化工学报 ›› 2022, Vol. 73 ›› Issue (12): 5438-5448.DOI: 10.11949/0438-1157.20221303
收稿日期:2022-09-27
修回日期:2022-11-24
出版日期:2022-12-05
发布日期:2023-01-17
通讯作者:
杨正金,徐铜文
作者简介:钱庆玲(1999—),女,硕士研究生,qianql@mail.ustc.edu.cn
基金资助:
Qingling QIAN( ), Qing ZHU, Zhengjin YANG(
), Qing ZHU, Zhengjin YANG( ), Tongwen XU(
), Tongwen XU( )
)
Received:2022-09-27
Revised:2022-11-24
Online:2022-12-05
Published:2023-01-17
Contact:
Zhengjin YANG, Tongwen XU
摘要:
二甲苯异构体的分离是一个非常重要但极具挑战性的工业过程,新型吸附剂材料的开发是实现其高效分离的关键。采用Noria和2,3,5,6-四氟对苯二腈制备了具有高比表面积的微孔Noria聚合物(MNP),并研究了MNP对二甲苯异构体的吸附分离能力。单组分二甲苯异构体在MNP上的吸附动力学及吸附等温线表明,MNP具有邻二甲苯优先吸附能力,Langmuir吸附等温线拟合得到邻二甲苯最大吸附量达344 mg·g-1。竞争吸附实验也表明MNP是一种邻二甲苯选择性吸附剂,邻二甲苯与间二甲苯的选择性最高可达2.4,邻二甲苯与对二甲苯选择性在1.9左右。动态穿透实验验证了MNP可以有效分离出邻二甲苯。此外,MNP具有极短的吸附平衡时间和优异的热稳定性,是一种具有潜力的邻二甲苯分离吸附剂。
中图分类号:
钱庆玲, 朱晴, 杨正金, 徐铜文. 微孔Noria聚合物用于二甲苯异构体吸附分离研究[J]. 化工学报, 2022, 73(12): 5438-5448.
Qingling QIAN, Qing ZHU, Zhengjin YANG, Tongwen XU. Microporous Noria polymer for selective adsorption and separation of xylene isomers[J]. CIESC Journal, 2022, 73(12): 5438-5448.

图2 (a) TFTPN、Noria和MNP的红外光谱图;(b)TFTPN和Noria的13C NMR谱图,以及MNP的13C CP/MAS固态NMR谱图;(c) Noria和MNP的PXRD谱图;(d) MNP的SEM图
Fig.2 (a) FT-IR spectra of TFTPN, Noria and MNP; (b) 13C NMR spectra of TFTPN and Noria, and 13C CP/MAS solid-state NMR spectra of MNP; (c) Powder X-ray diffraction patterns of Noria and MNP; (d) SEM image of MNP

图3 (a) Noria和MNP在77 K下的N2吸脱附等温线;(b) 采用非定域密度泛函理论方法计算的Noria和MNP的孔径分布
Fig.3 (a) N2 adsorption/desorption isotherms of Noria and MNP at 77 K; (b) The pore size distributions of Noria and MNP using the nonlocal density functional theory method
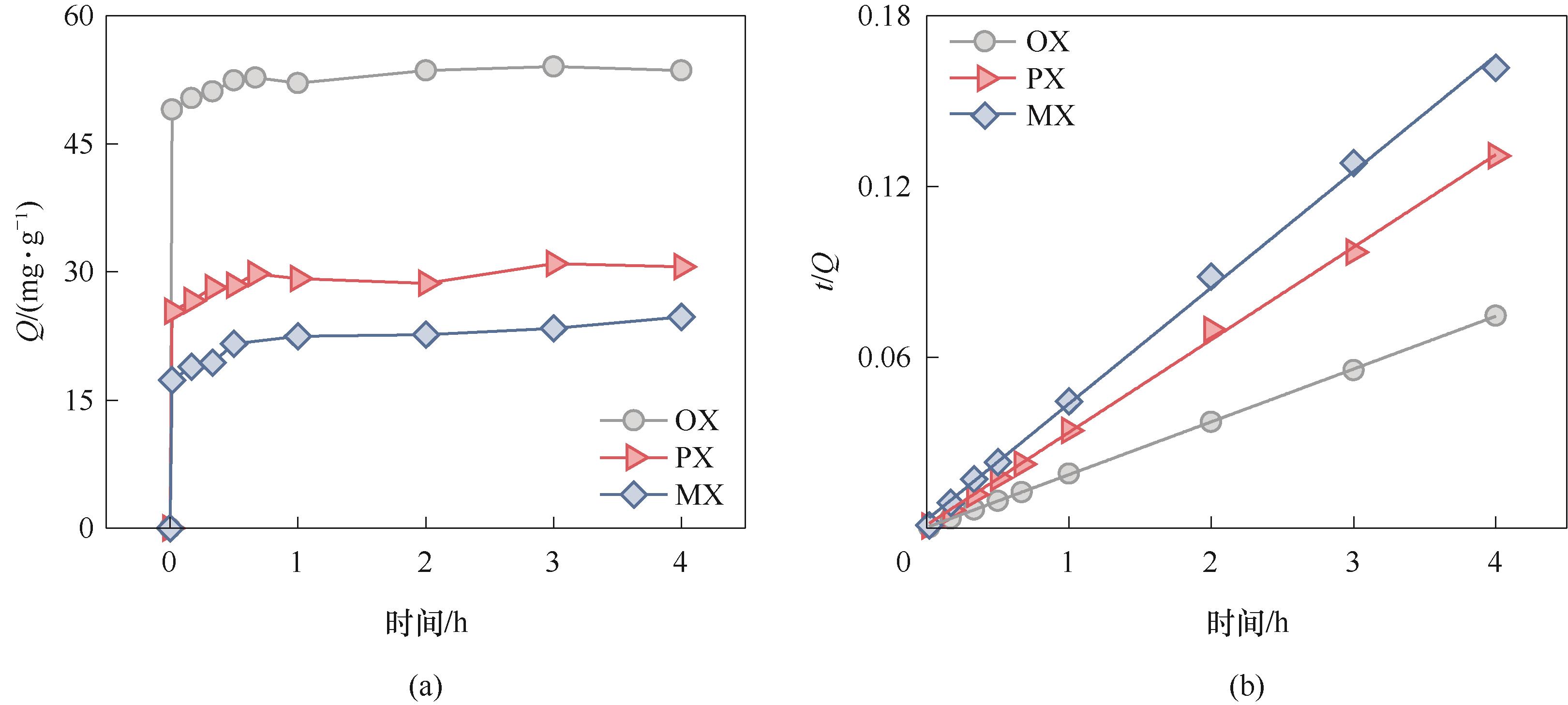
图5 (a) 单组分二甲苯在MNP上吸附量随时间的变化;(b) MNP的二甲苯吸附过程的准二级动力学模型拟合
Fig.5 (a) Time-dependent adsorption of single-xylene isomer on MNP; (b) Fitting the adsorption of xylene isomers on MNP to pseudo second-order kinetic model
| 二甲苯 | C0/ (mol·L-1) | 准一级动力学模型 | 准二级动力学模型 | 颗粒内扩散模型 | |||||
|---|---|---|---|---|---|---|---|---|---|
k1 / (g·mg-1·h-1) | Qe/ (mg·g-1) | R2 | k2 / (g·mg-1·h-1) | Qe / (mg·g-1) | R2 | k3 / (g·mg-1·h-1) | R2 | ||
| OX | 0.1 | 0.96 | 54.1 | 0.6020 | 1.31 | 53.9 | 0.9999 | 2.50 | 0.7511 |
| PX | 0.1 | 0.68 | 31.0 | 0.5597 | 0.90 | 30.8 | 0.9989 | 3.61 | 0.9004 |
