化工学报 ›› 2019, Vol. 70 ›› Issue (9): 3483-3494.DOI: 10.11949/0438-1157.20190169
收稿日期:2019-03-01
修回日期:2019-05-09
出版日期:2019-09-05
发布日期:2019-09-05
通讯作者:
苏庆泉
作者简介:李艺群(1990—),男,博士研究生,基金资助:
Yiqun LI1( ),Chunhuan LUO1,2,Na LI1,3,Qingquan SU1,2(
),Chunhuan LUO1,2,Na LI1,3,Qingquan SU1,2( )
)
Received:2019-03-01
Revised:2019-05-09
Online:2019-09-05
Published:2019-09-05
Contact:
Qingquan SU
摘要:
利用低品位的太阳能热、地热或者工业领域低温余热废热进行吸收式制冷是实现节能减排的一个有效途径。对具有良好制冷吸收特性的工质对CaCl2-LiCl/H2O进行了实验研究,通过测定不同质量比的CaCl2-LiCl/H2O溶液的饱和蒸气压,得出了CaCl2与LiCl的质量比为2∶1的工质对即CaCl2-LiCl(2∶1)/H2O的制冷吸收特性最佳。CaCl2-LiCl(2∶1)/H2O与常规的工质对LiBr/H2O相比,在大幅降低工质对成本的同时,在同一制冷工况下所需驱动热源温度即发生温度降低了5.8℃,而制冷COP提高0.041,?效率提高0.052。还系统测定了CaCl2-LiCl(2∶1)/H2O的结晶温度、密度、黏度、比热容、比焓以及腐蚀性。结果表明,相较于其他几种以CaCl2为主要成分的工质对,CaCl2-LiCl(2∶1)/H2O具有较低的黏性,且对碳钢和紫铜的腐蚀性也较小,可满足实际工程应用的要求。
中图分类号:
李艺群, 罗春欢, 李娜, 苏庆泉. 基于吸收式制冷循环的CaCl2-LiCl/H2O工质对研究[J]. 化工学报, 2019, 70(9): 3483-3494.
Yiqun LI, Chunhuan LUO, Na LI, Qingquan SU. Study on CaCl2-LiCl/H2O as working pair of absorption refrigeration cycle[J]. CIESC Journal, 2019, 70(9): 3483-3494.
| 成分 | 碳钢 | 紫铜 |
|---|---|---|
| C | 0.16 | — |
| Mn | 0.53 | — |
| Si | 0.3 | 0.006 |
| P | 0.035 | — |
| S | 0.04 | 0.01 |
| Zn | — | 0.005 |
| Pb | — | 0.05 |
| Sn | — | 0.05 |
| Fe | 余量 | 0.05 |
| Cu | — | 余量 |
表1 Q235普碳钢和T6紫铜的成分
Table 1 Composition of Q235 carbon steel and T6 copper
| 成分 | 碳钢 | 紫铜 |
|---|---|---|
| C | 0.16 | — |
| Mn | 0.53 | — |
| Si | 0.3 | 0.006 |
| P | 0.035 | — |
| S | 0.04 | 0.01 |
| Zn | — | 0.005 |
| Pb | — | 0.05 |
| Sn | — | 0.05 |
| Fe | 余量 | 0.05 |
| Cu | — | 余量 |
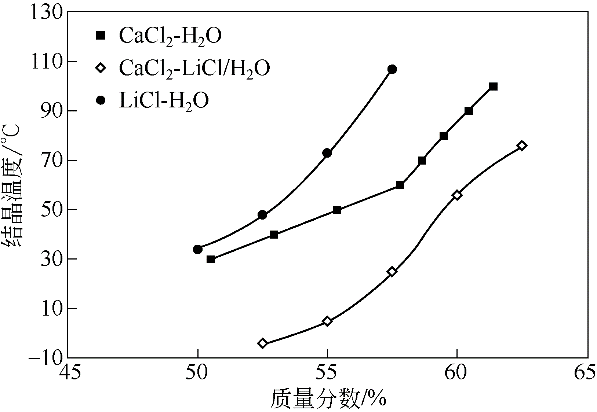
图5 相同质量分数的CaCl2-LiCl(2∶1)/H2O, CaCl2/H2O和LiCl/H2O的结晶温度对比
Fig.5 Comparison of crystallization temperatures of CaCl2-LiCl(2∶1)/H2O, CaCl2/H2O and LiCl/H2O at the same concentrations
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | p/kPa | T/℃ | p/kPa | T/℃ | p/kPa | T/℃ | p/kPa | T/℃ | p/kPa |
| 25.1 | 1.255 | 25.0 | 0.898 | 25.0 | 0.667 | 25.2 | 0.388 | ||
| 30.0 | 1.980 | 29.7 | 1.313 | 29.8 | 0.914 | 30.1 | 0.491 | ||
| 34.8 | 2.801 | 35.2 | 1.827 | 35.1 | 1.248 | 35.0 | 0.691 | ||
| 40.2 | 3.835 | 40.0 | 2.440 | 40.1 | 1.711 | 40.2 | 0.990 | ||
| 45.3 | 5.172 | 45.0 | 3.222 | 45.5 | 2.240 | 44.8 | 1.342 | ||
| 50.0 | 6.735 | 49.9 | 4.191 | 49.7 | 2.895 | 49.6 | 1.915 | ||
| 55.2 | 8.418 | 54.8 | 5.379 | 55.1 | 3.755 | 55.3 | 2.602 | ||
| 60.5 | 10.736 | 59.6 | 6.855 | 60.0 | 4.804 | 60.1 | 3.351 | ||
| 65.0 | 13.016 | 65.1 | 8.746 | 65.3 | 6.060 | 65.0 | 4.392 | ||
| 69.9 | 16.262 | 70.0 | 10.840 | 69.7 | 7.640 | 70.4 | 5.525 | 70.1 | 4.529 |
| 74.6 | 19.902 | 75.3 | 13.457 | 74.8 | 9.578 | 74.9 | 7.008 | 75.0 | 5.660 |
| 80.0 | 24.473 | 80.2 | 16.629 | 79.9 | 11.942 | 79.6 | 8.794 | 80.3 | 7.173 |
| 85.1 | 29.233 | 84.8 | 20.408 | 85.1 | 14.799 | 85.0 | 10.985 | 85.3 | 8.735 |
| 89.7 | 35.130 | 89.9 | 24.920 | 90.1 | 18.241 | 90.1 | 13.647 | 89.9 | 10.847 |
| 95.0 | 42.020 | 95.0 | 30.250 | 95.2 | 22.364 | 95.3 | 16.832 | 95.2 | 13.227 |
| 100.2 | 49.987 | 100.0 | 36.798 | 100.3 | 27.363 | 99.9 | 20.640 | 100.1 | 16.154 |
表2 CaCl2-LiCl(2∶1)/H2O的饱和蒸气压
Table 2 Saturated vapor pressures of CaCl2-LiCl(2∶1)/H2O
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | p/kPa | T/℃ | p/kPa | T/℃ | p/kPa | T/℃ | p/kPa | T/℃ | p/kPa |
