化工学报 ›› 2023, Vol. 74 ›› Issue (7): 2717-2734.DOI: 10.11949/0438-1157.20230193
涂玉明( ), 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗(
), 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗( )
)
收稿日期:2023-03-06
修回日期:2023-07-05
出版日期:2023-07-05
发布日期:2023-08-31
通讯作者:
任钟旗
作者简介:涂玉明(1995—),男,博士研究生,tuyumingbuct@yeah.net
基金资助:
Yuming TU( ), Gaoyan SHAO, Jianjie CHEN, Feng LIU, Shichao TIAN, Zhiyong ZHOU, Zhongqi REN(
), Gaoyan SHAO, Jianjie CHEN, Feng LIU, Shichao TIAN, Zhiyong ZHOU, Zhongqi REN( )
)
Received:2023-03-06
Revised:2023-07-05
Online:2023-07-05
Published:2023-08-31
Contact:
Zhongqi REN
摘要:
钙基催化剂是以钙氧化物为金属活性位点的固体碱催化剂,具有绿色高效、廉价易得等优点,有较好的催化性能,可广泛应用于生物柴油制备、废水处理和焦油裂解重整等领域,已受到国内外研究者们越来越广泛的关注。主要介绍了钙基催化剂类型、应用研究现状及作用机理,概述了其在各个领域的研究进展,分析归纳了钙基催化剂设计合成思路的历程和趋势:从CaO、Ca(OH)2等天然钙基催化剂到主动设计合成的负载型含Ca催化剂,指出了稳定高效钙基催化剂的设计合成是其实现进一步发展的关键。最后对钙基催化剂研究进展进行归纳总结,并对其未来发展及应用前景进行了展望。
中图分类号:
涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734.
Yuming TU, Gaoyan SHAO, Jianjie CHEN, Feng LIU, Shichao TIAN, Zhiyong ZHOU, Zhongqi REN. Advances in the design, synthesis and application of calcium-based catalysts[J]. CIESC Journal, 2023, 74(7): 2717-2734.

图1 A-SBC、30Ca/A-SBC- 600、30Ca/A-SBC-700、30Ca/A-SBC-800、20Ca/A-SBC-700、40Ca/A-SBC-700的XRD谱图[28]
Fig.1 XRD patterns of A-SBC, 30Ca/A-SBC- 600, 30Ca/A-SBC-700, 30Ca/A-SBC-800, 20Ca/A-SBC-700 and 40Ca/A-SBC-700[28]
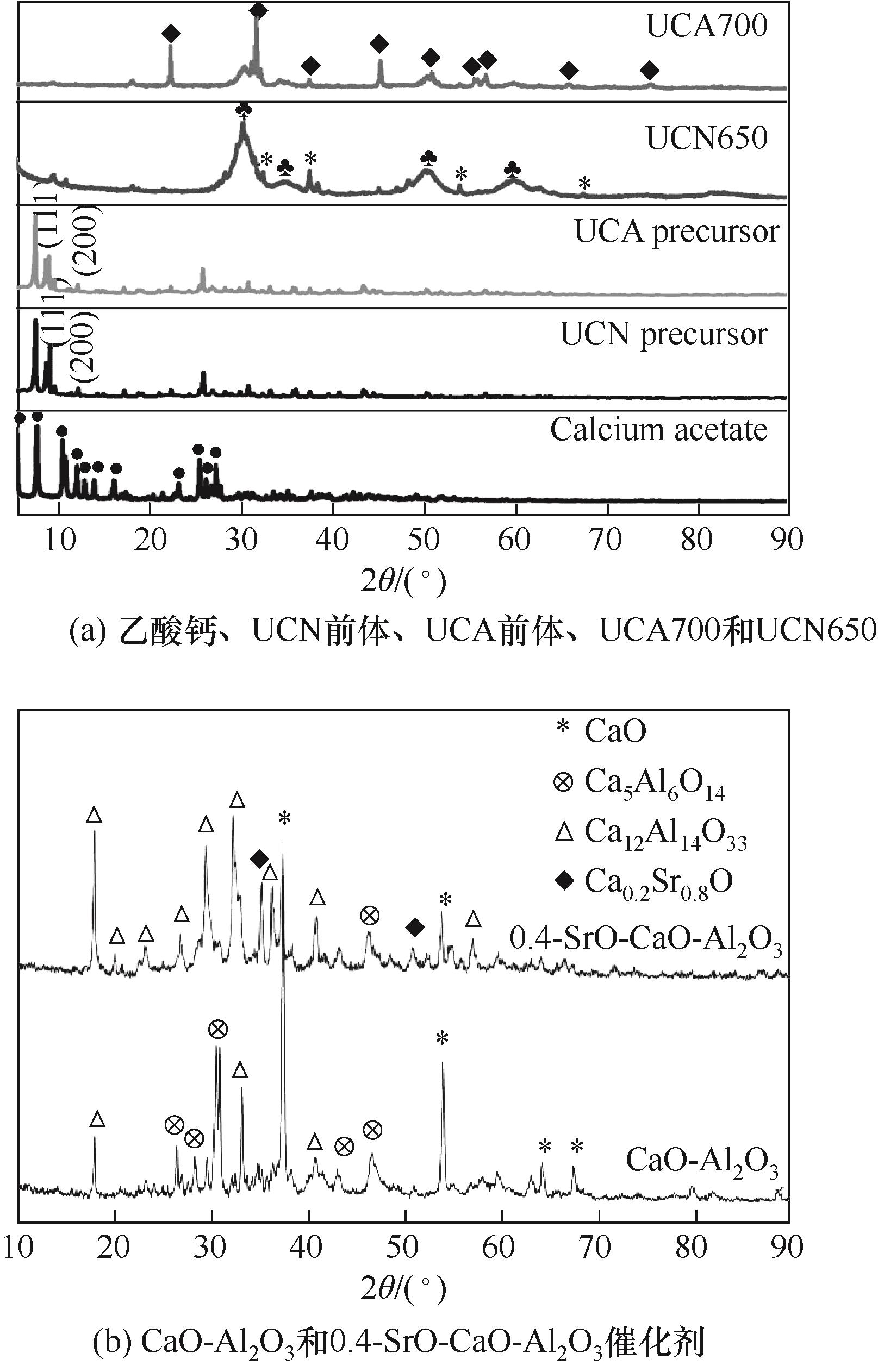
图3 乙酸钙、UCN前体、UCA前体、UCA700和UCN650(UCN、UCA分别代表在氮气和空气气氛中活化)(CaZrO3, Ca x Zr y O x+2y )[29], CaO-Al2O3和0.4-SrO-CaO-Al2O3催化剂的XRD谱图[31]
Fig.3 XRD patterns of calcium acetate, UCN precursor, UCA precursor, UCA700 and UCN650 (UCN, UCA represent activation in nitrogen and air atmosphere, respectively) (CaZrO3, Ca x Zr y O x+2y )[29], CaO-Al2O3 and 0.4-SrO-CaO-Al2O3 catalysts[31]
| 催化剂 | 转化率/% | 稳定性 |
|---|---|---|
| CaO[ | 87 | 多次使用后下降了30% |
| CaO/SBC[ | 93.4 | 多次使用后下降了9% |
| CaO-ZrO2[ | 97 | 使用3次后保持在93%以上 |
| CaO-MgO[ | 98.4 | 使用5次后保持在93%以上 |
| Ca/APB[ | 81.6 | 使用10次后基本保持稳定 |
| CaP-600[ | 99 | 微量浸出 |
表1 不同催化剂在生物柴油合成过程中的效果对比
Table 1 Comparison of the effects of different catalysts in the biodiesel synthesis process
| 催化剂 | 转化率/% | 稳定性 |
|---|---|---|
| CaO[ | 87 | 多次使用后下降了30% |
| CaO/SBC[ | 93.4 | 多次使用后下降了9% |
| CaO-ZrO2[ | 97 | 使用3次后保持在93%以上 |
| CaO-MgO[ | 98.4 | 使用5次后保持在93%以上 |
| Ca/APB[ | 81.6 | 使用10次后基本保持稳定 |
| CaP-600[ | 99 | 微量浸出 |
| 催化剂 | 废水类型 | COD/ (mg·L-1) | COD 去 除率/% |
|---|---|---|---|
| 煤基活性炭[ | 煤化工废水生化出水 | 283.8 | 32.16 |
| AC[ | 纺织废水 | 2570 | 50 |
| FeO x /AC[ | 炼油厂废水 | 54.6~256 | 40 |
| γ-Al2O3[ | 石化废水 | 750 | 46 |
| CuCo/CAFNi[ | 煤气化废水 | 70~80 | 60~65 |
| Mn x Ce1-x /γ-Al2O3[ | 焦化废水 | 150 | 45.6 |
| Al2O3-PDA-Ca x O y[ | 石化废水(生化出水) | 140~160 | 62 |
| Ca-C/Al2O3[ | 化工园区废水(生化出水) | 100~129 | 65 |
| Al2O3-PDA-SA-Ca x O y[ | 化工园区废水(生化出水) | 100~129 | 66 |
表2 不同催化剂处理实际废水的效果对比
Table 2 Comparison of the effectiveness of different catalysts in treating actual wastewater
| 催化剂 | 废水类型 | COD/ (mg·L-1) | COD 去 除率/% |
|---|---|---|---|
