化工学报 ›› 2019, Vol. 70 ›› Issue (11): 4153-4161.DOI: 10.11949/0438-1157.20190712
张金彦1( ),匡雯婕1,吉绍长2,曹小雪1,廖安平1,蓝平1(
),匡雯婕1,吉绍长2,曹小雪1,廖安平1,蓝平1( )
)
收稿日期:2019-06-24
修回日期:2019-08-15
出版日期:2019-11-05
发布日期:2019-11-05
通讯作者:
蓝平
作者简介:张金彦(1984—),女,博士,副教授,基金资助:
Jinyan ZHANG1( ),Wenjie KUANG1,Shaochang JI2,Xiaoxue CAO1,Anping LIAO1,Ping LAN1(
),Wenjie KUANG1,Shaochang JI2,Xiaoxue CAO1,Anping LIAO1,Ping LAN1( )
)
Received:2019-06-24
Revised:2019-08-15
Online:2019-11-05
Published:2019-11-05
Contact:
Ping LAN
摘要:
采用静态法测定了拉莫三嗪在正丙醇、异丙醇、正丁醇、异丁醇、丙酸乙酯、乙酸甲酯6种溶剂中的热力学行为,并采用Apelblat方程和λh方程进行了关联,其结果表明拉莫三嗪的溶解度随着温度的升高而升高,溶解行为由低到高为正丁醇>正丙醇>异丁醇>异丙醇,乙酸甲酯>丙酸乙酯,应用的方程拟合度较好,可用于单体拉莫三嗪热力学行为的预测。在此基础上,采用溶液法制备拉莫三嗪-邻苯二甲酰亚胺共晶,在298.15、303.15 K时,建立了共晶体在异丁醇、丙酸乙酯溶剂中的三元相图,结果表明同一溶剂体系内,随着温度的升高,拉莫三嗪-邻苯二甲酰亚胺共晶区域增大,有利于共晶的形成。同一温度下,拉莫三嗪-邻苯二甲酰亚胺共晶在异丁醇体系的对称性优于丙酸乙酯体系,表明拉莫三嗪-邻苯二甲酰亚胺共晶更易于在异丁醇体系中获得。
中图分类号:
张金彦, 匡雯婕, 吉绍长, 曹小雪, 廖安平, 蓝平. 拉莫三嗪-邻苯二甲酰亚胺药物共晶在有机溶剂中溶解度及三元相图测定[J]. 化工学报, 2019, 70(11): 4153-4161.
Jinyan ZHANG, Wenjie KUANG, Shaochang JI, Xiaoxue CAO, Anping LIAO, Ping LAN. Determination of solubility and ternary phase diagram of lamotrigine-phthalimide pharmaceutical cocrystal in pure solvent[J]. CIESC Journal, 2019, 70(11): 4153-4161.
| Solvent | T/K | xexp×103 | xcal×103(Apelblat) | xca×103 (λh) |
|---|---|---|---|---|
| isobutanol | 283.15 | 0.5193 | 0.5849 | 0.5401 |
| 288.15 | 0.7566 | 0.7510 | 0.7026 | |
| 293.15 | 0.8617 | 0.9503 | 0.9058 | |
| 298.15 | 1.3346 | 1.1859 | 1.1579 | |
| 303.15 | 1.5546 | 1.4609 | 1.4685 | |
| 308.15 | 1.7576 | 1.7776 | 1.8485 | |
| 313.15 | 2.0784 | 2.1380 | 2.3101 | |
| 318.15 | 2.4301 | 2.5433 | 2.8675 | |
| 323.15 | 3.0794 | 2.9941 | 3.5365 | |
| n-butyl alcohol | 283.15 | 1.4601 | 1.4496 | 1.4417 |
| 288.15 | 1.6682 | 1.6679 | 1.6739 | |
| 293.15 | 1.8872 | 1.9185 | 1.9349 | |
| 298.15 | 2.2309 | 2.2060 | 2.2273 | |
| 303.15 | 2.5958 | 2.5356 | 2.5541 | |
| 308.15 | 2.7752 | 2.9130 | 2.9183 | |
| 313.15 | 3.3956 | 3.3449 | 3.3234 | |
| 318.15 | 3.8945 | 3.8387 | 3.7730 | |
| 323.15 | 4.3699 | 4.4030 | 4.2712 | |
| n-propyl alcohol | 283.15 | 1.0406 | 0.9781 | 1.0198 |
| 288.15 | 1.2281 | 1.2437 | 1.2525 | |
| 293.15 | 1.4365 | 1.5542 | 1.5280 | |
| 298.15 | 1.9513 | 1.9108 | 1.8522 | |
| 303.15 | 2.2520 | 2.3135 | 2.2319 | |
| 308.15 | 2.7390 | 2.7607 | 2.6742 | |
| 313.15 | 3.3775 | 3.2497 | 3.1873 | |
| 318.15 | 3.6322 | 3.7764 | 3.7798 | |
| 323.15 | 4.3317 | 4.3354 | 4.4616 | |
| ethyl propionate | 283.15 | 0.5773 | 0.5804 | 0.5867 |
| 288.15 | 0.6544 | 0.6525 | 0.6520 | |
| 293.15 | 0.7394 | 0.7288 | 0.7228 | |
| 298.15 | 0.8150 | 0.8088 | 0.7995 | |
| 303.15 | 0.8622 | 0.8924 | 0.8826 | |
| 308.15 | 0.9753 | 0.9791 | 0.9724 | |
