化工学报 ›› 2019, Vol. 70 ›› Issue (12): 4625-4634.DOI: 10.11949/0438-1157.20190395
王志苗1,2( ),张洪起1,周立超1,李芳1,2,薛伟1,2(
),张洪起1,周立超1,李芳1,2,薛伟1,2( ),王延吉1,2(
),王延吉1,2( )
)
收稿日期:2019-04-16
修回日期:2019-08-23
出版日期:2019-12-05
发布日期:2019-12-05
通讯作者:
薛伟,王延吉
作者简介:王志苗(1985—),女,硕士,实验师,基金资助:
Zhimiao WANG1,2( ),Hongqi ZHANG1,Lichao ZHOU1,Fang LI1,2,Wei XUE1,2(
),Hongqi ZHANG1,Lichao ZHOU1,Fang LI1,2,Wei XUE1,2( ),Yanji WANG1,2(
),Yanji WANG1,2( )
)
Received:2019-04-16
Revised:2019-08-23
Online:2019-12-05
Published:2019-12-05
Contact:
Wei XUE,Yanji WANG
摘要:
采用微乳液法制备了Ce为助剂的Pd-Ce-O/SiO2催化剂,用于苯酚氧化羰基化合成碳酸二苯酯(DPC)反应。活性评价结果显示,催化剂性能随着Ce用量的增加而提高,当Ce/Pd摩尔比为10/1时,苯酚转化率为64.4%,碳酸二苯酯选择性为83.4%。利用XRD表征发现,部分Ce4+进入到PdO晶格中,使得失活Pd原子中的电子更容易向Ce转移,从而易于再生,表现出更好的催化性能。根据上述结果,设计制备了Pd-O/CeO2催化剂用于本反应,苯酚转化率和碳酸二苯酯选择性分别仅为24.0%和23.3%。表征发现,在Pd-Ce-O/SiO2催化剂表面,Pd物种主要是PdO,而Pd-O/CeO2表面的Pd物种则以PdO2为主。由于苯酚氧化羰基化反应的活性中心为Pd(Ⅱ),所以Pd-O/CeO2催化性能较差。并且,由于Pd与CeO2之间存在强相互作用,催化剂表面Pd含量较低,这也是Pd-O/CeO2催化活性较差的原因之一。
中图分类号:
王志苗, 张洪起, 周立超, 李芳, 薛伟, 王延吉. Ce在负载Pd催化苯酚氧化羰基化合成碳酸二苯酯反应中的作用[J]. 化工学报, 2019, 70(12): 4625-4634.
Zhimiao WANG, Hongqi ZHANG, Lichao ZHOU, Fang LI, Wei XUE, Yanji WANG. Role of Ce in supported Pd catalyst for oxidative carbonylation of phenol to diphenyl carbonate[J]. CIESC Journal, 2019, 70(12): 4625-4634.

图1 Pd-M-O/SiO2催化苯酚氧化羰基化反应性能(M=Co、Mn、Cu、Ce, M/Pd摩尔比为10/1)
Fig.1 Catalytic performance of Pd-M-O/SiO2 for oxidative carbonylation of phenol(reaction conditions: 100℃, 6.6 MPa, CO/O2=10/1, others were listed in Section 1.4)
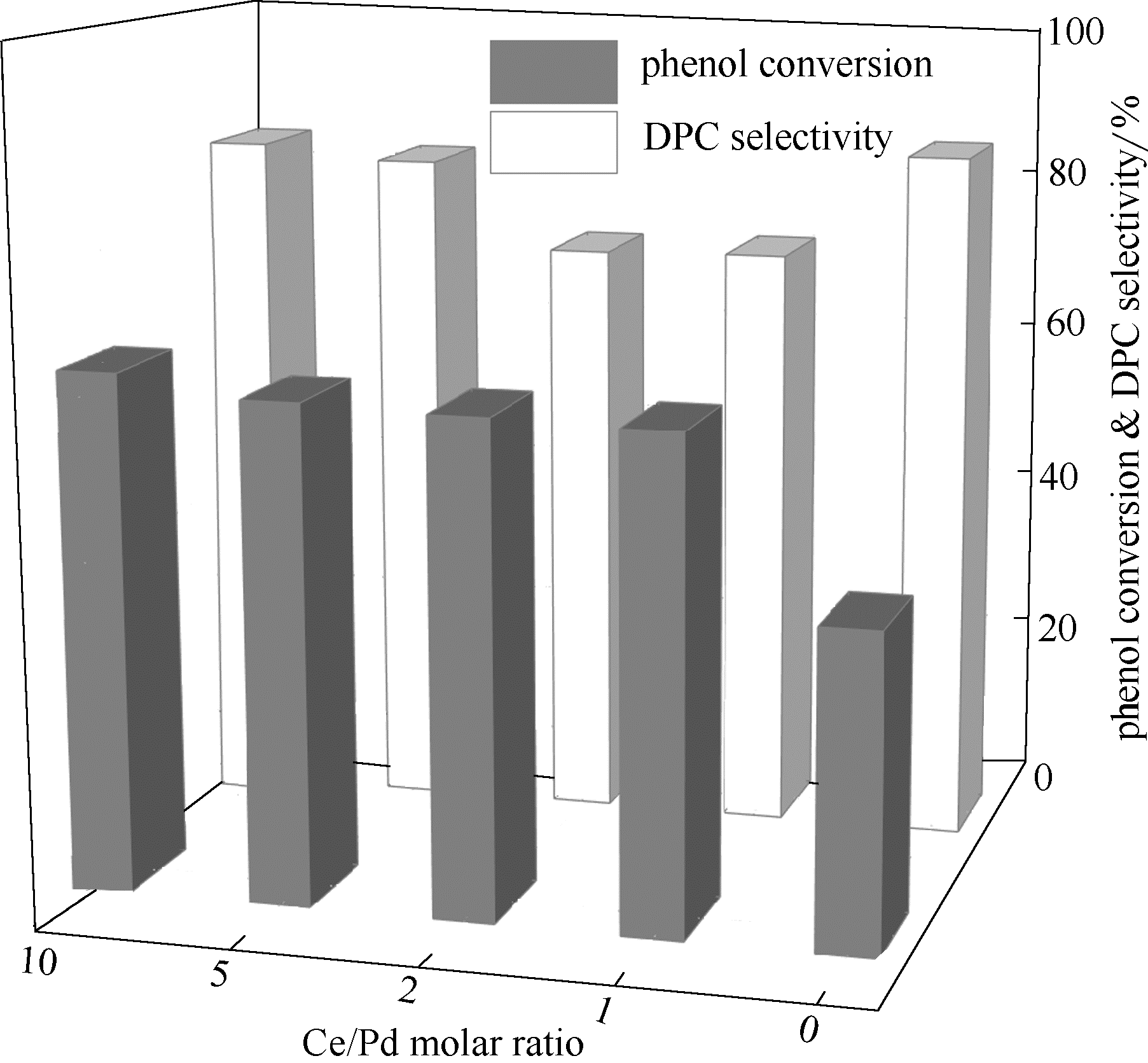
图2 Ce/Pd摩尔比对Pd-Ce-O/SiO2催化苯酚氧化羰基化反应的影响
Fig.2 Effect of Ce/Pd molar ratio on oxidative carbonylation of phenol over Pd-Ce-O/SiO2(reaction conditions: 100℃, 6.6 MPa, CO/O2=10/1, others were listed in Section 1.4)
| Pd-Ce-O/SiO2催化剂中Ce/Pd摩尔比 | PdO(101)晶面间距 / nm | 晶格参数 / nm | ||
|---|---|---|---|---|
| a | b | c | ||
| 不加Ce | 0.26032 | 0.30330 | 0.30330 | 0.53849 |
| 1∶1 | 0.26283 | 0.30413 | 0.30413 | 0.53347 |
| 2∶1 | 0.26360 | 0.30392 | 0.30392 | 0.53595 |
| 5∶1 | 0.26404 | 0.30423 | 0.30423 | 0.53322 |
| 10∶1 | 0.26572 | 0.30398 | 0.30398 | 0.54006 |
表1 具有不同Ce/Pd摩尔比Pd-Ce-O/SiO2催化剂中PdO(101)晶面间距和晶格参数 with different Ce/Pd molar ratio
Table 1 Interplanar spacing and lattice parameters of PdO(101) in Pd-Ce-O/SiO2 catalyst with different Ce/Pd molar ratio
| Pd-Ce-O/SiO2催化剂中Ce/Pd摩尔比 | PdO(101)晶面间距 / nm | 晶格参数 / nm | ||
|---|---|---|---|---|
| a | b | c | ||