| 煤基活性炭[ | 煤化工废水生化出水 | 283.8 | 32.16 |
| AC[ | 纺织废水 | 2570 | 50 |
| FeO x /AC[ | 炼油厂废水 | 54.6~256 | 40 |
| γ-Al2O3[ | 石化废水 | 750 | 46 |
| CuCo/CAFNi[ | 煤气化废水 | 70~80 | 60~65 |
| Mn x Ce1-x /γ-Al2O3[ | 焦化废水 | 150 | 45.6 |
| Al2O3-PDA-Ca x O y[ | 石化废水(生化出水) | 140~160 | 62 |
| Ca-C/Al2O3[ | 化工园区废水(生化出水) | 100~129 | 65 |
| Al2O3-PDA-SA-Ca x O y[ | 化工园区废水(生化出水) | 100~129 | 66 |
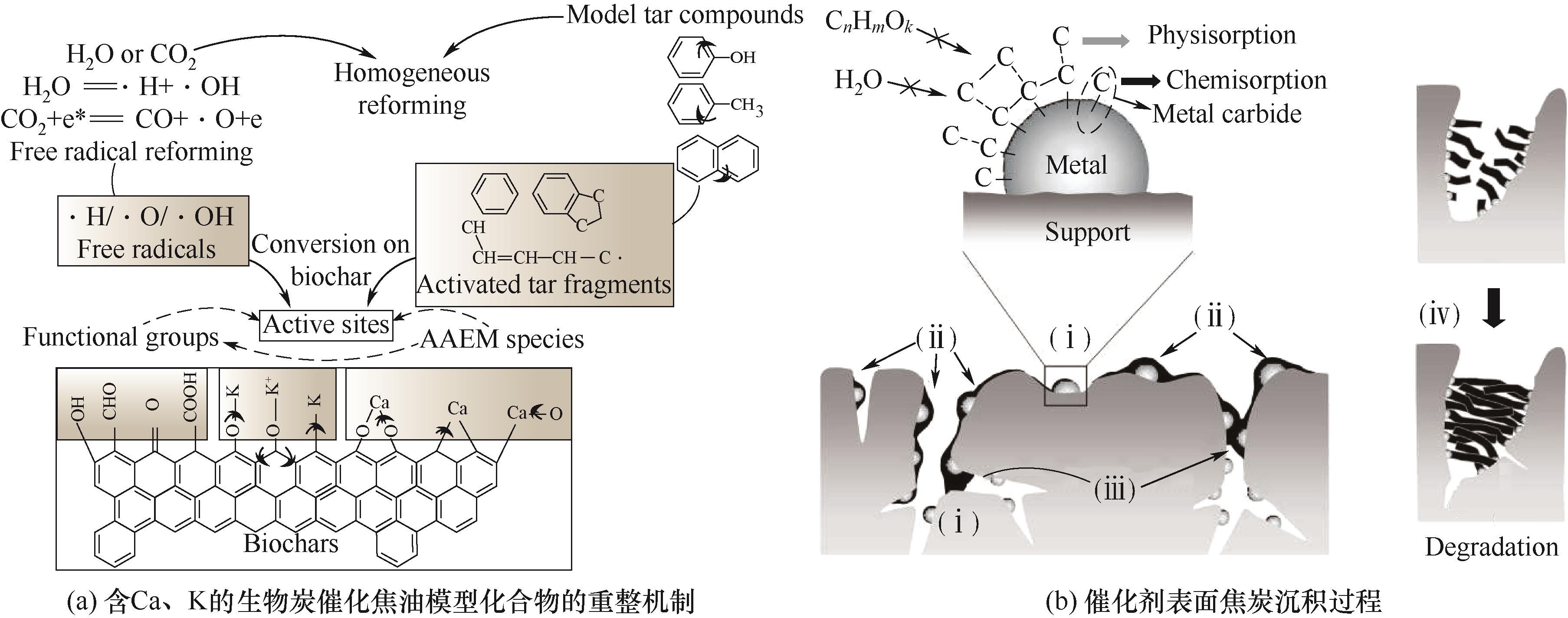
图10 含Ca、K的生物炭催化焦油模型化合物的重整机制(a)[70];催化剂表面焦炭沉积过程(b)[72](碳在催化活性表面颗粒上的化学吸附(ⅰ),焦炭包裹无机分子(ⅱ),焦炭沉积阻塞多孔结构(ⅲ),焦炭沉积导致的催化剂结构退化(ⅳ))
Fig.10 Mechanism of Ca- and K-containing biochar-catalyzed reformation of tar model compounds (a)[70]; Catalyst surface coke deposition process (b)[72]: chemisorption of carbon on catalytically active surface particles (ⅰ), coke encapsulation of inorganic molecules (ⅱ), coke deposition blocking porous structures (ⅲ), and catalyst structural degradation due to coke deposition (ⅳ)

图11 Ca基催化剂效果对比(a);Ca基催化剂的XRD谱图(b);反应机制示意图(c)[74]
Fig.11 Comparison of the effect of Ca-based catalysts (a); XRD pattern of Ca-based catalysts (b); Schematic representation of the reaction mechanism (c)[74]
| 催化剂 | 反应体系 | 反应温度/℃ | 转化率/% | H2摩尔分数/% |
|---|---|---|---|---|
| 白云石颗粒[ | 模拟焦油(乙酸、苯) | 850 | 99.8、18.7 | — |
| 白云石焙烧物[ | 生物质焦油 | 900 | 95.14 | — |
| Ca-K/生物炭[ | 模拟焦油(甲苯、萘) | 800 | 86~100 | 62 |
| Ca/CPC[ | 模拟焦油(甲苯) | 900 | 94.4 | 68.5 |
| Fe6Al4Ca1[ | 煤焦油 | 900 | 90 | 50 |
| Ni/生物炭[ | 生物质焦油 | 800 | 96.5 | — |
| Fe/生物炭[ | 生物质焦油 | 800 | 92.6 | 39.88 |
| Cu/生物炭[ | 生物质焦油 | 800 | 90.6 | — |
表3 不同催化剂在焦油裂解重整过程中的效果对比
Table 3 Comparison of the effects of different catalysts in tar cracking reforming process
| 催化剂 | 反应体系 | 反应温度/℃ | 转化率/% | H2摩尔分数/% |
|---|---|---|---|---|
| 白云石颗粒[ | 模拟焦油(乙酸、苯) | 850 | 99.8、18.7 | — |
| 白云石焙烧物[ | 生物质焦油 | 900 | 95.14 | — |
| Ca-K/生物炭[ | 模拟焦油(甲苯、萘) | 800 | 86~100 | 62 |
| Ca/CPC[ | 模拟焦油(甲苯) | 900 | 94.4 | 68.5 |
| Fe6Al4Ca1[ | 煤焦油 | 900 | 90 | 50 |
| Ni/生物炭[ | 生物质焦油 | 800 | 96.5 | — |
| Fe/生物炭[ | 生物质焦油 | 800 | 92.6 | 39.88 |
| Cu/生物炭[ | 生物质焦油 | 800 | 90.6 | — |
| 1 | Li X H, Jia Y S, Chen D H, et al. Study of bifunctional calcium-based catalysts for PCDD/Fs removal in medical waste pyrolysis-combustion process[J]. Chemical Engineering and Processing - Process Intensification, 2023, 188: 109377. |
| 2 | Adepoju T F, Ibeh M A, Udoetuk E N, et al. Quaternary blend of Carica papaya - Citrus sinesis - Hibiscus sabdariffa - waste used oil for biodiesel synthesis using CaO-based catalyst derived from binary mix of Lattorina littorea and Mactra coralline shell[J]. Renewable Energy, 2021, 171: 22-33. |
| 3 | 林启睿, 许增栋, 吴素芳. 纳米钙基吸附剂脱碳强化生物乙醇蒸汽重整制氢工艺[J]. 高校化学工程学报, 2018, 32(1): 163-169. |
| Lin Q R, Xu Z D, Wu S F. Hydrogen production via sorption enhanced steam reforming of bio-ethanol using nano Ca-based adsorbents[J]. Journal of Chemical Engineering of Chinese Universities, 2018, 32(1): 163-169. | |
| 4 | 张德静, 常飞, 薛伟, 等. 天然钙基催化剂在制备生物柴油中的研究进展[J]. 天然气化工(C1化学与化工), 2013, 38(2): 89-94. |