| 25.1 | 1.255 | 25.0 | 0.898 | 25.0 | 0.667 | 25.2 | 0.388 | ||
| 30.0 | 1.980 | 29.7 | 1.313 | 29.8 | 0.914 | 30.1 | 0.491 | ||
| 34.8 | 2.801 | 35.2 | 1.827 | 35.1 | 1.248 | 35.0 | 0.691 | ||
| 40.2 | 3.835 | 40.0 | 2.440 | 40.1 | 1.711 | 40.2 | 0.990 | ||
| 45.3 | 5.172 | 45.0 | 3.222 | 45.5 | 2.240 | 44.8 | 1.342 | ||
| 50.0 | 6.735 | 49.9 | 4.191 | 49.7 | 2.895 | 49.6 | 1.915 | ||
| 55.2 | 8.418 | 54.8 | 5.379 | 55.1 | 3.755 | 55.3 | 2.602 | ||
| 60.5 | 10.736 | 59.6 | 6.855 | 60.0 | 4.804 | 60.1 | 3.351 | ||
| 65.0 | 13.016 | 65.1 | 8.746 | 65.3 | 6.060 | 65.0 | 4.392 | ||
| 69.9 | 16.262 | 70.0 | 10.840 | 69.7 | 7.640 | 70.4 | 5.525 | 70.1 | 4.529 |
| 74.6 | 19.902 | 75.3 | 13.457 | 74.8 | 9.578 | 74.9 | 7.008 | 75.0 | 5.660 |
| 80.0 | 24.473 | 80.2 | 16.629 | 79.9 | 11.942 | 79.6 | 8.794 | 80.3 | 7.173 |
| 85.1 | 29.233 | 84.8 | 20.408 | 85.1 | 14.799 | 85.0 | 10.985 | 85.3 | 8.735 |
| 89.7 | 35.130 | 89.9 | 24.920 | 90.1 | 18.241 | 90.1 | 13.647 | 89.9 | 10.847 |
| 95.0 | 42.020 | 95.0 | 30.250 | 95.2 | 22.364 | 95.3 | 16.832 | 95.2 | 13.227 |
| 100.2 | 49.987 | 100.0 | 36.798 | 100.3 | 27.363 | 99.9 | 20.640 | 100.1 | 16.154 |
| i | Ai | Bi | Ci | AARD |
|---|---|---|---|---|
| 0 | -1.596×102 | -1.484×105 | 5.184×102 | 0.74% |
| 1 | 1.143×103 | 2.559×106 | 7.668×102 | |
| 2 | 1.362×104 | 2.625×103 | 4.173×102 | |
| 3 | 1.536×103 | -9.150×106 | -1.481×102 | |
| 4 | 8.118 | 2.110×106 | 8.453×10 |
表3 CaCl2-LiCl(2∶1)/H2O饱和蒸气压的回归参数和
Table 3 Regression parameters and AARD for saturated vapor pressures of CaCl2-LiCl(2∶1)/H2O
| i | Ai | Bi | Ci | AARD |
|---|---|---|---|---|
| 0 | -1.596×102 | -1.484×105 | 5.184×102 | 0.74% |
| 1 | 1.143×103 | 2.559×106 | 7.668×102 | |
| 2 | 1.362×104 | 2.625×103 | 4.173×102 | |
| 3 | 1.536×103 | -9.150×106 | -1.481×102 | |
| 4 | 8.118 | 2.110×106 | 8.453×10 |
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | ρ/(g·cm-3) | T/℃ | ρ/(g·cm-3) | T/℃ | ρ/(g·cm-3) | T/℃ | ρ/(g·cm-3) | T/℃ | ρ/(g·cm-3) |
| 30.0 | 1.8957 | 30.0 | 1.9434 | 30.1 | 1.9927 | 30.9 | 2.0393 | — | — |
| 39.8 | 1.8896 | 39.8 | 1.9374 | 39.9 | 1.9856 | 40.1 | 2.0331 | — | — |
| 50.0 | 1.8829 | 50.1 | 1.9309 | 50.0 | 1.9789 | 50.0 | 2.0267 | — | — |
| 60.0 | 1.8770 | 60.3 | 1.9241 | 60.0 | 1.9789 | 60.1 | 2.0203 | 60.0 | 2.0449 |
| 69.8 | 1.8708 | 69.9 | 1.9181 | 69.6 | 1.9672 | 69.9 | 2.0143 | 70.1 | 2.0371 |
| 79.9 | 1.8647 | 79.7 | 1.9125 | 79.6 | 1.9612 | 80.0 | 2.0085 | 80.0 | 2.0299 |
| 89.8 | 1.8590 | 89.7 | 1.9069 | 89.8 | 1.9550 | 89.9 | 2.0029 | 90.1 | 2.0248 |
| 99.9 | 1.8535 | 99.6 | 1.9012 | 99.6 | 1.9500 | 99.8 | 1.9978 | 99.9 | 2.0195 |
表4 CaCl2-LiCl(2∶1)/H2O的密度
Table 4 Densities of CaCl2-LiCl(2∶1)/H2O
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | ρ/(g·cm-3) | T/℃ | ρ/(g·cm-3) | T/℃ | ρ/(g·cm-3) | T/℃ | ρ/(g·cm-3) | T/℃ | ρ/(g·cm-3) |
| 30.0 | 1.8957 | 30.0 | 1.9434 | 30.1 | 1.9927 | 30.9 | 2.0393 | — | — |
| 39.8 | 1.8896 | 39.8 | 1.9374 | 39.9 | 1.9856 | 40.1 | 2.0331 | — | — |
| 50.0 | 1.8829 | 50.1 | 1.9309 | 50.0 | 1.9789 | 50.0 | 2.0267 | — | — |
| 60.0 | 1.8770 | 60.3 | 1.9241 | 60.0 | 1.9789 | 60.1 | 2.0203 | 60.0 | 2.0449 |
| 69.8 | 1.8708 | 69.9 | 1.9181 | 69.6 | 1.9672 | 69.9 | 2.0143 | 70.1 | 2.0371 |
| 79.9 | 1.8647 | 79.7 | 1.9125 | 79.6 | 1.9612 | 80.0 | 2.0085 | 80.0 | 2.0299 |