| 不加Ce | 0.26032 | 0.30330 | 0.30330 | 0.53849 |
| 1∶1 | 0.26283 | 0.30413 | 0.30413 | 0.53347 |
| 2∶1 | 0.26360 | 0.30392 | 0.30392 | 0.53595 |
| 5∶1 | 0.26404 | 0.30423 | 0.30423 | 0.53322 |
| 10∶1 | 0.26572 | 0.30398 | 0.30398 | 0.54006 |
| 催化剂 | Pd 含量/% (质量) | 比表 面积/(m2/g) | 催化反应性能② | ||
|---|---|---|---|---|---|
| XPS | 理论含量 | 苯酚转化率/% | DPC选择性/% | ||
| Pd-O/CeO2 | 0.58 | 1.0 | 14.5 | 24.0 | 23.3 |
| Pd-O/SiO2 | 0.90 | 1.0 | 37.1 | 38.4 | 85.4 |
| Pd-Ce-O/SiO2① | 0.74 | 1.0 | 49.6 | 64.4 | 83.4 |
表2 Pd基催化剂物理化学性质及催化苯酚氧化羰基化反应性能 for oxidative carbonylation of phenol
Table 2 Physicochemical properties of Pd-based catalysts and their catalytic performance for oxidative carbonylation of phenol
| 催化剂 | Pd 含量/% (质量) | 比表 面积/(m2/g) | 催化反应性能② | ||
|---|---|---|---|---|---|
| XPS | 理论含量 | 苯酚转化率/% | DPC选择性/% | ||
| Pd-O/CeO2 | 0.58 | 1.0 | 14.5 | 24.0 | 23.3 |
| Pd-O/SiO2 | 0.90 | 1.0 | 37.1 | 38.4 | 85.4 |
| Pd-Ce-O/SiO2① | 0.74 | 1.0 | 49.6 | 64.4 | 83.4 |
| 1 | 崔小明. 国内外聚碳酸酯的供需现状及发展前景分析[J]. 石油化工技术与经济, 2017, 33(1): 18-23. |
| Cui X M. Supply and demand status of polycarbonate at home and abroad and its development prospect analysis[J]. Technology & Economics in Petrochemicals, 2017, 33(1): 18-23. | |
| 2 | Gong J L, Ma X B, Wang S P. Phosgene-free approaches to catalytic synthesis of diphenyl carbonate and its intermediates[J]. Applied Catalysis A: General, 2007, 316: 1. |
| 3 | Figueiredo M C, Trieu V, Eiden S, et al. Spectroscopic investigation of the electrosynthesis of diphenyl carbonate from CO and phenol on gold electrodes[J]. ACS Catalysis, 2018, 8(4): 3087-3090. |
| 4 | 王延吉, 赵新强. 绿色催化过程与工艺[M]. 2版. 北京: 化学工业出版社, 2015. |
| Wang Y J, Zhao X Q. Green Catalytic Process and Technology [M]. 2nd ed. Beijing: Chemical Industry Press, 2015. | |
| 5 | 付嫱, 欧阳春, 曾毅, 等. 甲基苯基碳酸酯歧化反应研究进展[J]. 化工进展, 2017, 36(8): 2748-2755. |
| Fu Q, Ouyang C, Zeng Y, et al. Progresses in the research on disproportionation of methyl phenyl carbonate[J]. Chemical Industry and Engineering Progress, 2017, 36(8): 2748-2755. | |
| 6 | Tang R Z, Chen T, Chen Y, et al. Core-shell TiO2@SiO2 catalyst for transesterification of dimethyl carbonate and phenol to diphenyl carbonate[J]. Chinese Journal of Catalysis, 2014, 35(4): 457-461. |
| 7 | Yang X, Ma X B, Wang S P, et al. Transesterification of dimethyl oxalate with phenol over TiO2/SiO2: catalyst screening and reaction optimization[J]. AIChE Journal, 2008, 54(12): 3260-3272. |