| Zhang D J, Chang F, Xue W, et al. Application of natural calcium-based catalysts in synthesis of biodiesel: a review[J]. Natural Gas Chemical Industry, 2013, 38(2): 89-94. | |
| 5 | Tanpure S, Ghanwat V, Shinde B, et al. The eggshell waste transformed green and efficient synthesis of K-Ca (OH)2 catalyst for room temperature synthesis of chalcones[J]. Polycyclic Aromatic Compounds, 2022, 42(4): 1322-1340. |
| 6 | Kouzu M, Kasuno T, Tajika M, et al. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production[J]. Fuel, 2008, 87(12): 2798-2806. |
| 7 | Wei Z K, Xu C L, Li B X. Application of waste eggshell as low-cost solid catalyst for biodiesel production[J]. Bioresource Technology, 2009, 100(11): 2883-2885. |
| 8 | 黄继明, 刘润清, 韦恩光, 等. CaO/MgO复合固体碱催化剂催化降解PET[J]. 工程塑料应用, 2018, 46(7): 46-50. |
| Huang J M, Liu R Q, Wei E G, et al. Degradation behaviors of PET catalyzed by CaO/MgO composite as solid base catalyst[J]. Engineering Plastics Application, 2018, 46(7): 46-50. | |
| 9 | 付希, 王宜涛, 张月, 等. CaO-ZnO固体碱催化剂的制备及其催化大豆油酯交换反应的性能[J]. 石油化工, 2017, 46(5): 552-557. |
| Fu X, Wang Y T, Zhang Y, et al. Preparation of CaO-ZnO solid base catalysts and their catalytic performances for transesterification of soybean oil[J]. Petrochemical Technology, 2017, 46 (5): 552-557. | |
| 10 | Chang Y H, Tseng W C, Kaun C C, et al. Mg2Fe2O5 nanoparticle-decorated Ca2Fe2O5-CaFe2O4 heterostructure for efficient photocatalytic CO2 conversion[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(38): 12651-12658. |
| 11 | Kesić Ž, Lukić I, Zdujić M, et al. Assessment of CaTiO3, CaMnO3, CaZrO3 and Ca2Fe2O5 perovskites as heterogeneous base catalysts for biodiesel synthesis[J]. Fuel Processing Technology, 2016, 143: 162-168. |
| 12 | Bihanic C, Diliberto S, Pelissier F, et al. Eco-CaMnO x : a greener generation of eco-catalysts for eco-friendly oxidation processes[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(10): 4044-4057. |
| 13 | Kesserwan F, Ahmad M N, Khalil M, et al. Hybrid CaO/Al2O3 aerogel as heterogeneous catalyst for biodiesel production[J]. Chemical Engineering Journal, 2020, 385: 123834. |
| 14 | Sun H, Sun K, Wang F, et al. Catalytic self-activation of Ca-doped coconut shell for in situ synthesis of hierarchical porous carbon supported CaO transesterification catalyst[J]. Fuel, 2021, 285: 119192. |
| 15 | Lin Z Y, Huang H, Cheng L, et al. Tuning the p-orbital electron structure of s-block metal Ca enables a high-performance electrocatalyst for oxygen reduction[J]. Advanced Materials, 2021, 33(51): 2107103. |
| 16 | Tu Y M, Chen J J, Shao G Y, et al. Preparation and application of green calcium-based catalyst for advanced treatment of salty wastewater with ozone[J]. Journal of Cleaner Production, 2022, 362: 132464. |
| 17 | Chen J J, Tu Y M, Shao G Y, et al. Catalytic ozonation performance of calcium-loaded catalyst (Ca-C/Al2O3) for effective treatment of high salt organic wastewater[J]. Separation and Purification Technology, 2022, 301: 121937. |