| MX | 0.1 | 0.67 | 24.8 | 0.6382 | 0.56 | 24.5 | 0.9980 | 2.41 | 0.8033 |
表1 MNP吸附二甲苯异构体的动力学拟合参数
Table 1 Kinetic fitting results of xylene isomers adsorption on MNP
| 二甲苯 | C0/ (mol·L-1) | 准一级动力学模型 | 准二级动力学模型 | 颗粒内扩散模型 | |||||
|---|---|---|---|---|---|---|---|---|---|
k1 / (g·mg-1·h-1) | Qe/ (mg·g-1) | R2 | k2 / (g·mg-1·h-1) | Qe / (mg·g-1) | R2 | k3 / (g·mg-1·h-1) | R2 | ||
| OX | 0.1 | 0.96 | 54.1 | 0.6020 | 1.31 | 53.9 | 0.9999 | 2.50 | 0.7511 |
| PX | 0.1 | 0.68 | 31.0 | 0.5597 | 0.90 | 30.8 | 0.9989 | 3.61 | 0.9004 |
| MX | 0.1 | 0.67 | 24.8 | 0.6382 | 0.56 | 24.5 | 0.9980 | 2.41 | 0.8033 |

图6 (a) 25℃下MNP的单组分二甲苯的吸附等温线;(b) MNP在25℃下吸附单组分二甲苯的Langmuir吸附等温线拟合;(c) 温度对MNP的单组分二甲苯吸附量的影响
Fig.6 (a) Single-xylene isomer adsorption isotherms on MNP at 25℃; (b) Langmuir isotherms of single-xylene isomer adsorption on MNP at 25℃; (c) The effect of temperature on single-xylene isomer adsorption on MNP
| 二甲苯 | 温度/℃ | Langmuir 吸附等温线 | Freundlich 吸附等温线 | ||||
|---|---|---|---|---|---|---|---|
Qm/ (mg·g-1) | KL/ (L·mg-1) | R2 | KF / (mg·g-1·(L·mol-1)1/n ) | 1/n | R2 | ||
| OX | 25 | 344 | 2.60 | 0.9925 | 279.8 | 0.57 | 0.9799 |
| PX | 25 | 131 | 7.93 | 0.9951 | 131.4 | 0.36 | 0.8851 |
| MX | 25 | 118 | 18.00 | 0.9956 | 116.0 | 0.13 | 0.6579 |
表2 单组分二甲苯在MNP上吸附的Langmuir等温线拟合参数和Freundlich等温线拟合参数
Table 2 Parameters from fitting the adsorption isotherms of single-xylene isomer on MNP to Langmuir adsorption model and Freundlich adsorption model
| 二甲苯 | 温度/℃ | Langmuir 吸附等温线 | Freundlich 吸附等温线 | ||||
|---|---|---|---|---|---|---|---|
Qm/ (mg·g-1) | KL/ (L·mg-1) | R2 | KF / (mg·g-1·(L·mol-1)1/n ) | 1/n | R2 | ||
| OX | 25 | 344 | 2.60 | 0.9925 | 279.8 | 0.57 | 0.9799 |
| PX | 25 | 131 | 7.93 | 0.9951 | 131.4 | 0.36 | 0.8851 |
| MX | 25 | 118 | 18.00 | 0.9956 | 116.0 | 0.13 | 0.6579 |
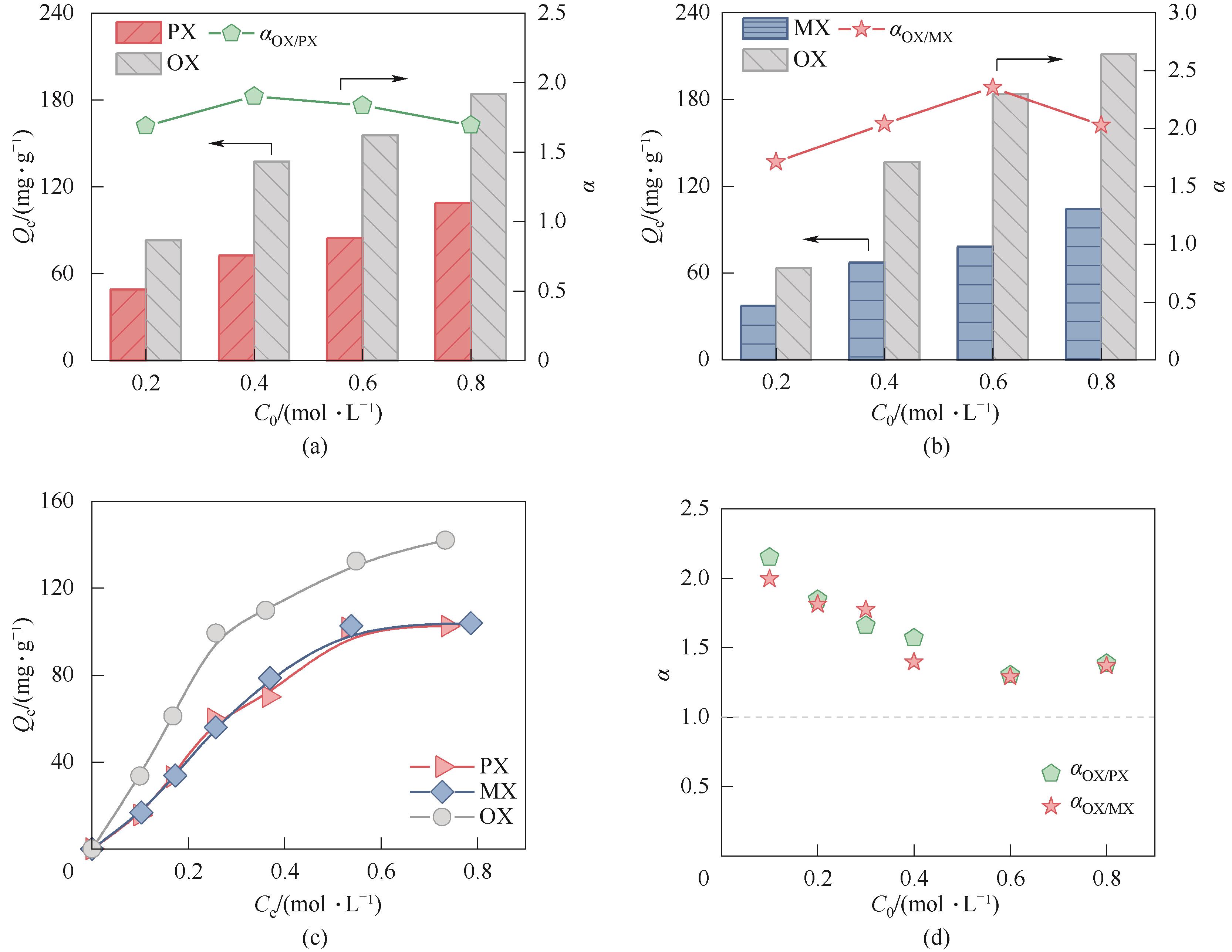
图7 (a) 25℃下MNP吸附分离OX和PX的二组分竞争性实验;(b) 25℃下MNP吸附分离OX和MX的二组分竞争性实验;(c) 25℃下MNP吸附分离二甲苯的三组分竞争性实验中的各组分吸附量;(d) 三组分竞争性实验中OX/PX和OX/MX选择性