| 8 | Yin X, Zeng Y, Yao J, et al. Kinetic modeling of the transesterification reaction of dimethyl carbonate and phenol in the reactive distillation reactor[J]. Industrial & Engineering Chemistry Research, 2014, 53(49): 19087-19093. |
| 9 | 薛伟, 张敬畅, 王延吉, 等. 新型超细包覆型催化剂的制备及催化苯酚氧化羰基化合成碳酸二苯酯[J]. 化工学报, 2004, 55(12): 2076-2081. |
| Xue W, Zhang J C, Wang Y J, et al. Preparation of novel ultrafine embedded catalyst for oxidative carbonylation of phenol to diphenyl carbonate[J]. Journal of Chemical Industry and Engineering (China), 2004, 55(12): 2076-2081. | |
| 10 | Lu W, Du Z P, Yuan H, et al. Synthesis of diphenyl carbonate over the magnetic catalysts Pd/La1-xPbxMnO3 (x= 0.2—0.7) [J]. Chinese Journal of Chemical Engineering, 2013, 21: 8-13. |
| 11 | Ronchin L, Vavasori A, Amadio E, et al. Oxidative carbonylation of phenols catalyzed by homogeneous and heterogeneous Pd precursors[J]. Journal of Molecular Catalysis A: Chemical, 2009, 298: 23-30. |
| 12 | Yang X J, Han J Y, Du Z P, et al. Effects of Pb dopant on structure and activity of Pd/K-OMS-2 catalysts for heterogeneous oxidative carbonylation of phenol[J]. Catalysis Communications, 2010, 11: 643-646. |
| 13 | Zhang Y L, Xiang S L, Wang G Q, et al. Preparation and application of coconut shell activated carbon immobilized palladium complexes[J]. Catalysis Science & Technology, 2014, 4: 1055-1063. |
| 14 | Yin C F, Zhou J, Chen Q M, et al. Deactivation causes of supported palladium catalysts for the oxidative carbonylation of phenol[J]. Journal of Molecular Catalysis A: Chemical, 2016, 424: 377-383. |
| 15 | Vavasori A, Toniolo L. Multistep electron-transfer catalytic system for the oxidative carbonylation of phenol to diphenyl carbonate[J]. Journal of Molecular Catalysis A: Chem., 1998, 139(2/3): 109-119. |
| 16 | Xue W, Zhang J C, Wang Y J, et al. Effect of promoter copper on the oxidative carbonylation of phenol over the ultrafine embedded catalyst Pd-Cu-O/SiO2[J]. Journal of Molecular Catalysis A: Chemical, 2005, 232(1/2): 77-81. |
| 17 | Liang Y H, Guo H X, Chen H P, et al. Effect of doping cerium in the support of catalyst Pd-Co/Cu-Co-Mn mixed oxides on the oxidative carbonylation of phenol[J]. Chinese Journal of Chemical Engineering, 2009, 17(3): 401-406. |
| 18 | 张光旭, 吴元欣, 马沛生, 等. 非均相催化一步合成碳酸二苯酯 (Ⅸ): Ce的添加方法对Pd-Sn催化剂性能的影响[J]. 化工学报, 2005, 56(1): 82-87. |