| 313.15 | 1.0975 | 1.0686 | 1.0695 | |
| 318.15 | 1.1540 | 1.1605 | 1.1745 | |
| 323.15 | 1.2507 | 1.2543 | 1.2880 | |
| methyl acetate | 283.15 | 0.7791 | 0.7088 | 0.7763 |
| 288.15 | 0.9716 | 0.9598 | 0.9772 | |
| 293.15 | 1.1703 | 1.2576 | 1.2206 | |
| 298.15 | 1.5689 | 1.5977 | 1.5137 | |
| 303.15 | 1.9761 | 1.9720 | 1.8644 | |
| 308.15 | 2.4009 | 2.3685 | 2.2814 | |
| 313.15 | 2.8242 | 2.7730 | 2.7747 | |
| 318.15 | 3.1267 | 3.1693 | 3.3552 | |
| 323.15 | 3.5420 | 3.5411 | 4.0352 | |
| isopropanol | 283.15 | 0.3764 | 0.3596 | 0.3653 |
| 288.15 | 0.4682 | 0.4659 | 0.4727 | |
| 293.15 | 0.5350 | 0.6007 | 0.6063 | |
| 298.15 | 0.8172 | 0.7710 | 0.7713 | |
| 303.15 | 1.0033 | 0.9853 | 0.9737 | |
| 308.15 | 1.2201 | 1.2539 | 1.2202 | |
| 313.15 | 1.6317 | 1.5891 | 1.5184 | |
| 318.15 | 1.9682 | 2.0059 | 1.8771 | |
| 323.15 | 2.5341 | 2.5224 | 2.3061 |
表1 拉莫三嗪在纯溶剂中在不同温度下的摩尔分数溶解度xa和计算值xcal
Table 1 Experimental and calculated molar fraction solubility x of lamotrigine in pure solvents at different temperatures
| Solvent | T/K | xexp×103 | xcal×103(Apelblat) | xca×103 (λh) |
|---|---|---|---|---|
| isobutanol | 283.15 | 0.5193 | 0.5849 | 0.5401 |
| 288.15 | 0.7566 | 0.7510 | 0.7026 | |
| 293.15 | 0.8617 | 0.9503 | 0.9058 | |
| 298.15 | 1.3346 | 1.1859 | 1.1579 | |
| 303.15 | 1.5546 | 1.4609 | 1.4685 | |
| 308.15 | 1.7576 | 1.7776 | 1.8485 | |
| 313.15 | 2.0784 | 2.1380 | 2.3101 | |
| 318.15 | 2.4301 | 2.5433 | 2.8675 | |
| 323.15 | 3.0794 | 2.9941 | 3.5365 | |
| n-butyl alcohol | 283.15 | 1.4601 | 1.4496 | 1.4417 |
| 288.15 | 1.6682 | 1.6679 | 1.6739 | |
| 293.15 | 1.8872 | 1.9185 | 1.9349 | |
| 298.15 | 2.2309 | 2.2060 | 2.2273 | |
| 303.15 | 2.5958 | 2.5356 | 2.5541 | |
| 308.15 | 2.7752 | 2.9130 | 2.9183 | |
| 313.15 | 3.3956 | 3.3449 | 3.3234 | |
| 318.15 | 3.8945 | 3.8387 | 3.7730 | |
| 323.15 | 4.3699 | 4.4030 | 4.2712 | |
| n-propyl alcohol | 283.15 | 1.0406 | 0.9781 | 1.0198 |
| 288.15 | 1.2281 | 1.2437 | 1.2525 | |
| 293.15 | 1.4365 | 1.5542 | 1.5280 | |
| 298.15 | 1.9513 | 1.9108 | 1.8522 | |
| 303.15 | 2.2520 | 2.3135 | 2.2319 | |
| 308.15 | 2.7390 | 2.7607 | 2.6742 | |
| 313.15 | 3.3775 | 3.2497 | 3.1873 | |
| 318.15 | 3.6322 | 3.7764 | 3.7798 | |
| 323.15 | 4.3317 | 4.3354 | 4.4616 | |
| ethyl propionate | 283.15 | 0.5773 | 0.5804 | 0.5867 |
| 288.15 | 0.6544 | 0.6525 | 0.6520 | |
| 293.15 | 0.7394 | 0.7288 | 0.7228 | |
| 298.15 | 0.8150 | 0.8088 | 0.7995 | |
| 303.15 | 0.8622 | 0.8924 | 0.8826 | |
| 308.15 | 0.9753 | 0.9791 | 0.9724 | |
| 313.15 | 1.0975 | 1.0686 | 1.0695 | |
| 318.15 | 1.1540 | 1.1605 | 1.1745 | |
| 323.15 | 1.2507 | 1.2543 | 1.2880 | |
| methyl acetate | 283.15 | 0.7791 | 0.7088 | 0.7763 |