Fig.7 (a) Competitive batch adsorption experiments on MNP conducted with a binary mixture of OX and PX at 25℃; (b) Competitive batch adsorption experiments on MNP conducted with a binary mixture of OX and MX at 25℃; (c) The adsorption capacity of each isomer on MNP in the competitive batch adsorption experiments conducted with a ternary mixture of PX, OX and MX at 25℃; (d) OX/PX and OX/MX selectivity in the ternary competitive batch adsorption experiments

图8 (a) 柱吸附分离装置图;(b) 25℃下MNP吸附分离OX、PX和MX三组分柱吸附实验中的穿透曲线
Fig.8 (a) Schematic representation of a homemade column separation device; (b) Breakthrough curves on MNP in the column adsorption experiment conducted with a ternary mixture of PX, OX and MX at 25℃
| 二甲苯 | 动力学尺寸/Å | 分子尺寸/Å | z/x | ||
|---|---|---|---|---|---|
| x | y | z | |||
| PX | 6.7 | 6.62 | 3.81 | 9.15 | 1.38 |
| OX | 7.4 | 7.27 | 3.83 | 7.83 | 1.08 |
| MX | 7.1 | 7.32 | 3.95 | 8.99 | 1.23 |
表3 二甲苯异构体的性质
Table 3 Properties of xylene isomers
| 二甲苯 | 动力学尺寸/Å | 分子尺寸/Å | z/x | ||
|---|---|---|---|---|---|
| x | y | z | |||
| PX | 6.7 | 6.62 | 3.81 | 9.15 | 1.38 |
| OX | 7.4 | 7.27 | 3.83 | 7.83 | 1.08 |
| MX | 7.1 | 7.32 | 3.95 | 8.99 | 1.23 |
| 22 | Zhang G W, Emwas A H, Shahul Hameed U F, et al. Shape-induced selective separation of ortho-substituted benzene isomers enabled by cucurbit[7]uril host macrocycles[J]. Chem, 2020, 6(5): 1082-1096. |
| 23 | Sun N, Wang S Q, Zou R Q, et al. Benchmark selectivity p-xylene separation by a non-porous molecular solid through liquid or vapor extraction[J]. Chemical Science, 2019, 10(38): 8850-8854. |
| 24 | Moosa B, Alimi L O, Shkurenko A, et al. A polymorphic azobenzene cage for energy-efficient and highly selective p-xylene separation[J]. Angewandte Chemie International Edition, 2020, 59(48): 21367-21371. |
| 25 | Du Plessis M, Nikolayenko V I, Barbour L J. Record-setting selectivity for p-xylene by an intrinsically porous zero-dimensional metallocycle[J]. Journal of the American Chemical Society, 2020, 142(10): 4529-4533. |
| 26 | Lee J S M, Briggs M E, Hasell T, et al. Hyperporous carbons from hypercrosslinked polymers[J]. Advanced Materials, 2016, 28(44): 9804-9810. |
| 27 | Alsbaiee A, Smith B J, Xiao L L, et al. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer[J]. Nature, 2016, 529(7585): 190-194. |
| 28 | He Y, Zhu X, Li Y K, et al. Efficient CO2 capture by triptycene-based microporous organic polymer with functionalized modification[J]. Microporous and Mesoporous Materials, 2015, 214: 181-187. |