| Zhang G X, Wu Y X, Ma P S, et al. Direct synthesis of diphenyl carbonate with heterogeneous catalysis reaction(Ⅸ): Effect of Ce loading methods on catalytic activity of catalyst[J]. Journal of Chemical Industry and Engineering (China), 2005, 56(1): 82-87. | |
| 19 | Yang X J, Yin C F, Han J Y, et al. Effect of oxygen species on the liquid product distribution in the oxidative carbonylation of phenol over Pd/M-OMS-2 catalysts[J]. Reac. Kinet. Mech. Cat., 2016, 117: 269-281. |
| 20 | Spezzati G, Su Y Q, Hofmann J P, et al. Atomically dispersed Pd-O species on CeO2(111) as highly active sites for low-temperature CO oxidation[J]. ACS Catalysis, 2017, 7: 6887-6891. |
| 21 | 刘逸锋, 沈本贤, 皮志鹏, 等. CeO2表面氧化转移FCC烟气中SO2的反应过程[J]. 化工学报, 2016, 67(12): 5015-5023. |
| Liu Y F, Shen B X, Pi Z P, et al. Oxidation transferring mechanism of SO2 in FCC flue gas over CeO2 surface[J]. CIESC Journal, 2016, 67(12): 5015-5023. | |
| 22 | 袁烨, 王志苗, 安华良, 等. Pd-O/CeO2纳米管催化苯酚氧化羰基化反应[J]. 催化学报, 2015, 36(7): 1142-1152. |
| Yuan Y, Wang Z M, An H L, et al. Oxidative carbonylation of phenol with a Pd-O/CeO2-nanotube catalyst[J]. Chinese Journal of Catalysis, 2015, 36(7): 1142-1152. | |
| 23 | Luo J Y, Meng M, Xian H, et al. The nanomorphology-controlled palladium-support interaction and the catalytic performance of Pd/CeO2 catalysts[J]. Catalysis Letters, 2009, 133: 328-333. |
| 24 | Brun M, Berthet A, Bertolini J C. XPS, AES and Auger parameter of Pd and PdO[J]. Journal of Electron Spectroscopy and Related Phenomena, 1999, 104: 55-60. |
| 25 | Wu G W, Wu Y X, Ma P S, et al. Direct synthesis of diphenyl carbonate over heterogeneous catalyst: effects of structure of substituted perovskite carrier on the catalyst activities[J]. Frontiers of Chemical Science and Engineering in China, 2007, 1(1): 59-64. |
| 26 | 张珍容, 张世英, 万隆, 等. Pd/Ce-HMS介孔材料的结构和表面化学态[J]. 化工学报, 2007, 58(3): 776-780. |
| Zhang Z R, Zhang S Y, Wan L, et al. Structure and surface chemical state of Pd/Ce containing hexagonal mesoporous silicas[J]. Journal of Chemical Industry and Engineering (China), 2007, 58(3): 776-780. | |
| 27 | Guimaraes A L, Dieguez D C, Schmal M. The effect of precursors salts on surface state of Pd/Al2O3 and Pd/CeO2/Al2O3 catalysts[J]. Annals of the Brazilian Academy of Sciences, 2004, 76(4): 825-832. |
| 28 | Luo M F, Hou Z Y, Yuan X X, et al. Characterization study of CeO2 supported Pd catalyst for low temperature carbon monoxide oxidation[J]. Catalysis Letters, 1998, 50(3/4): 205-209. |