| 89.8 | 1.8590 | 89.7 | 1.9069 | 89.8 | 1.9550 | 89.9 | 2.0029 | 90.1 | 2.0248 |
| 99.9 | 1.8535 | 99.6 | 1.9012 | 99.6 | 1.9500 | 99.8 | 1.9978 | 99.9 | 2.0195 |
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | η/(mPa·s) | T/℃ | η/(mPa·s) | T/℃ | η/(mPa·s) | T/℃ | η/(mPa·s) | T/℃ | η/(mPa·s) |
| 30.0 | 6.4417 | 30.0 | 10.0894 | 30.1 | 18.4371 | 30.9 | 33.5353 | — | — |
| 39.8 | 5.0810 | 39.8 | 7.9360 | 39.9 | 13.5747 | 40.1 | 23.8138 | — | — |
| 50.0 | 4.1293 | 50.1 | 6.2727 | 50.0 | 10.1141 | 50.0 | 17.5305 | — | — |
| 60.0 | 3.4514 | 60.3 | 5.0874 | 60.0 | 8.3263 | 60.1 | 13.6596 | 60.0 | 18.4415 |
| 69.8 | 2.9182 | 69.9 | 4.2625 | 69.6 | 6.6501 | 69.9 | 10.7005 | 70.1 | 14.0103 |
| 79.9 | 2.5188 | 79.7 | 3.5989 | 79.6 | 5.5813 | 80.0 | 8.7180 | 80.0 | 11.0275 |
| 89.8 | 2.1855 | 89.7 | 3.0674 | 89.8 | 4.7153 | 89.9 | 7.1871 | 90.1 | 9.0751 |
| 99.9 | 1.9162 | 99.6 | 2.6789 | 99.6 | 3.9853 | 99.8 | 6.0610 | 99.9 | 7.5790 |
表5 CaCl2-LiCl(2∶1)/H2O的黏度
Table 5 Viscosities of CaCl2-LiCl(2∶1)/H2O
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | η/(mPa·s) | T/℃ | η/(mPa·s) | T/℃ | η/(mPa·s) | T/℃ | η/(mPa·s) | T/℃ | η/(mPa·s) |
| 30.0 | 6.4417 | 30.0 | 10.0894 | 30.1 | 18.4371 | 30.9 | 33.5353 | — | — |
| 39.8 | 5.0810 | 39.8 | 7.9360 | 39.9 | 13.5747 | 40.1 | 23.8138 | — | — |
| 50.0 | 4.1293 | 50.1 | 6.2727 | 50.0 | 10.1141 | 50.0 | 17.5305 | — | — |
| 60.0 | 3.4514 | 60.3 | 5.0874 | 60.0 | 8.3263 | 60.1 | 13.6596 | 60.0 | 18.4415 |
| 69.8 | 2.9182 | 69.9 | 4.2625 | 69.6 | 6.6501 | 69.9 | 10.7005 | 70.1 | 14.0103 |
| 79.9 | 2.5188 | 79.7 | 3.5989 | 79.6 | 5.5813 | 80.0 | 8.7180 | 80.0 | 11.0275 |
| 89.8 | 2.1855 | 89.7 | 3.0674 | 89.8 | 4.7153 | 89.9 | 7.1871 | 90.1 | 9.0751 |
| 99.9 | 1.9162 | 99.6 | 2.6789 | 99.6 | 3.9853 | 99.8 | 6.0610 | 99.9 | 7.5790 |
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | cp /(kJ·kg-1·℃-1) | T/℃ | cp /(kJ·kg-1·℃-1) | T/℃ | cp /(kJ·kg-1·℃-1) | T/℃ | cp /(kJ·kg-1·℃-1) | T/℃ | cp /(kJ·kg-1·℃-1) |
| 30.0 | 2.68 | 30.0 | 2.56 | 30.0 | 2.43 | 30.0 | 2.28 | — | — |
| 40.0 | 2.69 | 40.0 | 2.57 | 40.0 | 2.45 | 40.0 | 2.30 | — | — |
| 50.0 | 2.70 | 50.0 | 2.59 | 50.0 | 2.46 | 50.0 | 2.31 | — | — |
| 60.0 | 2.71 | 60.0 | 2.60 | 60.0 | 2.48 | 60.0 | 2.34 | 60.0 | 2.21 |
| 70.0 | 2.74 | 70.0 | 2.63 | 70.0 | 2.50 | 70.0 | 2.35 | 70.0 | 2.23 |
| 80.0 | 2.76 | 80.0 | 2.65 | 80.0 | 2.52 | 80.0 | 2.38 | 80.0 | 2.26 |
| 90.0 | 2.78 | 90.0 | 2.68 | 90.0 | 2.55 | 90.0 | 2.40 | 90.0 | 2.28 |
| 100.0 | 2.80 | 100.0 | 2.70 | 100.0 | 2.57 | 100.0 | 2.42 | 100.0 | 2..29 |
表6 CaCl2-LiCl(2∶1)/H2O的比热容
Table 6 Specific heat capacity of CaCl2-LiCl(2∶1)/H2O
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | cp /(kJ·kg-1·℃-1) | T/℃ | cp /(kJ·kg-1·℃-1) | T/℃ | cp /(kJ·kg-1·℃-1) | T/℃ | cp /(kJ·kg-1·℃-1) | T/℃ | cp /(kJ·kg-1·℃-1) |
| 30.0 | 2.68 | 30.0 | 2.56 | 30.0 | 2.43 | 30.0 | 2.28 | — | — |
| 40.0 | 2.69 | 40.0 | 2.57 | 40.0 | 2.45 | 40.0 | 2.30 | — | — |
| 50.0 | 2.70 | 50.0 | 2.59 | 50.0 | 2.46 | 50.0 | 2.31 | — | — |
| 60.0 | 2.71 | 60.0 | 2.60 | 60.0 | 2.48 | 60.0 | 2.34 | 60.0 | 2.21 |
| 70.0 | 2.74 | 70.0 | 2.63 | 70.0 | 2.50 | 70.0 | 2.35 | 70.0 | 2.23 |
| 80.0 | 2.76 | 80.0 | 2.65 | 80.0 | 2.52 | 80.0 | 2.38 | 80.0 | 2.26 |
| 90.0 | 2.78 | 90.0 | 2.68 | 90.0 | 2.55 | 90.0 | 2.40 | 90.0 | 2.28 |
