化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4504-4510.DOI: 10.11949/0438-1157.20210281
收稿日期:2021-02-22
修回日期:2021-05-12
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
吴艳阳
作者简介:徐健元(1996—),男,硕士研究生,
Jianyuan XU( ),Yanyang WU(
),Yanyang WU( ),Jumei XU,Yangfeng PENG
),Jumei XU,Yangfeng PENG
Received:2021-02-22
Revised:2021-05-12
Online:2021-09-05
Published:2021-09-05
Contact:
Yanyang WU
摘要:
均三甲苯是一种重要的化工原料,通常存在于重整重芳烃中,其中均三甲苯与偏三甲苯、邻甲乙苯的分离较为困难。测定了2 kPa下均三甲苯(1)-偏三甲苯(2)、均三甲苯(1)-邻甲乙苯(3)两个体系的汽液相平衡数据,并分别采用NRTL和UNIQUAC活度系数模型对相平衡数据进行关联,回归获得二元交互参数,并进行了热力学一致性检验。结果表明,在2 kPa下,均三甲苯与邻甲乙苯无法通过普通精馏实现分离。通过对均三甲苯-偏三甲苯-邻甲乙苯体系进行烷基化反应,而后分别采用单塔减压精馏和双塔减压精馏对反应产物的分离进行精馏模拟,得出较优工艺为双塔连续减压精馏,两塔塔板数分别为50,回流比分别为7和8,此时均三甲苯纯度可达98%(质量)。该研究不仅补充了汽液相平衡数据库,也为低压下C9体系的分离工艺设计提供参考。
中图分类号:
徐健元, 吴艳阳, 徐菊美, 彭阳峰. 2 kPa下均三甲苯-偏三甲苯与均三甲苯-邻甲乙苯体系二元汽液相平衡数据研究及精馏模拟[J]. 化工学报, 2021, 72(9): 4504-4510.
Jianyuan XU, Yanyang WU, Jumei XU, Yangfeng PENG. Study on vapor-liquid equilibria and distillation simulation of 1,3,5-trimethylbenzene-1,2,4-trimethylbenzene and 1,3,5-trimethylbenzene-2-ethyltoluene at 2 kPa[J]. CIESC Journal, 2021, 72(9): 4504-4510.
| 原料 | 沸点(2 kPa)/K① | Antoine常数②[ | Antione常数适用的温度范围/K | ||
|---|---|---|---|---|---|
| A | B | C | |||
| 均三甲苯 | 329.41 | 7.07436 | 1569.622 | 209.578 | 322.15~466.15 |
| 偏三甲苯 | 332.43 | 7.04383 | 1573.267 | 208.564 | 325.15~471.15 |
| 邻甲乙苯 | 329.27 | 7.00314 | 1535.374 | 207.300 | 321.15~467.15 |
表1 实验原料的沸点及Antoine常数
Table 1 Boiling points and Antoine constants of experimental materials
| 原料 | 沸点(2 kPa)/K① | Antoine常数②[ | Antione常数适用的温度范围/K | ||
|---|---|---|---|---|---|
| A | B | C | |||
| 均三甲苯 | 329.41 | 7.07436 | 1569.622 | 209.578 | 322.15~466.15 |
| 偏三甲苯 | 332.43 | 7.04383 | 1573.267 | 208.564 | 325.15~471.15 |
| 邻甲乙苯 | 329.27 | 7.00314 | 1535.374 | 207.300 | 321.15~467.15 |
| T/K | ||||
|---|---|---|---|---|
| 均三甲苯(1)-偏三甲苯(2) | ||||
| 332.43 | 0.0000 | 0.0000 | — | 1.0000 |
| 332.05 | 0.0598 | 0.0759 | 1.1120 | 1.0016 |
| 331.79 | 0.1061 | 0.1329 | 1.1117 | 1.0014 |
| 331.52 | 0.1509 | 0.1875 | 1.1177 | 1.0013 |
| 331.32 | 0.1980 | 0.2404 | 1.1031 | 1.0011 |
| 331.05 | 0.2601 | 0.3076 | 1.0891 | 1.0026 |
| 330.88 | 0.3051 | 0.3547 | 1.0798 | 1.0035 |
| 330.67 | 0.3615 | 0.4096 | 1.0635 | 1.0099 |