| 18 | Shao G Y, Zhou Z Y, Tu Y M, et al. Calcium-based catalyst for ozone catalytic oxidation for advanced treatment of high salt organic wastewater[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 654: 130149. |
| 19 | Simsek S, Uslu S. Comparative evaluation of the influence of waste vegetable oil and waste animal oil-based biodiesel on diesel engine performance and emissions[J]. Fuel, 2020, 280: 118613. |
| 20 | Costa M J, Silva M R L, Ferreira E E A, et al. Enzymatic biodiesel production by hydroesterification using waste cooking oil as feedstock[J]. Chemical Engineering and Processing - Process Intensification, 2020, 157: 108131. |
| 21 | Jung J M, Oh J I, Kim J G, et al. Valorization of sewage sludge via non-catalytic transesterification[J]. Environment International, 2019, 131: 105035. |
| 22 | Yaşar F. Biodiesel production via waste eggshell as a low-cost heterogeneous catalyst: its effects on some critical fuel properties and comparison with CaO[J]. Fuel, 2019, 255: 115828. |
| 23 | Navas M B, Ruggera J F, Lick I D, et al. A sustainable process for biodiesel production using Zn/Mg oxidic species as active, selective and reusable heterogeneous catalysts[J]. Bioresources and Bioprocessing, 2020, 7(1): 1-13. |
| 24 | Xie W L, Li H T. Alumina-supported potassium iodide as a heterogeneous catalyst for biodiesel production from soybean oil[J]. Journal of Molecular Catalysis A: Chemical, 2006, 255(1/2): 1-9. |
| 25 | Karpagam R, Rani K, Ashokkumar B, et al. Green energy from Coelastrella sp. M-60: bio-nanoparticles mediated whole biomass transesterification for biodiesel production[J]. Fuel, 2020, 279: 118490. |
| 26 | Bedir Ö, Doğan T H. Comparison of catalytic activities of Ca-based catalysts from waste in biodiesel production[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2021, DOI: 10.1080/15567036.2021.1883159 . |
| 27 | Samart C, Chaiya C Y, Reubroycharoen P. Biodiesel production by methanolysis of soybean oil using calcium supported on mesoporous silica catalyst[J]. Energy Conversion and Management, 2010, 51(7): 1428-1431. |
| 28 | Wang Y Z, Li D N, Zhao D D, et al. Calcium-loaded municipal sludge-biochar as an efficient and stable catalyst for biodiesel production from vegetable oil[J]. ACS Omega, 2020, 5(28): 17471-17478. |
| 29 | Li H, Wang Y B, Ma X L, et al. Synthesis of CaO/ZrO2 based catalyst by using UiO-66(Zr) and calcium acetate for biodiesel production[J]. Renewable Energy, 2022, 185: 970-977. |
| 30 | Fan M M, Liu Y L, Zhang P B, et al. Blocky shapes Ca-Mg mixed oxides as a water-resistant catalyst for effective synthesis of biodiesel by transesterification[J]. Fuel Processing Technology, 2016, 149: 163-168. |
| 31 | Zhang Y J, Niu S L, Han K H, et al. Synthesis of the SrO-CaO-Al2O3 trimetallic oxide catalyst for transesterification to produce biodiesel[J]. Renewable Energy, 2021, 168: 981-990. |
| 32 | Wang S X, Shan R, Wang Y Z, et al. Synthesis of calcium materials in biochar matrix as a highly stable catalyst for biodiesel production[J]. Renewable Energy, 2019, 130: 41-49. |