| 288.15 | 0.9716 | 0.9598 | 0.9772 | |
| 293.15 | 1.1703 | 1.2576 | 1.2206 | |
| 298.15 | 1.5689 | 1.5977 | 1.5137 | |
| 303.15 | 1.9761 | 1.9720 | 1.8644 | |
| 308.15 | 2.4009 | 2.3685 | 2.2814 | |
| 313.15 | 2.8242 | 2.7730 | 2.7747 | |
| 318.15 | 3.1267 | 3.1693 | 3.3552 | |
| 323.15 | 3.5420 | 3.5411 | 4.0352 | |
| isopropanol | 283.15 | 0.3764 | 0.3596 | 0.3653 |
| 288.15 | 0.4682 | 0.4659 | 0.4727 | |
| 293.15 | 0.5350 | 0.6007 | 0.6063 | |
| 298.15 | 0.8172 | 0.7710 | 0.7713 | |
| 303.15 | 1.0033 | 0.9853 | 0.9737 | |
| 308.15 | 1.2201 | 1.2539 | 1.2202 | |
| 313.15 | 1.6317 | 1.5891 | 1.5184 | |
| 318.15 | 1.9682 | 2.0059 | 1.8771 | |
| 323.15 | 2.5341 | 2.5224 | 2.3061 |
| Solvent | A | B | C | R2 | RAD×102 | RMSD×105 |
|---|---|---|---|---|---|---|
| isobutanol | 144.63 | -9988.48 | -20.69 | 0.9841 | 0.0580 | 7.22 |
| ethyl propionate | 58.70 | -4460.85 | -8.93 | 0.9939 | 0.0116 | 1.46 |
| n-butyl alcohol | -99.34 | 2041.09 | 15.16 | 0.9949 | 0.0161 | 5.47 |
| methyl acetate | 517.02 | -26699.35 | -76.16 | 0.9968 | 0.0270 | 4.38 |
| n-propyl alcohol | 212.50 | -12743.95 | -30.89 | 0.9927 | 0.0321 | 6.68 |
| isopropanol | -85.47 | -256.36 | 13.89 | 0.9965 | 0.0360 | 3.34 |
表2 Apelblat模型拟合参数及均方差RMSD和相对平均偏差RAD
Table 2 Regressed parameters and RMSD, RAD for lamotrigine in six pure solvents by Apelblat equation
| Solvent | A | B | C | R2 | RAD×102 | RMSD×105 |
|---|---|---|---|---|---|---|
| isobutanol | 144.63 | -9988.48 | -20.69 | 0.9841 | 0.0580 | 7.22 |
| ethyl propionate | 58.70 | -4460.85 | -8.93 | 0.9939 | 0.0116 | 1.46 |
| n-butyl alcohol | -99.34 | 2041.09 | 15.16 | 0.9949 | 0.0161 | 5.47 |
| methyl acetate | 517.02 | -26699.35 | -76.16 | 0.9968 | 0.0270 | 4.38 |
| n-propyl alcohol | 212.50 | -12743.95 | -30.89 | 0.9927 | 0.0321 | 6.68 |
| isopropanol | -85.47 | -256.36 | 13.89 | 0.9965 | 0.0360 | 3.34 |
| Solvent | λ | h | R2 | RAD×102 | RMSD×105 |
|---|---|---|---|---|---|
| isobutanol | 0.3338 | 12835.36 | 0.9781 | 0.0935 | 23.71 |
| ethyl propionate | 0.0054 | 287329.59 | 0.9937 | 0.0179 | 2.01 |
| n-butyl alcohol | 0.0487 | 48581.73 | 0.9958 | 0.0206 | 7.79 |
| methyl acetate | 0.2125 | 17614.98 | 0.9943 | 0.0471 | 19.16 |
| n-propyl alcohol | 0.1503 | 22170.43 | 0.9921 | 0.0349 | 10.47 |
| isopropanol | 0.1979 | 21212.88 | 0.9852 | 0.0515 | 9.50 |
表3 λh模型拟合参数及均方差RMSD和相对平均偏差RAD
Table 3 Regressed parameters and RMSD, RAD for lamotrigine in six pure solvents by λh equation
| Solvent | λ | h | R2 | RAD×102 | RMSD×105 |
|---|---|---|---|---|---|
| isobutanol | 0.3338 | 12835.36 | 0.9781 | 0.0935 | 23.71 |
| ethyl propionate | 0.0054 | 287329.59 | 0.9937 | 0.0179 | 2.01 |