| 330.53 | 0.4042 | 0.4507 | 1.0539 | 1.0140 |
| 330.42 | 0.4451 | 0.4872 | 1.0403 | 1.0221 |
| 330.20 | 0.5276 | 0.5623 | 1.0242 | 1.0362 |
| 329.99 | 0.6235 | 0.6485 | 1.0102 | 1.0553 |
| 329.85 | 0.6970 | 0.7155 | 1.0041 | 1.0689 |
| 329.72 | 0.7698 | 0.7830 | 1.0014 | 1.0802 |
| 329.64 | 0.8240 | 0.8345 | 1.0012 | 1.0819 |
| 329.54 | 0.8950 | 0.9016 | 1.0009 | 1.0838 |
| 329.47 | 0.9489 | 0.9523 | 1.0006 | 1.0834 |
| 329.41 | 1.0000 | 1.0000 | 1.0000 | — |
| 均三甲苯(1)-邻甲乙苯(3) | ||||
| 329.27 | 0.0000 | 0.0000 | — | 1.0000 |
| 329.28 | 0.0557 | 0.0554 | 1.0000 | 1.0000 |
| 329.29 | 0.1108 | 0.1104 | 1.0000 | 1.0000 |
| 329.29 | 0.1497 | 0.1493 | 1.0000 | 1.0000 |
| 329.30 | 0.2009 | 0.2002 | 1.0000 | 1.0000 |
| 329.31 | 0.2689 | 0.2680 | 1.0000 | 1.0000 |
| 329.31 | 0.3054 | 0.3044 | 1.0000 | 1.0000 |
| 329.32 | 0.3440 | 0.3425 | 1.0000 | 1.0000 |
| 329.33 | 0.4197 | 0.4181 | 1.0000 | 1.0000 |
| 329.34 | 0.4776 | 0.4758 | 1.0000 | 1.0000 |
| 329.35 | 0.5842 | 0.5825 | 1.0000 | 1.0000 |
| 329.37 | 0.6935 | 0.6924 | 1.0000 | 1.0000 |
| 329.38 | 0.7743 | 0.7737 | 1.0000 | 1.0000 |
| 329.39 | 0.8349 | 0.8345 | 1.0000 | 1.0000 |
| 329.40 | 0.8893 | 0.8890 | 1.0000 | 1.0000 |
| 329.40 | 0.9283 | 0.9280 | 1.0000 | 1.0000 |
| 329.41 | 1.0000 | 1.0000 | 1.0000 | — |
表2 2 kPa下的汽液相平衡数据
Table 2 Vapor-liquid equilibria data at 2 kPa
| T/K | ||||
|---|---|---|---|---|
| 均三甲苯(1)-偏三甲苯(2) | ||||
| 332.43 | 0.0000 | 0.0000 | — | 1.0000 |
| 332.05 | 0.0598 | 0.0759 | 1.1120 | 1.0016 |
| 331.79 | 0.1061 | 0.1329 | 1.1117 | 1.0014 |
| 331.52 | 0.1509 | 0.1875 | 1.1177 | 1.0013 |
| 331.32 | 0.1980 | 0.2404 | 1.1031 | 1.0011 |
| 331.05 | 0.2601 | 0.3076 | 1.0891 | 1.0026 |
| 330.88 | 0.3051 | 0.3547 | 1.0798 | 1.0035 |
| 330.67 | 0.3615 | 0.4096 | 1.0635 | 1.0099 |
| 330.53 | 0.4042 | 0.4507 | 1.0539 | 1.0140 |
| 330.42 | 0.4451 | 0.4872 | 1.0403 | 1.0221 |
| 330.20 | 0.5276 | 0.5623 | 1.0242 | 1.0362 |
| 329.99 | 0.6235 | 0.6485 | 1.0102 | 1.0553 |
| 329.85 | 0.6970 | 0.7155 | 1.0041 | 1.0689 |
| 329.72 | 0.7698 | 0.7830 | 1.0014 | 1.0802 |
| 329.64 | 0.8240 | 0.8345 | 1.0012 | 1.0819 |
| 329.54 | 0.8950 | 0.9016 | 1.0009 | 1.0838 |
| 329.47 | 0.9489 | 0.9523 | 1.0006 | 1.0834 |
| 329.41 | 1.0000 | 1.0000 | 1.0000 | — |