| 33 | Acosta P I, Campedelli R R, Correa E L, et al. Efficient production of biodiesel by using a highly active calcium oxide prepared in presence of pectin as heterogeneous catalyst[J]. Fuel, 2020, 271: 117651. |
| 34 | Stamenković O S, Veljković V B, Todorović Z B, et al. Modeling the kinetics of calcium hydroxide catalyzed methanolysis of sunflower oil[J]. Bioresource Technology, 2010, 101(12): 4423-4430. |
| 35 | Tasić M B, Miladinović M R, Stamenković O S, et al. Kinetic modeling of sunflower oil methanolysis catalyzed by calcium-based catalysts[J]. Chemical Engineering & Technology, 2015, 38(9): 1550-1556. |
| 36 | Ramos M, Soares Dias A P, Teodoro F. Soybean oil ethanolysis over Ca based catalyst. Statistical optimization of reaction conditions[J]. Reaction Kinetics, Mechanisms and Catalysis, 2020, 130(1): 433-445. |
| 37 | Díaz-Muñoz L L, Reynel-Ávila H E, Mendoza-Castillo D I, et al. Optimization of flamboyant-based catalysts functionalized with calcium for fatty acid methyl esters production via transesterification[J]. Fuel, 2021, 302: 121125. |
| 38 | Mazaheri H, Ong H C, Masjuki H H, et al. Rice bran oil based biodiesel production using calcium oxide catalyst derived from Chicoreus brunneus shell[J]. Energy, 2018, 144: 10-19. |
| 39 | Karunadasa K S P, Manoratne C H, Pitawala H M T G A, et al. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in situ high-temperature X-ray powder diffraction[J]. Journal of Physics and Chemistry of Solids, 2019, 134: 21-28. |
| 40 | Suppes G J, Bockwinkel K, Lucas S, et al. Calcium carbonate catalyzed alcoholysis of fats and oils[J]. Journal of the American Oil Chemists' Society, 2001, 78(2): 139-146. |
| 41 | Wang J L, Bai Z Y. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater[J]. Chemical Engineering Journal, 2017, 312: 79-98. |
| 42 | Jia J B, Zhang P Y, Chen L. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures[J]. Applied Catalysis B: Environmental, 2016, 189: 210-218. |
| 43 | Feng X J, Yin C K, Lan H X, et al. Insights into the multiple roles of Ca2+ ion in peroxymonosulfate activation over Mn2O3 for organic pollutants degradation[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106669. |
| 44 | Abou-Elyazed A S, Sun Y, El-Nahas A M, et al. Solvent-free synthesis and characterization of Ca2+-doped UiO-66(Zr) as heterogeneous catalyst for esterification of oleic acid with methanol: a joint experimental and computational study[J]. Materials Today Sustainability, 2022, 18: 100110. |
| 45 | Liao M H, Chen J D, Li L X, et al. Effective degradation of nitrotoluenes in wastewater by heterogeneous catalytic ozonation in the presence of calcium oxide[C]//AIP Conference Proceedings. Hubei, 2017. |
| 46 | Hsu Y C, Chen J H, Yang H C. Calcium enhanced COD removal for the ozonation of phenol solution[J]. Water Research, 2007, 41(1): 71-78. |