| n-butyl alcohol | 0.0487 | 48581.73 | 0.9958 | 0.0206 | 7.79 |
| methyl acetate | 0.2125 | 17614.98 | 0.9943 | 0.0471 | 19.16 |
| n-propyl alcohol | 0.1503 | 22170.43 | 0.9921 | 0.0349 | 10.47 |
| isopropanol | 0.1979 | 21212.88 | 0.9852 | 0.0515 | 9.50 |

图4 拉莫三嗪-邻苯二甲酰亚胺共晶的单晶结构图:拉莫三嗪与邻苯二甲酰亚胺形成的超分子合成子(a),拉莫三嗪-邻苯二甲酰亚胺共晶ac平面的结构图(b)
Fig.4 Recognition pattern observed in crystal structure of LAM-PTL: partial packing diagram of LAM-PTL showing supramolecular synthons with applied graph-set analysis (a), packing diagram of LAM-PTL viewed in ac plane (b)
| Composition of liquid phase at 298.15 K | Composition of liquid phase at 303.15 K | ||||||
|---|---|---|---|---|---|---|---|
| LAM | PLT | Isobutanol | Equilibrium solid phase | LAM | PLT | Isobutanol | Equilibrium solid phase |
| 0 | 0.00388 | 0.99612 | PTL | 0 | 0.00499 | 0.99501 | PTL |
| 0.00091 | 0.00403 | 0.99506 | PTL | 0.00085 | 0.00500 | 0.99415 | PTL |
| 0.00132 | 0.00422 | 0.99446 | PTL | 0.00113 | 0.00505 | 0.99382 | PTL |
| 0.00144 | 0.00418 | 0.99439 | PTL | 0.00143 | 0.00488 | 0.99369 | PTL |
| 0.00195 | 0.00411 | 0.99394 | PTL+1∶1LAM/PTL | 0.00233 | 0.00461 | 0.99307 | PTL+1∶1LAM/PTL |
| 0.0037 | 0.00215 | 0.99414 | 1∶1LAM/PTL | 0.00292 | 0.00390 | 0.99318 | 1∶1LAM/PTL |
| 0.00445 | 0.00185 | 0.99370 | LAM+1∶1LAM/PTL | 0.00404 | 0.00243 | 0.99353 | 1∶1LAM/PTL |
| 0.00468 | 0.00148 | 0.99385 | LAM | 0.00521 | 0.00222 | 0.99257 | LAM+1∶1LAM/PTL |
| 0.00455 | 0.00114 | 0.99431 | LAM | 0.00539 | 0.00112 | 0.99349 | LAM |
| 0.00453 | 0.00097 | 0.99450 | LAM | 0.00546 | 0.00147 | 0.99307 | LAM |
| 0.0046 | 0 | 0.99540 | LAM | 0.00535 | 0 | 0.99465 | LAM |
表4 298.15 K、303.15 K时拉莫三嗪+邻苯二甲酰亚胺+异丁醇体系的固液平衡实验数据(质量分数)
Table 4 Experimental solid-liquid equilibrium data (mass fraction) for LAM + PTL + isobutanol at 298.15 K and 303.15 K
| Composition of liquid phase at 298.15 K | Composition of liquid phase at 303.15 K | ||||||
|---|---|---|---|---|---|---|---|
| LAM | PLT | Isobutanol | Equilibrium solid phase | LAM | PLT | Isobutanol | Equilibrium solid phase |
| 0 | 0.00388 | 0.99612 | PTL | 0 | 0.00499 | 0.99501 | PTL |
| 0.00091 | 0.00403 | 0.99506 | PTL | 0.00085 | 0.00500 | 0.99415 | PTL |
| 0.00132 | 0.00422 | 0.99446 | PTL | 0.00113 | 0.00505 | 0.99382 | PTL |
| 0.00144 | 0.00418 | 0.99439 | PTL | 0.00143 | 0.00488 | 0.99369 | PTL |
| 0.00195 | 0.00411 | 0.99394 | PTL+1∶1LAM/PTL | 0.00233 | 0.00461 | 0.99307 | PTL+1∶1LAM/PTL |
| 0.0037 | 0.00215 | 0.99414 | 1∶1LAM/PTL | 0.00292 | 0.00390 | 0.99318 | 1∶1LAM/PTL |
| 0.00445 | 0.00185 | 0.99370 | LAM+1∶1LAM/PTL | 0.00404 | 0.00243 | 0.99353 | 1∶1LAM/PTL |
| 0.00468 | 0.00148 | 0.99385 | LAM | 0.00521 | 0.00222 | 0.99257 | LAM+1∶1LAM/PTL |