| 均三甲苯(1)-邻甲乙苯(3) | ||||
| 329.27 | 0.0000 | 0.0000 | — | 1.0000 |
| 329.28 | 0.0557 | 0.0554 | 1.0000 | 1.0000 |
| 329.29 | 0.1108 | 0.1104 | 1.0000 | 1.0000 |
| 329.29 | 0.1497 | 0.1493 | 1.0000 | 1.0000 |
| 329.30 | 0.2009 | 0.2002 | 1.0000 | 1.0000 |
| 329.31 | 0.2689 | 0.2680 | 1.0000 | 1.0000 |
| 329.31 | 0.3054 | 0.3044 | 1.0000 | 1.0000 |
| 329.32 | 0.3440 | 0.3425 | 1.0000 | 1.0000 |
| 329.33 | 0.4197 | 0.4181 | 1.0000 | 1.0000 |
| 329.34 | 0.4776 | 0.4758 | 1.0000 | 1.0000 |
| 329.35 | 0.5842 | 0.5825 | 1.0000 | 1.0000 |
| 329.37 | 0.6935 | 0.6924 | 1.0000 | 1.0000 |
| 329.38 | 0.7743 | 0.7737 | 1.0000 | 1.0000 |
| 329.39 | 0.8349 | 0.8345 | 1.0000 | 1.0000 |
| 329.40 | 0.8893 | 0.8890 | 1.0000 | 1.0000 |
| 329.40 | 0.9283 | 0.9280 | 1.0000 | 1.0000 |
| 329.41 | 1.0000 | 1.0000 | 1.0000 | — |
| 模型 | 模型参数 | AAD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 均三甲苯(1)-偏三甲苯(2) | ||||||||||
| NRTL | 6.6444 | -1.8407 | -2160.15 | 613.62 | 0.3 | 0.020 | 4.73×10-7 | 2.15×10-5 | 0.0025 | |
| UNIQUAC | -3.8399 | 2.6059 | 1254.69 | -856.9 | — | 0.020 | 4.76×10-7 | 2.14×10-5 | 0.0025 | |
| 均三甲苯(1)-邻甲乙苯(3) | ||||||||||
| NRTL | -7.4030 | 7.7726 | 2411.95 | -2533.05 | 0.3 | 0.003 | 1.04×10-9 | 1.43×10-5 | 0.0064 | |
| UNIQUAC | 0.0884 | -0.0830 | -35.97 | 34.13 | — | 0.003 | 1.03×10-9 | 1.43×10-5 | 0.0064 | |
表3 2 kPa下系统活度系数模型参数关联及回归偏差
Table 3 Activity coefficient parameters correlation and regression deviation at 2 kPa
| 模型 | 模型参数 | AAD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 均三甲苯(1)-偏三甲苯(2) | ||||||||||
| NRTL | 6.6444 | -1.8407 | -2160.15 | 613.62 | 0.3 | 0.020 | 4.73×10-7 | 2.15×10-5 | 0.0025 | |
| UNIQUAC | -3.8399 | 2.6059 | 1254.69 | -856.9 | — | 0.020 | 4.76×10-7 | 2.14×10-5 | 0.0025 | |
| 均三甲苯(1)-邻甲乙苯(3) | ||||||||||
| NRTL | -7.4030 | 7.7726 | 2411.95 | -2533.05 | 0.3 | 0.003 | 1.04×10-9 | 1.43×10-5 | 0.0064 | |
| UNIQUAC | 0.0884 | -0.0830 | -35.97 | 34.13 | — | 0.003 | 1.03×10-9 | 1.43×10-5 | 0.0064 | |
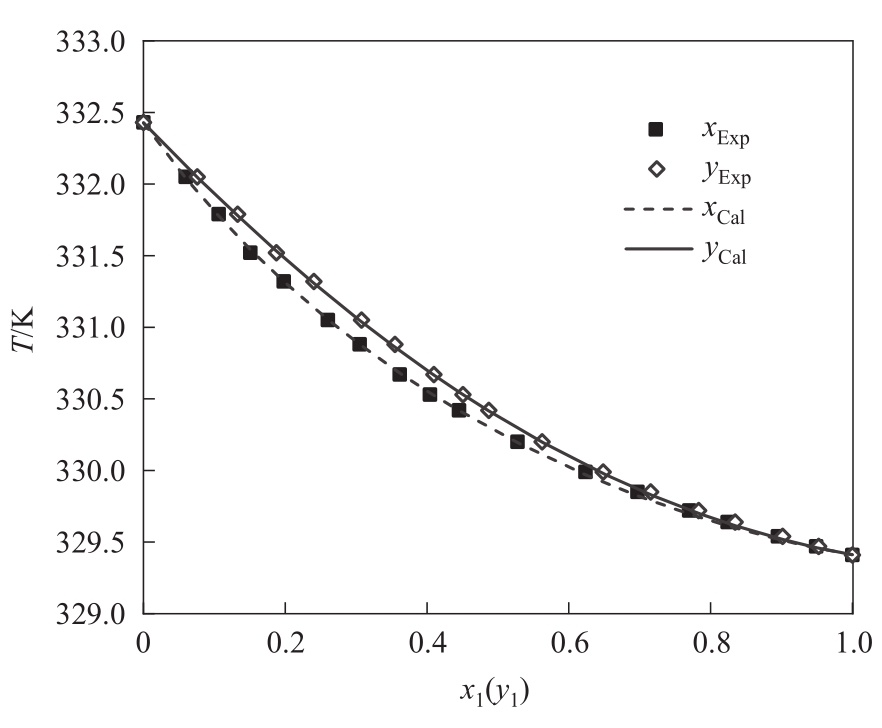
图4 均三甲苯(1)-偏三甲苯(2)体系的模拟值(NRTL模型)与实验值对比
Fig.4 Comparison of the VLE data from experimental data and regression data by NRTL for the system of 1,3,5-trimethylbenzene (1) - 1,2,4-trimethylbenzene (2) at 2 kPa