| 29 | Wang H, Liu C Z, Ma X F, et al. Porous multifunctional phenylcarbamoylated-β-cyclodextrin polymers for rapid removal of aromatic organic pollutants[J]. Environmental Science and Pollution Research, 2022, 29(10): 13893-13904. |
| 30 | Tan H L, Chen Q B, Chen T T, et al. Selective adsorption and separation of xylene isomers and benzene/cyclohexane with microporous organic polymers POP-1[J]. ACS Applied Materials & Interfaces, 2018, 10(38): 32717-32725. |
| 31 | Li L Y, Guo L D, Olson D H, et al. Discrimination of xylene isomers in a stacked coordination polymer[J]. Science, 2022, 377(6603): 335-339. |
| 32 | Kudo H, Hayashi R, Mitani K, et al. Molecular waterwheel (Noria) from a simple condensation of resorcinol and an alkanedial[J]. Angewandte Chemie International Edition, 2006, 45(47): 7948-7952. |
| 33 | Giri A, Patil N N, Patra A. Porous noria polymer: a cage-to-network approach toward a robust catalyst for CO2 fixation and nitroarene reduction[J]. Chemical Communications, 2021, 57(36): 4404-4407. |
| 1 | Yang Y X, Bai P, Guo X H. Separation of xylene isomers: a review of recent advances in materials[J]. Industrial & Engineering Chemistry Research, 2017, 56(50): 14725-14753. |
| 2 | 殷梦凡, 唐政, 张睿, 等. 离子液体液液萃取分离正辛烷/邻二甲苯[J]. 化工学报, 2021, 72(12): 6282-6290. |
| Yin M F, Tang Z, Zhang R, et al. Separation of n-octane and o-xylene by liquid-liquid extraction with ionic liquids[J]. CIESC Journal, 2021, 72(12): 6282-6290. | |
| 3 | Sholl D S, Lively R P. Seven chemical separations to change the world[J]. Nature, 2016, 532(7600): 435-437. |
| 4 | Lusi M, Barbour L J. Solid-vapor sorption of xylenes: prioritized selectivity as a means of separating all three isomers using a single substrate[J]. Angewandte Chemie International Edition, 2012, 51(16): 3928-3931. |
| 5 | Torres-Knoop A, Krishna R, Dubbeldam D. Separating xylene isomers by commensurate stacking of p-xylene within channels of MAF-X8[J]. Angewandte Chemie International Edition, 2014, 53(30): 7774-7778. |
| 6 | 陈亮, 肖剑, 谢在库, 等. 对二甲苯悬浮熔融结晶动力学[J]. 化工学报, 2009, 60(11): 2787-2791. |