| 0.00455 | 0.00114 | 0.99431 | LAM | 0.00539 | 0.00112 | 0.99349 | LAM |
| 0.00453 | 0.00097 | 0.99450 | LAM | 0.00546 | 0.00147 | 0.99307 | LAM |
| 0.0046 | 0 | 0.99540 | LAM | 0.00535 | 0 | 0.99465 | LAM |
| Composition of liquid phase at 298.15 K | Composition of liquid phase at 303.15 K | ||||||
|---|---|---|---|---|---|---|---|
| LAM | PLT | Ethyl propionate | Equilibrium solid phase | LAM | PLT | Ethyl propionate | Equilibrium solid phase |
| 0 | 0.00867 | 0.99133 | PTL | 0 | 0.00987 | 0.99013 | PTL |
| 0.00086 | 0.00844 | 0.99070 | PTL | 0.001 | 0.00920 | 0.9898 | PTL |
| 0.00102 | 0.00854 | 0.99044 | PTL | 0.00121 | 0.00943 | 0.98935 | PTL |
| 0.00103 | 0.00874 | 0.99023 | PTL+1∶1LAM/PTL | 0.00119 | 0.00958 | 0.98923 | PTL+1∶1LAM/PTL |
| 0.00128 | 0.00743 | 0.99129 | 1∶1LAM/PTL | 0.00125 | 0.00752 | 0.99123 | 1∶1LAM/PTL |
| 0.00165 | 0.00550 | 0.99284 | 1∶1LAM/PTL | 0.00195 | 0.00559 | 0.99246 | 1∶1LAM/PTL |
| 0.00238 | 0.00387 | 0.99374 | LAM+1∶1LAM/PTL | 0.00247 | 0.00441 | 0.99312 | LAM+1∶1LAM/PTL |
| 0.00211 | 0.00136 | 0.99653 | LAM | 0.00226 | 0.00148 | 0.99625 | LAM |
| 0.00214 | 0.00122 | 0.99664 | LAM | 0.00242 | 0.00121 | 0.99637 | LAM |
| 0.00213 | 0.00100 | 0.99687 | LAM | 0.00232 | 0.00098 | 0.99670 | LAM |
| 0.00214 | 0.00080 | 0.99706 | LAM | 0.00233 | 0.00081 | 0.99686 | LAM |
| 0.00204 | 0 | 0.99796 | LAM | 0.00216 | 0 | 0.99784 | LAM |
表5 298.15、303.15 K时拉莫三嗪+邻苯二甲酰亚胺+丙酸乙酯体系的固液平衡实验数据(质量分数)
Table 5 Experimental solid-liquid equilibrium data (mass fraction) for LAM + PTL +ethyl propionate at 298.15 K and 303.15 K
| Composition of liquid phase at 298.15 K | Composition of liquid phase at 303.15 K | ||||||
|---|---|---|---|---|---|---|---|
| LAM | PLT | Ethyl propionate | Equilibrium solid phase | LAM | PLT | Ethyl propionate | Equilibrium solid phase |
| 0 | 0.00867 | 0.99133 | PTL | 0 | 0.00987 | 0.99013 | PTL |
| 0.00086 | 0.00844 | 0.99070 | PTL | 0.001 | 0.00920 | 0.9898 | PTL |
| 0.00102 | 0.00854 | 0.99044 | PTL | 0.00121 | 0.00943 | 0.98935 | PTL |
| 0.00103 | 0.00874 | 0.99023 | PTL+1∶1LAM/PTL | 0.00119 | 0.00958 | 0.98923 | PTL+1∶1LAM/PTL |
| 0.00128 | 0.00743 | 0.99129 | 1∶1LAM/PTL | 0.00125 | 0.00752 | 0.99123 | 1∶1LAM/PTL |
| 0.00165 | 0.00550 | 0.99284 | 1∶1LAM/PTL | 0.00195 | 0.00559 | 0.99246 | 1∶1LAM/PTL |
| 0.00238 | 0.00387 | 0.99374 | LAM+1∶1LAM/PTL | 0.00247 | 0.00441 | 0.99312 | LAM+1∶1LAM/PTL |
| 0.00211 | 0.00136 | 0.99653 | LAM | 0.00226 | 0.00148 | 0.99625 | LAM |
| 0.00214 | 0.00122 | 0.99664 | LAM | 0.00242 | 0.00121 | 0.99637 | LAM |
| 0.00213 | 0.00100 | 0.99687 | LAM | 0.00232 | 0.00098 | 0.99670 | LAM |
| 0.00214 | 0.00080 | 0.99706 | LAM | 0.00233 | 0.00081 | 0.99686 | LAM |