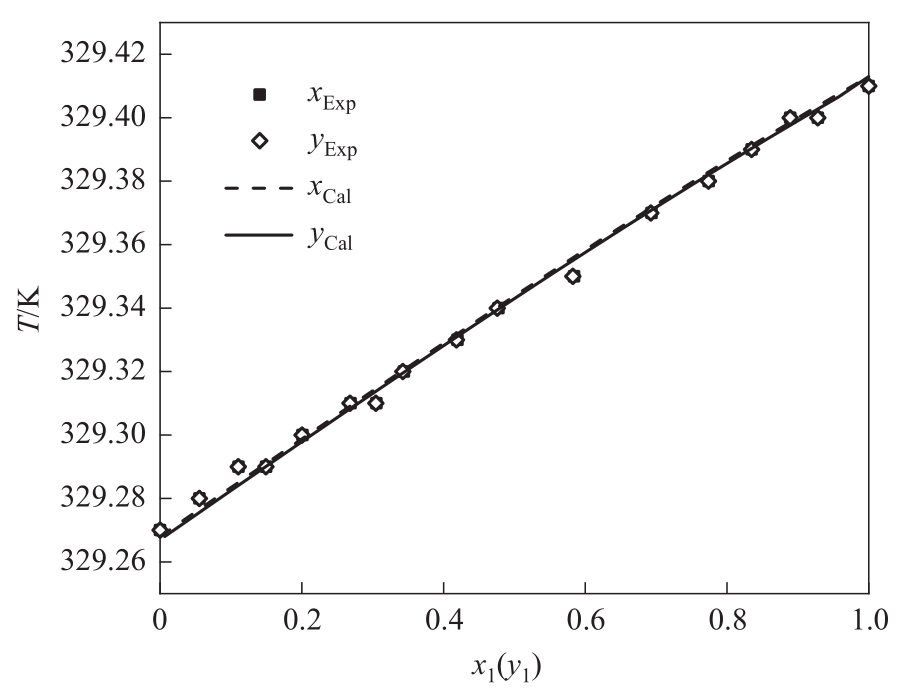
图5 均三甲苯(1)-邻甲乙苯(3)体系的模拟值(NRTL模型)与实验值对比
Fig.5 Comparison of the VLE data from experimental data and regression data by NRTL for the system of 1,3,5-trimethylbenzene (1) - 2-ethyltoluene (3) at 2 kPa
| 参数 | 单塔减压精馏 | 双塔连续减压精馏 | |
|---|---|---|---|
| T11 | T21 | T22 | |
| 塔顶压力/kPa | 2 | 2 | 2 |
| 塔板数n | 120 | 50 | 50 |
| 回流比R | 12 | 7 | 8 |
| 塔顶采出率 | 0.70 | 0.65 | 0.60 |
| 再沸器热负荷QR/kW | 97.4296 | 56.8002 | 37.1880 |
表4 精馏塔参数
Table 4 Parameters of distillation columns
| 参数 | 单塔减压精馏 | 双塔连续减压精馏 | |
|---|---|---|---|
| T11 | T21 | T22 | |
| 塔顶压力/kPa | 2 | 2 | 2 |
| 塔板数n | 120 | 50 | 50 |
| 回流比R | 12 | 7 | 8 |
| 塔顶采出率 | 0.70 | 0.65 | 0.60 |
| 再沸器热负荷QR/kW | 97.4296 | 56.8002 | 37.1880 |
| 1 | 唐卫东. 连续重整重芳烃综合利用工艺的探讨[J]. 石油炼制与化工, 2006, 37(11): 35-39. |
| Tang W D. Perspectives on comprehensive utilization of heavy aromatics from CCR unit[J]. Petroleum Processing and Petrochemicals, 2006, 37(11): 35-39. | |
| 2 | 高丽霞, 刘植昌, 高金森, 等. 离子液体中苯与C9芳烃烷基转移反应[J]. 化学反应工程与工艺, 2005, 21(3): 274-279. |
| Gao L X, Liu Z C, Gao J S, et al. Transalkylation of C9 aromatic hydrocarbon and benzene in ionic liquids[J]. Chemical Reaction Engineering and Technology, 2005, 21(3): 274-279. | |
| 3 | 陈伴生. 重整C9芳烃的综合利用: 利用烷基化分离方法提纯均三甲苯[D]. 南京: 东南大学, 2006: 5-6. |
| Chen B S. Complete utilization of C9 aromatics mixture—purify mesitylene by alkylation distillation[D]. Nanjing: Southeast University, 2006: 5-6. | |
| 4 | 冯海强, 傅吉全. 采用分离集成技术从碳九芳烃中提取均三甲苯[J]. 化工进展, 2011, 30(3): 478-482. |
| Feng H Q, Fu J Q. Extraction of mesitylene from C9 arene by integrated separation technology[J]. Chemical Industry and Engineering Progress, 2011, 30(3): 478-482. | |
| 5 | 李伟宏, 任海伦, 张敬, 等. 一种差压热耦合萃取精馏精制均三甲苯的方法: 104591952A[P]. 2015-05-06. |
| Li W H, Ren H L, Zhang J, et al. Method for refining mesitylene by virtue of differential pressure thermal coupling rectification: 104591952A[P]. 2015-05-06. | |
| 6 | 冯海强, 傅吉全. 萃取精馏分离均三甲苯的实验和模拟[J]. 石油化工, 2011, 40(2): 157-160. |
| Feng H Q, Fu J Q. Experiment and simulation of separating mesitylene by extractive distillation[J]. Petrochemical Technology, 2011, 40(2): 157-160. | |
| 7 | 徐广宇, 徐建兵, 廖丽萍, 等. 2, 4, 6-三甲基苯甲酸的合成[J]. 化工进展, 2007, 26(3): 430-432. |