| 100.0 | 2.80 | 100.0 | 2.70 | 100.0 | 2.57 | 100.0 | 2.42 | 100.0 | 2..29 |
| i | Ai | Bi | Ci | AARD |
|---|---|---|---|---|
| 0 | 2.2760×10 | 2.9488×10-2 | 1.8683×10-4 | 0.19% |
| 1 | 3.6877×10 | -1.2945×10-1 | 8.6798×10-4 | |
| 2 | -6.9317×102 | 1.4542×10-1 | -9.6057×10-4 |
表7 CaCl2-LiCl(2∶1)/H2O比热容的回归参数和平均绝对相对偏差
Table 7 Regression parameters and AARD for specific heat capacities of CaCl2-LiCl(2∶1)/H2O
| i | Ai | Bi | Ci | AARD |
|---|---|---|---|---|
| 0 | 2.2760×10 | 2.9488×10-2 | 1.8683×10-4 | 0.19% |
| 1 | 3.6877×10 | -1.2945×10-1 | 8.6798×10-4 | |
| 2 | -6.9317×102 | 1.4542×10-1 | -9.6057×10-4 |
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | h/(kJ·kg-1) | T/℃ | h/(kJ·kg-1) | T/℃ | h/(kJ·kg-1) | T/℃ | h/(kJ·kg-1) | T/℃ | h/(kJ·kg-1) |
| 30.0 | 321.29 | 30.0 | 310.46 | 30.0 | 304.27 | 30.0 | 298.49 | — | — |
| 40.0 | 348.13 | 40.0 | 336.07 | 40.0 | 328.64 | 40.0 | 321.34 | — | — |
| 50.0 | 375.14 | 50.0 | 361.88 | 50.0 | 353.22 | 50.0 | 344.40 | — | — |
| 60.0 | 402.33 | 60.0 | 387.89 | 60.0 | 378.01 | 60.0 | 367.66 | 60.0 | 353.23 |
| 70.0 | 429.71 | 70.0 | 414.10 | 70.0 | 403.00 | 70.0 | 391.14 | 70.0 | 375.46 |
| 80.0 | 457.26 | 80.0 | 440.51 | 80.0 | 428.21 | 80.0 | 414.82 | 80.0 | 397.92 |
| 90.0 | 484.99 | 90.0 | 467.12 | 90.0 | 453.63 | 90.0 | 438.72 | 90.0 | 420.60 |
| 100.0 | 512.91 | 100.0 | 493.93 | 100.0 | 479.26 | 100.0 | 462.83 | 100.0 | 443.52 |
表8 CaCl2-LiCl(2∶1)/H2O的比焓
Table 8 Specific enthalpies of CaCl2-LiCl(2∶1)/H2O
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | h/(kJ·kg-1) | T/℃ | h/(kJ·kg-1) | T/℃ | h/(kJ·kg-1) | T/℃ | h/(kJ·kg-1) | T/℃ | h/(kJ·kg-1) |
| 30.0 | 321.29 | 30.0 | 310.46 | 30.0 | 304.27 | 30.0 | 298.49 | — | — |
| 40.0 | 348.13 | 40.0 | 336.07 | 40.0 | 328.64 | 40.0 | 321.34 | — | — |
| 50.0 | 375.14 | 50.0 | 361.88 | 50.0 | 353.22 | 50.0 | 344.40 | — | — |
| 60.0 | 402.33 | 60.0 | 387.89 | 60.0 | 378.01 | 60.0 | 367.66 | 60.0 | 353.23 |
| 70.0 | 429.71 | 70.0 | 414.10 | 70.0 | 403.00 | 70.0 | 391.14 | 70.0 | 375.46 |
| 80.0 | 457.26 | 80.0 | 440.51 | 80.0 | 428.21 | 80.0 | 414.82 | 80.0 | 397.92 |
| 90.0 | 484.99 | 90.0 | 467.12 | 90.0 | 453.63 | 90.0 | 438.72 | 90.0 | 420.60 |
| 100.0 | 512.91 | 100.0 | 493.93 | 100.0 | 479.26 | 100.0 | 462.83 | 100.0 | 443.52 |
| i | Ai | Bi | Ci | Di | AARD |
|---|---|---|---|---|---|
| 0 | -1.4508×103 | 5.7582×103 | -1.1136×104 | 8.1644×103 | 0.02% |
| 1 | 1.4530×101 | -7.3293×101 | 1.4604×102 | -1.0071×10-2 | |
| 2 | -1.9842×10-2 | 1.2769×10-1 | -2.6044×10-1 | 1.7708×10-1 |
表9 CaCl2-LiCl(2∶1)/H2O比焓的回归参数和平均绝对相对偏差
Table 9 Regression parameters and AARD for specific enthalpies of CaCl2-LiCl(2∶1)/H2O
| i | Ai | Bi | Ci | Di | AARD |
|---|---|---|---|---|---|
| 0 | -1.4508×103 | 5.7582×103 | -1.1136×104 | 8.1644×103 | 0.02% |
| 1 | 1.4530×101 | -7.3293×101 | 1.4604×102 | -1.0071×10-2 | |
| 2 | -1.9842×10-2 | 1.2769×10-1 | -2.6044×10-1 | 1.7708×10-1 |
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | s/(kJ·kg-1·℃-1) | T/℃ | s/(kJ·kg-1·℃-1) | T/℃ | s/(kJ·kg-1·℃-1) | T/℃ | s/(kJ·kg-1·℃-1) | T/℃ | s/(kJ·kg-1·℃-1) |
| 30.0 | 1.6839 | 30.0 | 1.6936 | 30.0 | 1.7027 | 30.0 | 1.7132 | — | — |
| 40.0 | 1.7710 | 40.0 | 1.7768 | 40.0 | 1.7819 | 40.0 | 1.7875 | — | — |
| 50.0 | 1.8558 | 50.0 | 1.8578 | 50.0 | 1.8595 | 50.0 | 1.8592 | — | — |
