2): 1009-1017. |
| [13] | 殷梦凡, 唐政, 张睿, 刘植昌, 刘海燕, 徐春明, 孟祥海. 离子液体液液萃取分离正辛烷/邻二甲苯[J]. 化工学报, 2021, 72(12): 6282-6290. |
| [14] | 郭达, 祁贵生, 刘有智, 焦纬洲, 闫文超, 高雨松. 错流旋转填料床的质、热同传性能及传热机理研究[J]. 化工学报, 2021, 72(11): 5543-5551. |
| [15] | 张正义,张千,楼紫阳,刘伟,朱宇楠,袁春波,于潇,赵天涛. 催化臭氧氧化处理渗滤液RO浓液的氧化特性及光谱分析[J]. 化工学报, 2021, 72(10): 5362-5371. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号