| Xu G Y, Xu J B, Liao L P, et al. Synthesis of 2, 4, 6-trimethylbenzoic acid[J]. Chemical Industry and Engineering Progress, 2007, 26(3): 430-432. | |
| 8 | 项纯, 林光伟, 杨志萍, 等. 一种液相氧化法制备均苯三甲酸/偏苯三甲酸的方法: 110143862A[P]. 2019-08-20. |
| Xiang C, Lin G W, Yang Z P, et al. Liquid phase oxidation method for preparing trimesic acid/trimellitic acid: 110143862A[P]. 2019-08-20. | |
| 9 | 曹正国, 李江华, 王福, 等. 均三甲苯低温液相连续氧化生产均苯三甲酸的方法: 110642699A[P]. 2020-01-03. |
| Cao Z G, Li J H, Wang F, et al. Method for producing trimesic acid through low-temperature liquid-phase continuous oxidation of mesitylene: 110642699A[P]. 2020-01-03. | |
| 10 | 赵开鹏, 韩松. 重整C9芳烃的综合利用[J]. 石油化工, 1999, 28(7): 57-67. |
| Zhao K P, Han S. Comprehensive utilization of reformed C9 aromatics[J]. Petrochemical Technology, 1999, 28(7): 57-67. | |
| 11 | 王明. 偏三甲苯异构化制取均三甲苯研究[D]. 天津: 天津大学, 2014: 2-4. |
| Wang M. Research on the production of 1, 3, 5-trimethylbenzene by the isomerization of 1, 2, 4-trimethylbenzene[D]. Tianjin: Tianjin University, 2014: 2-4. | |
| 12 | 匡华. C9芳烃中均三甲苯与邻甲乙苯的分离研究[J]. 化学反应工程与工艺, 2004, 20(2): 184-187. |
| Kuang H. Separation of mesitylene and o-methylethylbenzene from C9 aromatic[J]. Chemical Reaction Engineering and Technology, 2004, 20(2): 184-187. | |
| 13 | Zhu M L, Sun J S, Tian Y F, et al. Design and application of a highly efficient separation technology for C9 arenes[J]. Asia-Pacific Journal of Chemical Engineering, 2007, 2(4): 278-281. |
| 14 | 郭乃龄, 郑金璇, 张杏芳, 等. 均三甲苯-偏三甲苯-连三甲苯汽液平衡的研究[J]. 化学工程, 1983, 11(4): 27-32. |
| Guo N L, Zheng J X, Zhang X F, et al. Research on vapor-liquid equilibrium of 1, 3, 5-trimethylbenzene-1, 2, 4-trimethylbenze-1, 2, 3-trimethylbenzene[J]. Chemical Engineering (China), 1983, 11(4): 27-32. | |
| 15 | Fu J Q, Liu X J, Fu D. Study on vapor liquid equilibrium for C9 separation by azeotropic distillation[J]. China Petroleum Processing & Petrochemical Technology, 2012, 14(1): 80-86. |
| 16 | 肖文, 周大军. 重整C9芳烃中提取高纯度均三甲苯的实验研究[J]. 石油学报(石油加工), 2010, 26(S1): 93-97. |
| Xiao W, Zhou D J. Experimental study on manufacturing high purity mesitylene from C9 arene cut of reforming[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2010, 26(S1): 93-97. | |
| 17 | 程能林. 溶剂手册[M]. 4版. 北京: 化学工业出版社, 2007: 216-218. |