| 0.00204 | 0 | 0.99796 | LAM | 0.00216 | 0 | 0.99784 | LAM |

图5 三元相图的局部放大图:拉莫三嗪+邻苯二甲酰亚胺+异丁醇 298.15 K (a) 和 303.15 K (b),拉莫三嗪+邻苯二甲酰亚胺+丙酸乙酯298.15 K (c) 和 303.15 K (d)
Fig.5 Ternary phase diagrams for LAM +PTL+ isobutanol at 298.15 K (a) and 303.15 K (b), LAM + PTL+ ethyl propionate at 298.15 K (c) and 303.15 K (d)
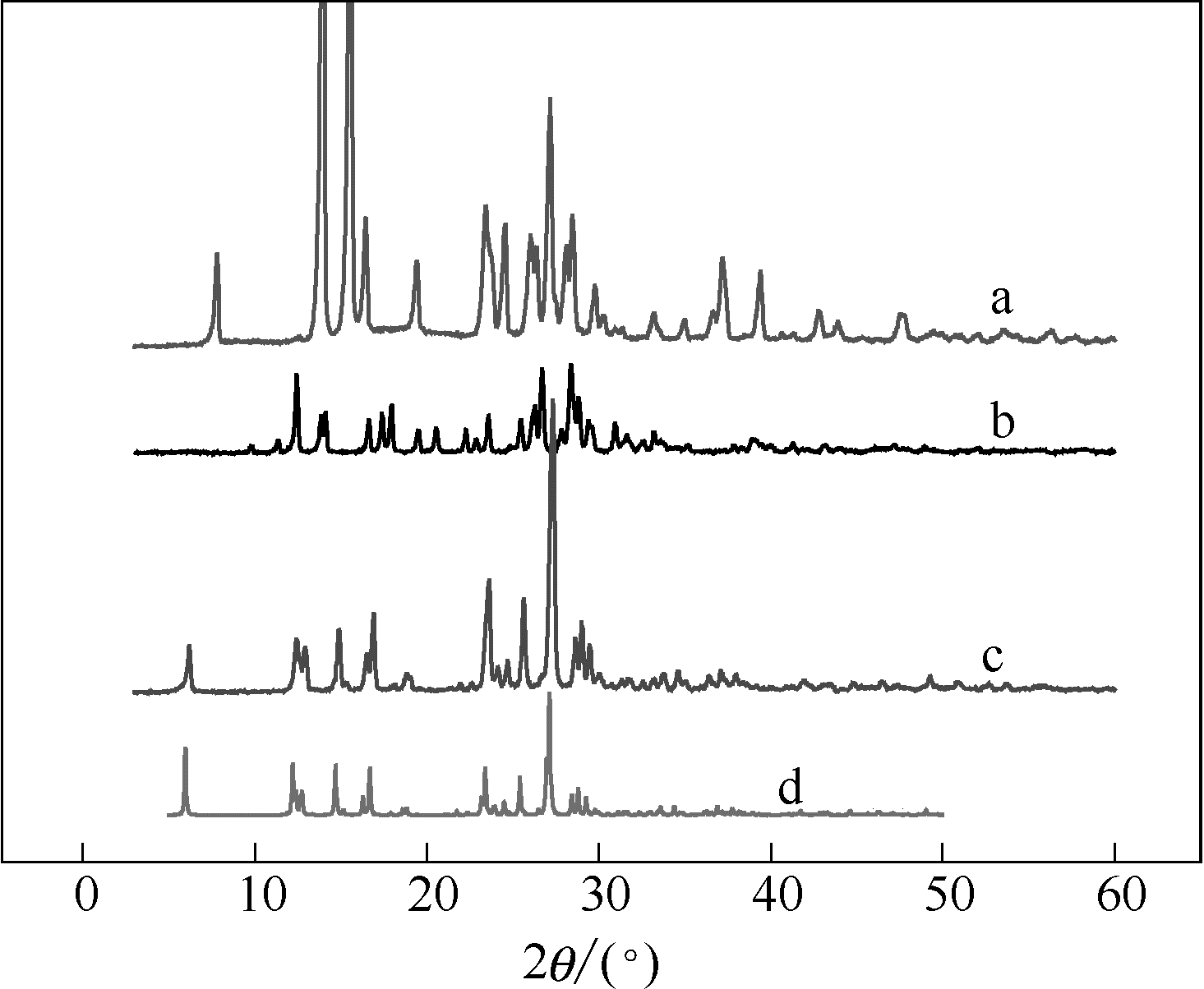
图6 XRD谱图:邻苯二甲酰亚胺(a);拉莫三嗪(b);实验中得到的拉莫三嗪-邻苯二甲酰亚胺共晶(c);模拟得到的拉莫三嗪-邻苯二甲酰亚胺共晶(d)
Fig.6 XRD patterns of PTL (a), LAM (b), experimental LAM-PTL cocrystal (c), calculated LAM-PTL cocrystal (d)
| 1 | DuggiralaN K, PerryM L, ÖrnA, et al. Pharmaceutical cocrystals: along the path to improved medicines[J]. Chemical Communications, 2015, 52(4): 640-655. |
| 2 | KorotkovaE I, KratochvílB. Pharmaceutical cocrystals[J]. Procedia Chemistry, 2014, 10: 473-476. |
| 3 | ZellerB L, SaleebF Z, LudescherR D. Trends in development of porous carbohydrate food ingredients for use in flavor encapsulation[J]. Trends in Food Science & Technology, 1998, 9(11/12): 389-394. |
| 4 | DeladinoL, NavarroA S, MartinoM N. Microstructure of minerals and yerba mate extract co-crystallized with sucrose[J]. Journal of Food Engineering, 2010, 96(3): 410-415. |
| 5 | ZhuW, ZhuL, SunL, et al. Uncovering the intramolecular emission and tuning the nonlinear optical properties of organic materials by cocrystallization[J]. Angewandte Chemie, 2016, 128(45): 14105-14384. |
| 6 | SangeethaM, MathammalR. Establishment of the structural and enhanced physicochemical properties of the cocrystal-2-benzyl amino pyridine with oxalic acid[J]. Journal of Molecular Structure, 2017, 1143: 192-203. |
| 7 | NayakM, JanaA, FleckM, et al. A unique example of a three component cocrystal of metal complexes[J]. CrystEngComm, 2010, 12(5): 1416-1421. |
| 8 | HazraS, KonerR, NayakM, et al. Cocrystallized dinuclearmononuclear CuII3NaI and double-decker-triple-decker CuII5KI3 complexes derived from N, N -ethylenebis (3-ethoxysalicylaldimine) [J]. Cryst. Growth Des., 2009, 9(8): 3603-3608. |
| 9 | AakeröyC B, SalmonD J. Building cocrystals with molecular sense and supramolecular sensibility[J]. CrystEngComm, 2005, 7(72): 439-448. |
| 10 | YoshimuraM, MiyakeM, KawatoT, et al. Impact of the dissolution profile of the cilostazol cocrystal with supersaturation on the oral bioavailability[J]. Cryst. Growth Des., 2017, 17(2): 550-557. |
| 11 | BhandaruJ S, MalothuN, AkkinepallyR R. Characterization and solubility studies of pharmaceutical cocrystals of eprosartan mesylate[J]. Cryst. Growth Des., 2015, 15(3): 1173-1179. |
| 12 | ChenY, LiL, YaoJ, et al. Improving the solubility and bioavailability of apixaban via apixaban-oxalic acid cocrystal[J]. Cryst. Growth Des., 2016, 16(5): 2923-2930. |