| Chen L, Xiao J, Xie Z K, et al. Suspension melt crystallization kinetics of p-xylene[J]. CIESC Journal, 2009, 60(11): 2787-2791. | |
| 7 | 杨明磊, 魏民, 胡蓉, 等. 二甲苯模拟移动床分离过程建模与仿真[J]. 化工学报, 2013, 64(12): 4335-4341. |
| Yang M L, Wei M, Hu R, et al. Modeling of simulated moving bed for xylene separation[J]. CIESC Journal, 2013, 64(12): 4335-4341. | |
| 8 | Zhang G W, Hua B, Dey A, et al. Intrinsically porous molecular materials (IPMs) for natural gas and benzene derivatives separations[J]. Accounts of Chemical Research, 2021, 54(1): 155-168. |
| 9 | Santacesaria E, Morbidelli M, Danise P, et al. Separation of xylenes on Y zeolites(1): Delermination of the adsorption equilibrium parameters, selectivities, and mass-transfer coefficients through finite bath experiments[J]. Industrial & Engineering Chemistry Process Design and Development, 1982, 21(3): 440-445. |
| 34 | Rasouli M, Yaghobi N, Chitsazan S, et al. Influence of monovalent cations ion-exchange on zeolite ZSM-5 in separation of para-xylene from xylene mixture[J]. Microporous and Mesoporous Materials, 2012, 150: 47-54. |
| 35 | Jiang J W, Sandler S I. Shape versus inverse-shape selective adsorption of alkane isomers in carbon nanotubes[J]. The Journal of Chemical Physics, 2006, 124(2): 024717. |
| 10 | Minceva M, Rodrigues A E. Understanding and revamping of industrial scale SMB units for p-xylene separation[J]. AIChE Journal, 2007, 53(1): 138-149. |
| 11 | Rasouli M, Yaghobi N, Chitsazan S, et al. Effect of nanocrystalline zeolite NaY on meta-xylene separation[J]. Microporous and Mesoporous Materials, 2012, 152: 141-147. |
| 12 | Zhao Y J, Zhao H F, Liu D H. Selective adsorption and separation of o-xylene using an aluminum-based metal-organic framework[J]. Industrial & Engineering Chemistry Research, 2021, 60(47): 17143-17149. |
| 13 | Yang L P, Liu H B, Xing J C, et al. Separation of xylene isomers in the anion-pillared square grid material SIFSIX-1-Cu[J]. Chemistry—a European Journal, 2021, 27(20): 6187-6190. |
| 14 | Li X L, Wang J H, Bai N N, et al. Refinement of pore size at sub-angstrom precision in robust metal-organic frameworks for separation of xylenes[J]. Nature Communications, 2020, 11: 4280. |