| Cheng N L. Handbook of Solvents[M]. 4th ed. Beijing: Chemical Industry Press, 2007: 216-218. | |
| 18 | Yaws C L, Yang H C. To estimate vapor pressure easily[J]. Hydrocarbon Processing, 1989, 68(10): 65-66. |
| 19 | Xu J M, Li S T, Zeng Z X, et al. Isobaric vapor-liquid equilibrium for binary system of isoamyl DL-lactate and isoamyl alcohol at 25.0, 50.0, and 101.3 kPa[J]. Journal of Chemical & Engineering Data, 2020, 65(1): 81-87. |
| 20 | 徐勉, 吴亮衡, 张杰. 2,4-TDI/共沸剂二元体系的汽液平衡数据的测定及共沸组成分析[J]. 化工学报, 2016, 67(5): 1673-1679. |
| Xu M, Wu L H, Zhang J. Measurement of vapor-liquid equilibrium data and azeotropic analysis for 2, 4-toluene diisocyanate + entrainer[J]. CIESC Journal, 2016, 67(5): 1673-1679. | |
| 21 | Song Y Z, Wang Y, Zhang X B, et al. Isobaric vapor-liquid equilibrium measurements of binary systems of dimethyl carbonate with dimethyl sulfoxide, anisole, and diethyl oxalate at 101.3 kPa[J]. Journal of Chemical & Engineering Data, 2021, 66(3): 1367-1375. |
| 22 | 宋卓栋, 张作毅, 潘学龙, 等. 聚甲醛二甲醚体系汽液平衡数据的测定与关联[J]. 化工学报, 2020, 71: 1-6. |
| Song Z D, Zhang Z Y, Pan X L, et al. Determination and correlation of vapor-liquid equilibrium for polyoxymethylene dimethyl ether system[J]. CIESC Journal, 2020, 71: 1-6. | |
| 23 | 周峰, 陈长旭, 许春建. 乙酸异戊酯+异戊醇和乙酸异戊酯+正己醇体系汽液平衡[J]. 化工学报, 2017, 68(2): 560-566. |
| Zhou F, Chen C X, Xu C J. Isobaric vapor-liquid equilibrium for binary systems of isoamyl acetate + isoamyl alcohol and isoamyl acetate + n-hexanol at 50.00 and 101.33 kPa[J]. CIESC Journal, 2017, 68(2): 560-566. | |
| 24 | Herington E F G. A thermodynamic test for the internal consistency of experimental data on volatility ratios[J]. Nature, 1947, 160(4070): 610-611. |
| 25 | Zhong Y, Wu Y Y, Zhu J W, et al. Thermodynamics in separation for the ternary system 1, 2-ethanediol + 1, 2-propanediol + 2, 3-butanediol[J]. Industrial & Engineering Chemistry Research, 2014, 53(30): 12143-12148. |
| 26 | 谈金辉, 徐菊美, 施云海. 常压下乙醇-水-醋酸钾系统汽液平衡数据的测定与关联[J]. 化工学报, 2020, 71(8): 3444-3451. |
| Tan J H, Xu J M, Shi Y H. Measurement and correlation of vapor-liquid equilibria for ethanol-water-potassium acetate system under atmospheric pressure[J]. CIESC Journal, 2020, 71(8): 3444-3451. | |