| 60.0 | 1.9387 | 60.0 | 1.9371 | 60.0 | 1.9353 | 60.0 | 1.9298 | 60.0 | 1.9398 |
| 70..0 | 2.0210 | 70..0 | 2.0152 | 70..0 | 2.0088 | 70..0 | 1.9995 | 70..0 | 2.0054 |
| 80.0 | 2.0991 | 80.0 | 2.0921 | 80.0 | 2.0813 | 80.0 | 2.0689 | 80.0 | 2.0701 |
| 90.0 | 2.1769 | 90.0 | 2.1667 | 90.0 | 2.1524 | 90.0 | 2.1358 | 90.0 | 2.1338 |
| 100.0 | 2.2531 | 100.0 | 2.2400 | 100.0 | 2.2221 | 100.0 | 2.2014 | 100.0 | 2.1949 |
表10 CaCl2-LiCl(2∶1)/H2O的比熵
Table 10 Specific entropies of CaCl2-LiCl(2∶1)/H2O
| w=40.0% | w=45.0% | w=50.0% | w=55.0% | w=60.0% | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/℃ | s/(kJ·kg-1·℃-1) | T/℃ | s/(kJ·kg-1·℃-1) | T/℃ | s/(kJ·kg-1·℃-1) | T/℃ | s/(kJ·kg-1·℃-1) | T/℃ | s/(kJ·kg-1·℃-1) |
| 30.0 | 1.6839 | 30.0 | 1.6936 | 30.0 | 1.7027 | 30.0 | 1.7132 | — | — |
| 40.0 | 1.7710 | 40.0 | 1.7768 | 40.0 | 1.7819 | 40.0 | 1.7875 | — | — |
| 50.0 | 1.8558 | 50.0 | 1.8578 | 50.0 | 1.8595 | 50.0 | 1.8592 | — | — |
| 60.0 | 1.9387 | 60.0 | 1.9371 | 60.0 | 1.9353 | 60.0 | 1.9298 | 60.0 | 1.9398 |
| 70..0 | 2.0210 | 70..0 | 2.0152 | 70..0 | 2.0088 | 70..0 | 1.9995 | 70..0 | 2.0054 |
| 80.0 | 2.0991 | 80.0 | 2.0921 | 80.0 | 2.0813 | 80.0 | 2.0689 | 80.0 | 2.0701 |
| 90.0 | 2.1769 | 90.0 | 2.1667 | 90.0 | 2.1524 | 90.0 | 2.1358 | 90.0 | 2.1338 |
| 100.0 | 2.2531 | 100.0 | 2.2400 | 100.0 | 2.2221 | 100.0 | 2.2014 | 100.0 | 2.1949 |
| i | Ai | Bi | Ci | Di | AARD |
|---|---|---|---|---|---|
| 0 | 1.5469×102 | -1.0106×103 | 2.1465×103 | -1.5011×103 | 0.98% |
| 1 | -1.2590×100 | 8.1964×100 | -1.7371×101 | 1.2146×101 | |
| 2 | 3.4252×10-3 | -2.2055×10-2 | 4.6621×10-2 | -3.2573×10-2 | |
| 3 | -3.0971×10-6 | 1.9836×10-5 | -4.1854×10-5 | 2.9198×10-5 |
表11 CaCl2-LiCl(2∶1)/H2O比熵的回归参数和平均绝对相对偏差
Table 11 Regression parameters and AARD for specific entropies of CaCl2-LiCl(2∶1)/H2O
| i | Ai | Bi | Ci | Di | AARD |
|---|---|---|---|---|---|
| 0 | 1.5469×102 | -1.0106×103 | 2.1465×103 | -1.5011×103 | 0.98% |
| 1 | -1.2590×100 | 8.1964×100 | -1.7371×101 | 1.2146×101 | |
| 2 | 3.4252×10-3 | -2.2055×10-2 | 4.6621×10-2 | -3.2573×10-2 | |
| 3 | -3.0971×10-6 | 1.9836×10-5 | -4.1854×10-5 | 2.9198×10-5 |
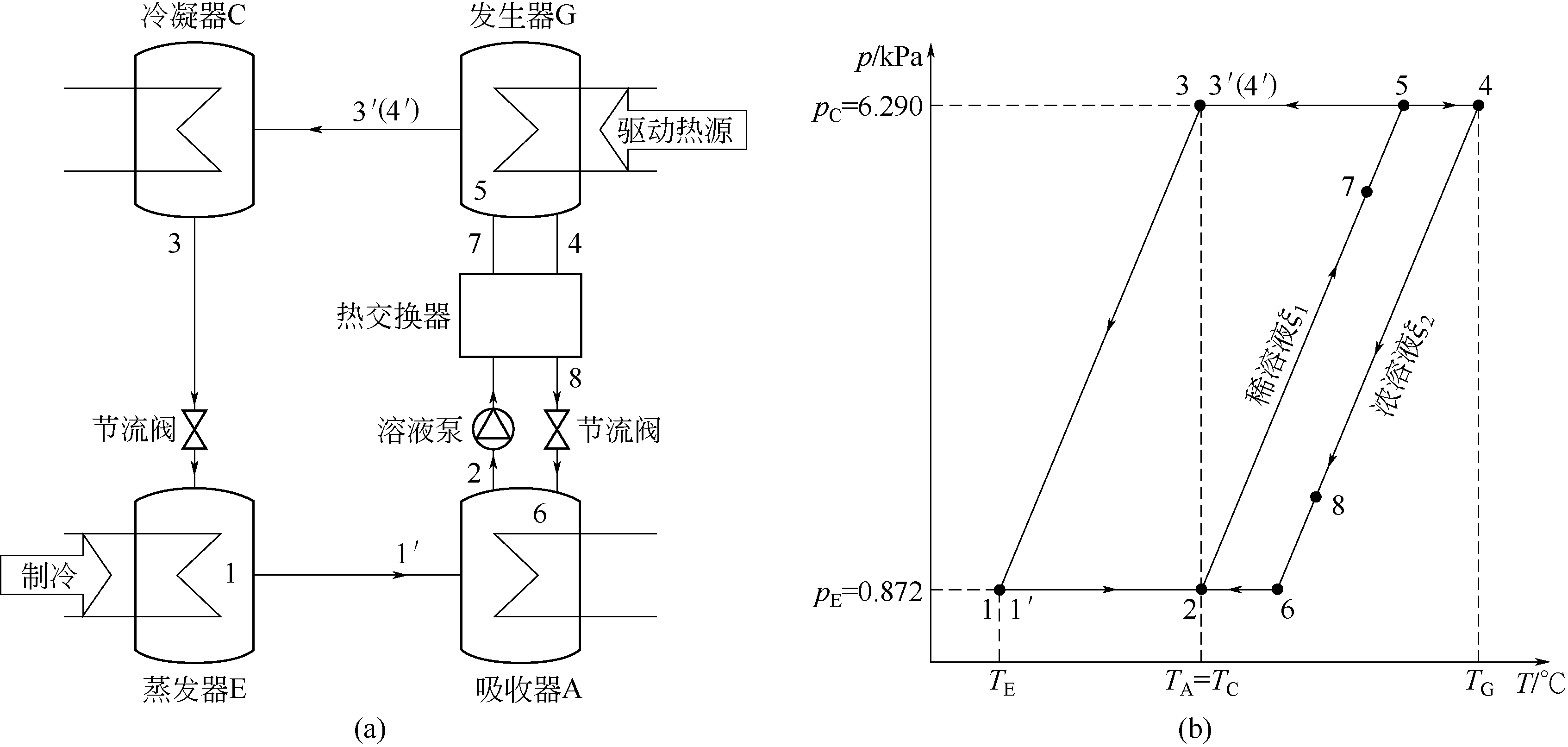
图10 吸收式制冷循环工作原理图(a)和吸收式制冷循环的p-T图(b)
Fig.10 Schematic diagramforabsorption refrigeration system (a) and p-T diagramfor anabsorption refrigeration system under standard operating conditions (b)
| 工质对 | 稀溶液 | 浓溶液 |
|---|---|---|
| ① CaCl2-LiCl (2∶1)/H2O | 54.20% | 57.20% |
| ② LiBr/H2O | 56.40% | 59.40% |
| ③ CaCl2-LiBr-LiNO3-KNO3(16.2∶2∶2∶1)/H2O | 58.50% | 61.50% |
| ④ CaCl2-LiNO3-LiBr (8.72∶1∶1)/H2O | 57.30% | 60.30% |
| ⑤ CaCl2-LiBr (1.35∶1)/H2O | 55.80% | 58.80% |
表12 标准制冷工况下各种工质对溶液的总质量分数
Table 12 Concentrations of various working pairs under the same refrigeration conditions
| 工质对 | 稀溶液 | 浓溶液 |
|---|---|---|
| ① CaCl2-LiCl (2∶1)/H2O | 54.20% | 57.20% |
| ② LiBr/H2O | 56.40% | 59.40% |
| ③ CaCl2-LiBr-LiNO3-KNO3(16.2∶2∶2∶1)/H2O | 58.50% | 61.50% |
| ④ CaCl2-LiNO3-LiBr (8.72∶1∶1)/H2O | 57.30% | 60.30% |
| ⑤ CaCl2-LiBr (1.35∶1)/H2O | 55.80% | 58.80% |
| 点 | 物质 | p/k Pa | T/℃ | w/% | h/ (kJ·kg-1) | s/ (kJ·kg-1·℃-1) | D/ (kg·s-1) |
|---|---|---|---|---|---|---|---|
| 1 | 冷凝水 | 0.872 | 5.0 | 0 | 439.6 | 1.6082 | 1 |
| 1′ | 水蒸气 | 0.872 | 5.0 | 0 | 2927.9 | 10.5598 | 1 |