| 15 | He Z J, Yang Y X, Bai P, et al. Metal-organic framework MIL-53(Cr) as a superior adsorbent: highly efficient separation of xylene isomers in liquid phase[J]. Journal of Industrial and Engineering Chemistry, 2019, 77: 262-272. |
| 16 | Mukherjee S, Joarder B, Manna B, et al. Framework-flexibility driven selective sorption of p-xylene over other isomers by a dynamic metal-organic framework[J]. Scientific Reports, 2014, 4: 5761. |
| 17 | Huang W, Jiang J, Wu D Y, et al. A highly stable nanotubular MOF rotator for selective adsorption of benzene and separation of xylene isomers[J]. Inorganic Chemistry, 2015, 54(22): 10524-10526. |
| 18 | Duan L H, Dong X Y, Wu Y Y, et al. Adsorption and diffusion properties of xylene isomers and ethylbenzene in metal-organic framework MIL-53(Al)[J]. Journal of Porous Materials, 2013, 20(2): 431-440. |
| 19 | Vermoortele F, Maes M, Moghadam P Z, et al. p-Xylene-selective metal-organic frameworks: a case of topology-directed selectivity[J]. Journal of the American Chemical Society, 2011, 133(46): 18526-18529. |
| 20 | Lennox M J, Düren T. Understanding the kinetic and thermodynamic origins of xylene separation in UiO-66(Zr) via molecular simulation[J]. The Journal of Physical Chemistry C, 2016, 120(33): 18651-18658. |
| 21 | Jie K C, Liu M, Zhou Y J, et al. Near-ideal xylene selectivity in adaptive molecular pillar[n]arene crystals[J]. Journal of the American Chemical Society, 2018, 140(22): 6921-6930. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [7] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [8] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [9] | 刘杰, 吴立盛, 李锦锦, 罗正鸿, 周寅宁. 含乙烯基胺酯键聚醚类可逆交联聚合物的制备及性能研究[J]. 化工学报, 2023, 74(7): 3051-3057. |
| [10] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [11] | 朱兴驰, 郭志远, 纪志永, 汪婧, 张盼盼, 刘杰, 赵颖颖, 袁俊生. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| [12] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [13] | 杨琴, 秦传鉴, 李明梓, 杨文晶, 赵卫杰, 刘虎. 用于柔性传感的双形状记忆MXene基水凝胶的制备及性能研究[J]. 化工学报, 2023, 74(6): 2699-2707. |
| [14] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [15] | 顾浩, 张福建, 刘珍, 周文轩, 张鹏, 张忠强. 力电耦合作用下多孔石墨烯膜时间维度的脱盐性能及机理研究[J]. 化工学报, 2023, 74(5): 2067-2074. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号