| 13 | SugandhaK, KaityS, MukherjeeS, et al. Solubility enhancement of ezetimibe by a cocrystal engineering technique[J]. Cryst. Growth Des., 2014, 14(9): 4475-4486. |
| 14 | ChildsS L, KandiP, LingireddyS R. Formulation of a danazol cocrystal with controlled supersaturation plays an essential role in improving bioavailability[J]. Molecular Pharmaceutics, 2013, 10(8): 3112-3127. |
| 15 | BrittainH G. Cocrystal systems of pharmaceutical interest: 2010[J]. Cryst. Growth Des., 2012, 12(2): 1046-1054. |
| 16 | BrittainH G. Cocrystal systems of pharmaceutical interest: 2011[J]. Cryst. Growth Des., 2012, 12(11): 5823-5832. |
| 17 | ShazaD, JacekK, GamidiR K, et al. A new 1∶1 drug-drug cocrystal of theophylline and aspirin: discovery, characterization, and construction of ternary phase diagrams[J]. Cryst. Growth Des., 2018, 18(12): 7526-7532. |
| 18 | TaoQ, ChenJ M, MaL, et al. Phenazopyridine cocrystal and salts that exhibit enhanced solubility and stability[J]. Cryst. Growth Des., 2012, 12(6): 3144-3152. |
| 19 | MaenoY, FukamiT, KawahataM, et al. Novel pharmaceutical cocrystal consisting of paracetamol and trimethylglycine, a new promising cocrystal former[J]. International Journal of Pharmaceutics, 2014, 473(1/2): 179-186. |
| 20 | ShayanfarA, JouybanA. Physicochemical characterization of a new cocrystal of ketoconazole[J]. Powder Technology, 2014, 262: 242-248. |
| 21 | ShayanfarA, VelagaS, JouybanA. Solubility of carbamazepine, nicotinamide and carbamazepine-nicotinamide cocrystal in ethanol-water mixtures[J]. Fluid Phase Equilibria, 2014, 363: 97-105. |
| 22 | NanZ, WangM, YanB. In situ investigation on the formation mechanism of MCM-41 mesoporous silica by microcalorimetry[J]. Journal of Chemical & Engineering Data, 2009, 54(1): 83-89. |
| 23 | ChadhaR, SainiA, AroraP, et al. Multicomponent solids of lamotrigine with some selected coformers and their characterization by thermoanalytical, spectroscopic and X-ray diffraction methods[J]. CrystEngComm, 2011, 13(20): 6271-6284. |
| 24 | LekšićE, PavlovićG, MestrovićE. Cocrystals of lamotrigine based on coformers involving carbonyl group discovered by hot-stage microscopy and DSC screening[J]. Cryst. Growth Des., 2012, 12(4): 1847-1858. |
| 25 | KaurR, CavanaghK L, RodrestrovićE. Cocrystals of lamotrigine based on coformers involving carbonyl group discovered by hot-stage microscopy and dsc screening[J]. Cryst. Growth Des., 2017, 17(10): 5012-5016. |
| 26 | 边林. 药物共晶的设计合成与表征[D]. 天津: 天津大学, 2013. |
| BianL. Design, synthesis and characterization of drug eutectic [D]. Tianjin: Tianjin University, 2013. | |