| 2 | 稀溶液 | 0.872 | 37.0 | 54.2 | 314.7 | 1.7639 | 19.1 |
| 3 | 冷凝水 | 6.290 | 37.0 | 0 | 573.5 | 2.0639 | 1 |
| 4 | 浓溶液 | 6.290 | 75.2 | 57.2 | 397.1 | 2.0360 | 18.1 |
| 4′ | 水蒸气 | 6.290 | 75.2 | 0 | 3058.5 | 10.0539 | 1 |
| 5 | 稀溶液 | 6.290 | 72.2 | 54.2 | 398.5 | 2.0168 | 19.1 |
| 6 | 浓溶液 | 6.290 | 39.9 | 57.2 | 316.8 | 1.7908 | 18.1 |
| 7 | 稀溶液 | — | 64.8 | 54.2 | 380.8 | 1.9654 | 19.1 |
| 8 | 浓溶液 | — | 44.6 | 57.2 | 327.3 | 1.8274 | 18.1 |
表13 基于CaCl2-LiCl(2∶1)/H2O的吸收式制冷循环的各点状态参数
Table 13 Typical points’ state parameters in absorption refrigeration cycle using CaCl2-LiCl(2∶1)/H2O as
| 点 | 物质 | p/k Pa | T/℃ | w/% | h/ (kJ·kg-1) | s/ (kJ·kg-1·℃-1) | D/ (kg·s-1) |
|---|---|---|---|---|---|---|---|
| 1 | 冷凝水 | 0.872 | 5.0 | 0 | 439.6 | 1.6082 | 1 |
| 1′ | 水蒸气 | 0.872 | 5.0 | 0 | 2927.9 | 10.5598 | 1 |
| 2 | 稀溶液 | 0.872 | 37.0 | 54.2 | 314.7 | 1.7639 | 19.1 |
| 3 | 冷凝水 | 6.290 | 37.0 | 0 | 573.5 | 2.0639 | 1 |
| 4 | 浓溶液 | 6.290 | 75.2 | 57.2 | 397.1 | 2.0360 | 18.1 |
| 4′ | 水蒸气 | 6.290 | 75.2 | 0 | 3058.5 | 10.0539 | 1 |
| 5 | 稀溶液 | 6.290 | 72.2 | 54.2 | 398.5 | 2.0168 | 19.1 |
| 6 | 浓溶液 | 6.290 | 39.9 | 57.2 | 316.8 | 1.7908 | 18.1 |
| 7 | 稀溶液 | — | 64.8 | 54.2 | 380.8 | 1.9654 | 19.1 |
| 8 | 浓溶液 | — | 44.6 | 57.2 | 327.3 | 1.8274 | 18.1 |
| CaCl2-LiCl(2∶1)/H2O | LiBr/H2O | CaCl2-LiBr-LiNO3-KNO3 (16.2∶2∶2∶1) /H2O | CaCl2-LiNO3-LiBr (8.72∶1∶1)/H2O | CaCl2-LiBr(1.35∶1)/H2O |
|---|---|---|---|---|
| 0.803 | 0.762 | 0.793 | 0.805 | 0.788 |
表14 采用不同工质对的吸收式制冷循环COP
Table 14 COP of absorption refrigeration cycles using different working pairs
| CaCl2-LiCl(2∶1)/H2O | LiBr/H2O | CaCl2-LiBr-LiNO3-KNO3 (16.2∶2∶2∶1) /H2O | CaCl2-LiNO3-LiBr (8.72∶1∶1)/H2O | CaCl2-LiBr(1.35∶1)/H2O |
|---|---|---|---|---|
| 0.803 | 0.762 | 0.793 | 0.805 | 0.788 |
| 过程 | ?损失/kW | |
|---|---|---|
| CaCl2-LiCl(2∶1)/H2O | LiBr/H2O | |
| 蒸发过程 | 0.5 | 0.5 |
| 冷凝过程 | 0.3 | 0.8 |
| 吸收过程 | 49.2 | 55.3 |
| 发生过程 | 310.9 | 332.7 |
| 热交换过程 | 25.7 | 31.4 |
表15 采用CaCl2-LiCl(2∶1)/H2O和LiBr/H2O时各部件的?损失
Table 15 Exergy destruction in each part of absorption refrigeration cycle using CaCl2-LiCl(2∶1)/H2O or
| 过程 | ?损失/kW | |
|---|---|---|
| CaCl2-LiCl(2∶1)/H2O | LiBr/H2O | |
| 蒸发过程 | 0.5 | 0.5 |
| 冷凝过程 | 0.3 | 0.8 |
| 吸收过程 | 49.2 | 55.3 |
| 发生过程 | 310.9 | 332.7 |
| 热交换过程 | 25.7 | 31.4 |
| 1 | 国家发展改革委, 科技部, 工业和信息化部, 等 . “十三五”节能环保产业发展规划[J]. 有色冶金节能, 2017, 33(1): 1-7+13. |
| National Development and Reform Commission, Ministry of Science and Technology, Ministry of Industry and Information Technology, et al . The 13th five-year plan for energy conservation and environmental protection industries[J]. Energy Saving of Non-Ferrous Metallurgy, 2017, 33(1): 1-7+13. | |
| 2 | 王长庆 . 吸收式制冷技术的应用与发展[J]. 能源技术, 2000, 21(1): 31-35. |
| Wang C Q . Application and development of absorption refrigeration technology[J]. Power & Energy, 2000, 21(1): 31-35. | |
| 3 | Inoue N , Irie K , Fukusumi Y . Absorption heat pump: US 7464562[P]. 2008-12-16. |
| 4 | Srikhirin P , Aphornratana S , Chungpaibulpatana S . A review of absorption refrigeration technologies[J]. Renew. Sust. Energ. Rev., 2001, 5(4): 343-372. |
| 5 | Bellos E , Tzivanidis C , Antonopoulos K A . Exergetic, energetic and financial evaluation of a solar driven absorption cooling system with various collector types[J]. Appl. Therm. Eng., 2016, 102: 749-759. |