| 47 | 全学军, 晏云鹏, 程治良, 等. Ca(OH)2催化臭氧去除废水有机污染物的方法: 103351051A[P]. 2015-04-29. |
| Quan X J, Yan Y P, Cheng Z L, et al. Method for removing organic pollutants in waste water by taking Ca(OH)2 as catalyst of ozone: 103351051A[P]. 2015-04-29. | |
| 48 | Lee W J, Bao Y P, Hu X, et al. Hybrid catalytic ozonation-membrane filtration process with CeO x and MnO x impregnated catalytic ceramic membranes for micropollutants degradation[J]. Chemical Engineering Journal, 2019, 378: 121670. |
| 49 | Han P W, Lv H X, Li X G, et al. Perovskite CaZrO3 for efficient ozonation treatment of organic pollutants in wastewater[J]. Catalysis Science & Technology, 2021, 11(11): 3697-3705. |
| 50 | Wang Y X, Xie Y B, Sun H Q, et al. Hierarchical shape-controlled mixed-valence calcium manganites for catalytic ozonation of aqueous phenolic compounds[J]. Catalysis Science & Technology, 2016, 6(9): 2918-2929. |
| 51 | Wang S H, Zhou L L, Zheng M, et al. Catalytic ozonation over Ca2Fe2O5 for the degradation of quinoline in an aqueous solution[J]. Industrial & Engineering Chemistry Research, 2022, 61(19): 6343-6353. |
| 52 | Zhou Z Y, Kong D L, Zhu H Y, et al. Preparation and adsorption characteristics of an ion-imprinted polymer for fast removal of N i ( Ⅱ ) ions from aqueous solution[J]. Journal of Hazardous Materials, 2018, 341: 355-364. |
| 53 | Kong D L, Qiao N, Wang N, et al. Facile preparation of a nano-imprinted polymer on magnetite nanoparticles for the rapid separation of lead ions from aqueous solution[J]. Physical Chemistry Chemical Physics, 2018, 20(18): 12870-12878. |
| 54 | Kong D L, Qiao N, Liu H, et al. Fast and efficient removal of copper using sandwich-like graphene oxide composite imprinted materials[J]. Chemical Engineering Journal, 2017, 326: 141-150. |
| 55 | Liu C, Chen X X, Zhang J, et al. Advanced treatment of bio-treated coal chemical wastewater by a novel combination of microbubble catalytic ozonation and biological process[J]. Separation and Purification Technology, 2018, 197: 295-301. |
| 56 | Bilińska L, Blus K, Bilińska M, et al. Industrial textile wastewater ozone treatment: catalyst selection[J]. Catalysts, 2020, 10(6): 611. |
| 57 | Chen C M, Chen H S, Guo X, et al. Advanced ozone treatment of heavy oil refining wastewater by activated carbon supported iron oxide[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 2782-2791. |
| 58 | Vittenet J, Aboussaoud W, Mendret J, et al. Catalytic ozonation with γ-Al2O3 to enhance the degradation of refractory organics in water[J]. Applied Catalysis A: General, 2015, 504: 519-532. |
| 59 | Wei K J, Cao X X, Gu W C, et al. Ni-induced C-Al2O3-framework (NiCAF) supported core-multishell catalysts for efficient catalytic ozonation: a structure-to-performance study[J]. Environmental Science & Technology, 2019, 53(12): 6917-6926. |
| 60 | He C, Wang J B, Wang C R, et al. Catalytic ozonation of bio-treated coking wastewater in continuous pilot- and full-scale system: efficiency, catalyst deactivation and in situ regeneration[J]. Water Research, 2020, 183: 116090. |