| 27 | LangeL, SadowskiG. Thermodynamic modeling for efficient cocrystal formation[J]. Crystal Growth & Design, 2015, 15, 4406-4416. |
| 28 | ZhangJ, DangL, WeiH. Solubility of 5-(dithiolan-3-yl) pentanoic acid in the mixed solvents of cyclohexane + ethyl acetate, heptane+ethylacetate, andhexane+ethylacetate[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 4025-4028. |
| 29 | BalujaS, AlnayabE A M, HiraparaA. Solubility and solution thermodynamics of hippuric acid in various solvents from 298.15 K to 328.15 K[J]. Journal of Molecular Liquids, 2017, 238: 84-88. |
| 30 | ChenLZ, SongL, LanGC, et al. Solubility and metastable zone width measurement of 3,4-bis(3-nitrofurazan-4-yl)furoxan(dntf) in ethanol+water[J]. Chinese Journal of Chemical Engineering, 2017, 25(5): 646-651. |
| 31 | XieY, ShiH W, DuC B, et al. Solubility determination and modeling for 4,4-dihydroxydiphenyl sulfone in mixed solvents of(acetone, ethylacetate, oracetonitrile)+methanol and acetone+ ethanol from (278.15 to 313.15) K[J]. Journal of Chemical & Engineering Data, 2016, 61(10): 3519-3526. |
| 32 | YangH, RasmusonA C. Solubility of butylparaben in methanol, ethanol, propanol, ethyl acetate, acetone, and acetonitrile[J]. Journal of Chemical & Engineering Data, 2010, 55(11): 5091-5093. |
| 33 | LeiZ Y, HuY H, YangW G, et al. Solubility of 2-(2,4,6trichlorophenoxy)ethyl bromide in methanol, ethanol, propanol, isopropanol, acetonitrile, n-heptane, and acetone[J]. Journal of Chemical & Engineering Data, 2011, 56(5): 2714-2719. |
| 34 | BuchowskiH, KsiazczakA, PietrzykS. ChemInform abstract: solvent activity along a saturation line and solubility of hydrogen bonding solids [J]. Journal of Physical Chemistry, 1980, 84(9): 975-979. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 常明慧, 王林, 苑佳佳, 曹艺飞. 盐溶液蓄能型热泵循环特性研究[J]. 化工学报, 2023, 74(S1): 329-337. |
| [3] | 张化福, 童莉葛, 张振涛, 杨俊玲, 王立, 张俊浩. 机械蒸汽压缩蒸发技术研究现状与发展趋势[J]. 化工学报, 2023, 74(S1): 8-24. |
| [4] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [5] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [6] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [7] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [8] | 卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638. |
| [9] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [10] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [11] | 毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041. |
| [12] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [13] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [14] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [15] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号