| 6 | Leonzio G . Solar systems integrated with absorption heat pumps and thermal energy storages: state of art[J]. Renew. Sust. Energ. Rev., 2017, 70: 492-505. |
| 7 | Alobaid M , Hughes B , Calautit J K , et al . A review of solar driven absorption cooling with photovoltaic thermal systems[J]. Renew. Sust. Energ. Rev., 2017, 76: 728-742. |
| 8 | Misra R D , Sahoo P K , Sahoo S , et al . Thermoeconomic optimization of a single effect water/LiBr vapour absorption refrigeration system[J]. Int. J. Refrig., 2003, 26(2): 158-169. |
| 9 | Kaynakli O , Kilic M . Theoretical study on the effect of operating conditions on performance of absorption refrigeration system[J]. Energ. Convers. Manage., 2007, 48(2): 599-607. |
| 10 | 车德勇, 孙佰仲, 国文学, 等 . 溴化锂吸收式制冷技术的应用与发展[J]. 东北电力大学学报, 2003, 23(6): 36-40. |
| Che D Y , Sun B Z , Guo W X , et al . Application and development of lithium bromide absorption refrigeration technology[J].J. Northeast. Dianli Uiniv., 2003, 23(6): 36-40. | |
| 11 | Lin P , Wang R Z , Xia Z Z . Numerical investigation of a two-stage air-cooled absorption refrigeration system for solar cooling: cycle analysis and absorption cooling performances[J]. Renew. Energ., 2011, 36(5): 1401-1412. |
| 12 | Malinina O S , Baranenko A V , Zaitsev A V . Influence of the average daily outdoor air parameters on the efficiency of solar lithium bromide-water absorption refrigeration machine[C]//AIP Conference Proceedings. AIP Publishing, 2018, 2007(1): 030040. |
| 13 | Mortazavi M , Schmid M , Moghaddam S . Compact and efficient generator for low grade solar and waste heat driven absorption systems[J]. Appl. Energ., 2017, 198: 173-179. |
| 14 | Wang M , Ferreira C A I . Absorption heat pump cycles with NH3–ionic liquid working pairs[J]. Appl. Energ., 2017, 204: 819-830. |
| 15 | Luo C H , Chen K , Li Y Q , et al . Crystallization temperature, vapor pressure, density, viscosity, and specific heat capacity of the LiNO3/[BMIM] Cl/H2O ternary system[J]. J. Chem. Eng. Data, 2017, 62(10): 3043-3052. |
| 16 | Luo C H , Li Y Q , Chen K , et al . Thermodynamic properties and corrosivity of a new absorption heat pump working pair: lithium nitrate+ 1-butyl-3-methylimidazolium bromide+ water[J]. Fluid Phase Equilib., 2017, 451: 25-39. |
| 17 | Luo C H , Li Y Q , Li N , et al . Thermophysical properties of lithium nitrate+ 1-ethyl-3-methylimidazolium diethylphosphate+ water system[J]. J. Chem. Thermodyn., 2018, 126: 160-170. |
| 18 | 李娜, 罗春欢, 苏庆泉 . CaCl2-LiBr-LiNO3-KNO3/H2O工质对的热物性和腐蚀性[J]. 过程工程学报, 2018, 18(4): 764-768. |
| Li N , Luo C H , Su Q Q . Thermophysical properties and corrosivity of CaCl2-LiBr-LiNO3-KNO3/H2O working pair[J]. Chin. J. Proc. Eng., 2018, 18(4): 764-768. | |
| 19 | Li N , Luo C H , Su Q Q . A working pair of CaCl2–LiBr–LiNO3/H2O and its application in a single-stage solar-driven absorption refrigeration cycle[J]. Int. J. Refrig., 2018, 86: 1-13. |
| 20 | 李娜, 罗春欢, 苏庆泉 . CaCl2-LiBr (1.35: 1)/H2O工质对的热物性及应用[J]. 工程科学学报, 2018, 40(2): 167-176. |
| Li N , Luo C H , Su Q Q . Thermophysical properties and applications of CaCl2-LiBr(1.35:1)/ H2O as a working pair[J]. Chin. J. Eng., 2018, 40(2): 167-176. | |