| 27 | Yang S K, Zhang Y P, Wang X J, et al. Isobaric VLE data for 1-methyl-3-ethylbenzene or 1-methyl-4-ethylbenzene + 1, 2, 4-trimethylbenzene at 20 kPa[J]. The Journal of Chemical Thermodynamics, 2013, 60: 52-56. |
| 28 | Yang S K, Zhang Y P, Wang X J, et al. Isobaric vapor-liquid equilibrium data for the system of 1-methyl-2-ethylbenzene + 1, 2, 4-trimethylbenzene at 10 kPa[J]. Journal of Chemical & Engineering Data, 2013, 58(2): 398-401. |
| 29 | 张卫江, 付强, 李汝贤, 等. 异丁烯烷基化法分离提纯均三甲苯[J]. 天津大学学报, 2005, 38(6): 518-522. |
| Zhang W J, Fu Q, Li R X, et al. Production of high-purity 1, 3, 5-trimethyl benzene by isobutene alkylation[J]. Journal of Tianjin University, 2005, 38(6): 518-522. | |
| 30 | 张卫江, 张雪梅, 韩振为, 等. 偏三甲苯生产均三甲苯工艺[J]. 化工学报, 2002, 53(3): 274-279. |
| Zhang W J, Zhang X M, Han Z W, et al. Technology of production of 1, 3, 5-trimethylbenzene from 1, 2, 4-trimethylbenzene[J]. CIESC Journal, 2002, 53(3): 274-279. | |
| 31 | 任慧勇, 杨卫兰, 张蓓. 中国重整C9+重芳烃分离和利用机会分析[J]. 现代化工, 2020, 40(8): 11-14, 20. |
| Ren H Y, Yang W L, Zhang P. Opportunities for separation and utilization of C9+ heavy aromatics from reformation in China[J]. Modern Chemical Industry, 2020, 40(8): 11-14, 20. |
| [1] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [2] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [3] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [4] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [5] | 汪尔奇, 彭书舟, 杨震, 段远源. 含HFO混合体系气液相平衡的理论模型评价[J]. 化工学报, 2023, 74(8): 3216-3225. |
| [6] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [7] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [8] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [9] | 高金明, 郭玉娇, 鄂承林, 卢春喜. 一种封闭罩内顺流多旋臂气液分离器的分离特性研究[J]. 化工学报, 2023, 74(7): 2957-2966. |
| [10] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| [11] | 朱兴驰, 郭志远, 纪志永, 汪婧, 张盼盼, 刘杰, 赵颖颖, 袁俊生. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| [12] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [13] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [14] | 孙永尧, 高秋英, 曾文广, 王佳铭, 陈艺飞, 周永哲, 贺高红, 阮雪华. 面向含氮油田伴生气提质利用的膜耦合分离工艺设计优化[J]. 化工学报, 2023, 74(5): 2034-2045. |
| [15] | 张正, 何永平, 孙海东, 张荣子, 孙正平, 陈金兰, 郑一璇, 杜晓, 郝晓刚. 蛇形流场电控离子交换装置用于选择性提锂[J]. 化工学报, 2023, 74(5): 2022-2033. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号