| 61 | 任钟旗, 陈健杰, 周智勇, 等. 用于含盐有机废水深度处理的钙基碳包覆臭氧氧化催化剂: 115228495A[P]. 2022-10-25. |
| Ren Z Q, Chen J J, Zhou Z Y, et al. Calcium-based carbon-coated ozone oxidation catalyst for advanced treatment of salt-containing organic wastewater: 115228495A[P]. 2022-10-25. | |
| 62 | 任钟旗, 邵高燕, 周智勇, 等. 用于含盐有机废水深度处理的Ca基臭氧催化氧化催化剂及其制备方法: 115055174A[P]. 2022-09-16. |
| Ren Z Q, Shao G Y, Zhou Z Y, et al. Ca-based catalytic ozonation catalyst for deep treatment of salt-containing organic wastewater and preparation method of Ca-based catalytic ozonation catalyst: 115055174A[P]. 2022-09-16. | |
| 63 | Frainetti A J, Klinghoffer N B. Recent experimental advances on the utilization of biochar as a tar reforming catalyst: a review[J]. International Journal of Hydrogen Energy, 2023, 48(22): 8022-8044. |
| 64 | Abdoulmoumine N, Adhikari S, Kulkarni A, et al. A review on biomass gasification syngas cleanup[J]. Applied Energy, 2015, 155: 294-307. |
| 65 | 王聪哲, 许桂英. 天然非均相焦油裂解催化剂研究进展[J]. 现代化工, 2018, 38(12): 34-38, 40. |
| Wang C Z, Xu G Y. Study progress in natural heterogeneous catalysts for tar cracking[J]. Modern Chemical Industry, 2018, 38(12): 34-38, 40. | |
| 66 | 巩伟, 阴秀丽, 谢建军, 等. 多孔白云石颗粒催化裂解生物质焦油的动力学研究[J]. 太阳能学报, 2010, 31(7): 800-805. |
| Gong W, Yin X L, Xie J J, et al. Kinetic study of tar catalytic cracking over porous granular dolomite catalyst[J]. Acta Energiae Solaris Sinica, 2010, 31(7): 800-805. | |
| 67 | 孙云娟, 蒋剑春. 生物质热解气化产物中焦油的催化裂解研究[J]. 林产化学与工业, 2007, 27(5): 15-20. |
| Sun Y J, Jiang J C. Study on catalytic cracking of tar derived from biomass pyrolysis and gasification[J]. Chemistry and Industry of Forest Products, 2007, 27(5): 15-20. | |
| 68 | Guan G Q, Chen G, Kasai Y, et al. Catalytic steam reforming of biomass tar over iron- or nickel-based catalyst supported on calcined scallop shell[J]. Applied Catalysis B: Environmental, 2012, 115/116: 159-168. |
| 69 | Guo F Q, Jia X P, Liang S, et al. Development of biochar-based nanocatalysts for tar cracking/reforming during biomass pyrolysis and gasification[J]. Bioresource Technology, 2020, 298: 122263. |
| 70 | Feng D D, Zhao Y J, Zhang Y, et al. Effects of K and Ca on reforming of model tar compounds with pyrolysis biochars under H2O or CO2 [J]. Chemical Engineering Journal, 2016, 306: 422-432. |
| 71 | Feng D D, Zhao Y J, Zhang Y, et al. Roles and fates of K and Ca species on biochar structure during in situ tar H2O reforming over nascent biochar[J]. International Journal of Hydrogen Energy, 2017, 42(34): 21686-21696. |
| 72 | Ochoa A, Bilbao J, Gayubo A G, et al. Coke formation and deactivation during catalytic reforming of biomass and waste pyrolysis products: a review[J]. Renewable and Sustainable Energy Reviews, 2020, 119: 109600. |
| 73 | Dong Q, Li H J, Zhang S P, et al. Biomass tar cracking and syngas production using rice husk char-supported nickel catalysts coupled with microwave heating[J]. RSC Advances, 2018, 8(71): 40873-40882. |