| 21 | Safarov J T . Vapor pressure of heat transfer fluids of absorption refrigeration machines and heat pumps: binary solutions of lithium nitrate with methanol[J]. J. Chem. Thermodyn., 2005, 37(12): 1261-1267. |
| 22 | Kim J S , Lee H . Solubilities, vapor pressures, densities, and viscosities of the LiBr+ LiI+ HO (CH2)3OH+ H2O system[J]. J.Chem. Eng. Data, 2001, 46(1): 79-83. |
| 23 | Park Y , Kim J S , Lee H , et al . Density, vapor pressure, solubility, and viscosity for water+ lithium bromide+ lithium nitrate+ 1, 3-propanediol[J]. J.Chem. Eng. Data, 1997, 42(1): 145-148. |
| 24 | He Z , Zhao Z , Zhang X , et al . Thermodynamic properties of new heat pump working pairs: 1,3-dimethylimidazolium dimethylphosphate and water, ethanol and methanol[J]. Fluid Phase Equilib., 2010, 298(1): 83-91. |
| 25 | 陈东, 谢继红 . 热泵技术及其应用[M]. 北京: 化学工业出版社, 2008: 206-220. |
| Chen D , Xie J H . Technology and Application of Heat Pump[M]. Beijing: Chemical Industry Press, 2008: 206-220. | |
| 26 | Yang D T , Zhu Y J , Liu S C , et al . Thermodynamic properties of a ternary AHP working pair: lithium bromide + 1-ethyl-3-methylimidazolium chloride+ H2O[J]. J. Chem. Eng. Data, 2019, 64(2): 574-583. |
| 27 | Aprhornratana S , Eames I W . Thermodynamic analysis of absorption refrigeration cycles using the second law of thermodynamics method[J]. Int. J. Refrig., 1995, 18(4): 244-252. |
| 28 | Şencan A , Yakut K A , Kalogirou S A . Exergy analysis of lithium bromide/water absorption systems[J]. Renew. Energ., 2005, 30(5): 645-657. |
| 29 | Patel H A , Patel L N , Jani D , et al . Energetic analysis of single stage lithium bromide water absorption refrigeration system[J]. Procedia Technology, 2016, 23: 488-495. |
| 30 | 戴永庆 . 溴化锂吸收式制冷技术及应用[M]. 北京: 机械工业出版社, 2001: 49-67. |
| Dai Y Q . LiBr Absorption Refrigeration Technology and Application[M]. Beijing: China Machine Press, 2001: 49-67. | |
| 31 | 王林 . 小型吸收式制冷机原理与应用[M]. 北京: 中国建筑工业出版社, 2011: 93-109. |
| Wang L . Principle and Application of Small-sized Absorption Refrigerator[M]. Beijing: China Architecture & Building Press, 2011: 93-109. |
| [1] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [2] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [3] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [4] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [5] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [6] | 李艳辉, 丁邵明, 白周央, 张一楠, 于智红, 邢利梅, 高鹏飞, 王永贞. 非常规服役超临界锅炉的微纳尺度腐蚀动力学模型建立及应用[J]. 化工学报, 2023, 74(6): 2436-2446. |
| [7] | 雷博雯, 吴建华, 吴启航. R290低压比热泵高补气过热度循环研究[J]. 化工学报, 2023, 74(5): 1875-1883. |
| [8] | 周必茂, 许世森, 王肖肖, 刘刚, 李小宇, 任永强, 谭厚章. 烧嘴偏转角度对气化炉渣层分布特性的影响[J]. 化工学报, 2023, 74(5): 1939-1949. |
| [9] | 张永泉, 玄伟伟. 碱金属/(FeO+CaO+MgO)对硅酸盐灰熔渣结构和黏度的影响机理[J]. 化工学报, 2023, 74(4): 1764-1771. |
| [10] | 苏晓丹, 朱干宇, 李会泉, 郑光明, 孟子衡, 李防, 杨云瑞, 习本军, 崔玉. 湿法磷酸半水工艺考察与石膏结晶过程研究[J]. 化工学报, 2023, 74(4): 1805-1817. |
| [11] | 靳志远, 单国荣, 潘鹏举. AM/AMPS/SSS三元共聚物的制备及耐温耐盐性能[J]. 化工学报, 2023, 74(2): 916-923. |
| [12] | 张家庆, 蒋榕培, 史伟康, 武博翔, 杨超, 刘朝晖. 煤基/石油基火箭煤油高参数黏温特性与组分特性研究[J]. 化工学报, 2023, 74(2): 653-665. |
| [13] | 陈毓明, 历伟, 严翔, 王靖岱, 阳永荣. 初生态聚乙烯聚集态结构调控研究进展[J]. 化工学报, 2023, 74(2): 487-499. |
| [14] | 苏伟怡, 丁佳慧, 李春利, 王洪海, 姜艳军. 酶促反应结晶研究进展[J]. 化工学报, 2023, 74(2): 617-629. |
| [15] | 周璇, 李孟亚, 孙杰, 岑振凯, 吕强三, 周立山, 王海涛, 韩丹丹, 龚俊波. 添加剂对氨基酸晶体生长的影响[J]. 化工学报, 2023, 74(2): 500-510. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号