| 29 | Sanchez M G, Gazquez J L. Oxygen vacancy model in strong metal-support interaction[J]. Journal of Catalysis, 1987, 104(1): 120-135. |
| 30 | 杨成, 任杰, 孙予罕. CeO2和La2O3改性Pd/r-Al2O3甲醇低温分解催化剂的研究(Ⅰ): CeO2改性Pd/γ-Al2O3催化剂的结构和性能[J]. 催化学报, 2001, 22(3): 283-286. |
| Yang C, Ren J, Sun Y H. Study of CeO2- and La2O3-Modified Pd/γ-Al2O3 catalyst for methanol decomposition at low temperature(Ⅰ): Structure and properties of CeO2-modified Pd/γ-Al2O3 catalyst[J]. Chinese Journal of Catalysis, 2001, 22(3): 283-286. | |
| 31 | Zhu H Q, Qin Z F, Shan W J, et al. Pd/CeO2-TiO2 catalyst for CO oxidation at low temperature: a TPR study with H2 and CO as reducing agents[J]. Journal of Catalysis, 2004, 225(2): 267-277. |
| 32 | Cargnello M, Doan-Nguyen V V T, Gordon T R, et al. Control of metal nanocrystal size reveal metal-support interface role for ceria catalysts[J]. Science, 2013, 341(6147): 771-773. |
| 33 | Boronin A I, Slavinskaya E M, Danilova I G, et al. Investigation of palladium interaction with cerium oxide and its state in catalysts for low-temperature CO oxidation[J]. Catalysis Today, 2009, 144: 201-211. |
| 34 | Wang Z, Qu Z P, Quan X, et al. Selective catalytic oxidation of ammonia to nitrogen over CuO-CeO2 mixedoxides prepared by surfactant-templated method[J]. Applied Catalysis B: Environmental, 2013, 134: 153-166. |
| [1] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [2] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [5] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [6] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [7] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [8] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [9] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [10] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [11] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [12] | 朱风, 陈凯琳, 黄小凤, 鲍银珠, 李文斌, 刘嘉鑫, 吴玮强, 高王伟. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| [13] | 王辰, 史秀锋, 武鲜凤, 魏方佳, 张昊虹, 车寅, 吴旭. 氧化还原法制备Mn3O4催化剂及其甲苯催化氧化性能与机理研究[J]. 化工学报, 2023, 74(6): 2447-2457. |
| [14] | 李勇, 高佳琦, 杜超, 赵亚丽, 李伯琼, 申倩倩, 贾虎生, 薛晋波. Ni@C@TiO2核壳双重异质结的构筑及光热催化分解水产氢[J]. 化工学报, 2023, 74(6): 2458-2467. |
| [15] | 周继鹏, 何文军, 李涛. 异形催化剂上乙烯催化氧化失活动力学反应工程计算[J]. 化工学报, 2023, 74(6): 2416-2426. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号