| 74 | Gu J, Wang S X, Lu T, et al. Synthesis and evaluation of pyrolysis waste peat char supported catalyst for steam reforming of toluene[J]. Renewable Energy, 2020, 160: 964-973. |
| 75 | Wang C P, Yan H, Yu Y B, et al. Chemical looping reforming of coal tar vapor on the surface of CaO-modified Fe-based oxygen carrier[J]. Energy & Fuels, 2020, 34(7): 8534-8542. |
| 76 | Shen Y F, Yoshikawa K. Tar conversion and vapor upgrading via in situ catalysis using silica-based nickel nanoparticles embedded in rice husk char for biomass pyrolysis/gasification[J]. Industrial & Engineering Chemistry Research, 2014, 53(27): 10929-10942. |
| 77 | Guo F Q, Li X L, Liu Y, et al. Catalytic cracking of biomass pyrolysis tar over char-supported catalysts[J]. Energy Conversion and Management, 2018, 167: 81-90. |
| 78 | Wang H M, Bing W H, Chen C Y, et al. Geometric effect promoted hydrotalcites catalysts towards aldol condensation reaction[J]. Chinese Journal of Catalysis, 2020, 41(8): 1279-1287. |
| 79 | Luo X, Wang Y Y, Xie X L, et al. Catalytic ozonation of cinnamaldehyde to benzaldehyde over Ca(OH)2 [J]. ChemistrySelect, 2021, 6(20): 5052-5060. |
| 80 | Luo X, Hou Y R, Xie X L, et al. Role of water on ozonation of cinnamaldehyde to benzaldehyde under Ca(OH)2 catalysis: a combined in situ DRIFTS and DFT study[J]. Applied Surface Science, 2021, 569: 151071. |
| 81 | Wu J F, Su T M, Jiang Y X, et al. In situ DRIFTS study of O3 adsorption on CaO, γ-Al2O3, CuO, α-Fe2O3 and ZnO at room temperature for the catalytic ozonation of cinnamaldehyde[J]. Applied Surface Science, 2017, 412: 290-305. |
| 82 | Sharma A, Lee B K. A novel nanocomposite of Ca(OH)2-incorporated zeolite as an additive to reduce atmospheric emissions of PM and VOCs during asphalt production[J]. Environmental Science: Nano, 2017, 4(3): 613-624. |
| 83 | Hu E L, Shang S M, Chiu K. Removal of reactive dyes in textile effluents by catalytic ozonation pursuing on-site effluent recycling[J]. Molecules, 2019, 24(15): 2755. |
| 84 | Cao H B, Xing L L, Wu G G, et al. Promoting effect of nitration modification on activated carbon in the catalytic ozonation of oxalic acid[J]. Applied Catalysis B: Environmental, 2014, 146: 169-176. |
| [1] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [5] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [6] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [7] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [8] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [9] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [10] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [11] | 段重达, 姚小伟, 朱家华, 孙静, 胡南, 李广悦. 环境因素对克雷白氏杆菌诱导碳酸钙沉淀的影响[J]. 化工学报, 2023, 74(8): 3543-3553. |
| [12] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [13] | 刘春雨, 周桓宇, 马跃, 岳长涛. CaO调质含油污泥干燥特性及数学模型[J]. 化工学报, 2023, 74(7): 3018-3027. |
| [14] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [15] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号