化工学报 ›› 2023, Vol. 74 ›› Issue (3): 1033-1041.DOI: 10.11949/0438-1157.20221438
毛元敬1( ), 杨智1(
), 杨智1( ), 莫松平1, 郭浩2, 陈颖1, 罗向龙1, 陈健勇1, 梁颖宗1
), 莫松平1, 郭浩2, 陈颖1, 罗向龙1, 陈健勇1, 梁颖宗1
收稿日期:2022-11-03
修回日期:2022-12-26
出版日期:2023-03-05
发布日期:2023-04-19
通讯作者:
杨智
作者简介:毛元敬(2000—),男,硕士研究生,2112002077@mail2.gdut.edu.cn
基金资助:
Yuanjing MAO1( ), Zhi YANG1(
), Zhi YANG1( ), Songping MO1, Hao GUO2, Ying CHEN1, Xianglong LUO1, Jianyong CHEN1, Yingzong LIANG1
), Songping MO1, Hao GUO2, Ying CHEN1, Xianglong LUO1, Jianyong CHEN1, Yingzong LIANG1
Received:2022-11-03
Revised:2022-12-26
Online:2023-03-05
Published:2023-04-19
Contact:
Zhi YANG
摘要:
统计缔合流体理论(SAFT)状态方程对长链烷醇的热物性研究具有重要意义,而状态方程参数的获取是流体热物性研究的基础。基于SAFT-VR Mie状态方程,采用Levenberg-Marquardt算法并结合相平衡、过冷液相密度和声速性质的参数回归策略,获取C6~C10烷醇的状态方程模型参数。进一步评估SAFT-VR Mie状态方程对C6~C10烷醇在宽温度和压力范围内的相平衡和热力学偏导特性的预测性能,并与PC-SAFT状态方程进行比较。结果表明,SAFT-VR Mie对五种烷醇整体具有更优的饱和蒸气压、饱和液相密度、蒸发焓、过冷液相密度、比定压热容和声速预测性能,平均预测偏差分别为0.74%、0.82%、3.02%、0.54%、2.88%和2.31%。同时,SAFT-VR Mie具有可靠的外推预测能力,其对高压声速的预测结果与实验数据具有较好的一致性。此外,SAFT-VR Mie对压力-密度导数的不合理描述是造成声速预测偏差的主要原因。改进分子间单体和缔合相互作用能够有效提高比热容的预测精度,为长链烷醇缔合流体的热物性预测提供更好的理论关联模型。
中图分类号:
毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041.
Yuanjing MAO, Zhi YANG, Songping MO, Hao GUO, Ying CHEN, Xianglong LUO, Jianyong CHEN, Yingzong LIANG. Estimation of SAFT-VR Mie equation of state parameters and thermodynamic properties of C6—C10 alcohols[J]. CIESC Journal, 2023, 74(3): 1033-1041.

图1 SAFT-VR Mie状态方程分子构型和参数回归流程示意图
Fig.1 Schematic diagram of the molecular configuration and parameter regression procedure of SAFT-VR Mie equation of state (EoS)
| Substances | m | σ | (ε/k) | r | (εAB/k)/K | λr | AARD/% | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ps | ρs | ΔHvap | ρl | cP | u | |||||||
| 1-hexanol (C6) | 2.4364 | 4.2407 | 315.79 | 0.28852 | 3102.4 | 11.9366 | 0.81 | 1.06 | 1.64 | 0.23 | 1.60 | 2.09 |
| 1-heptanol (C7) | 2.4415 | 4.4413 | 336.05 | 0.26707 | 3404.3 | 12.2372 | 0.81 | 0.73 | 3.11 | 0.57 | 2.00 | 2.12 |
| 1-octanol (C8) | 2.7639 | 4.4240 | 342.37 | 0.29096 | 3180.6 | 12.9708 | 0.67 | 0.61 | 3.21 | 0.64 | 3.17 | 3.61 |
| 1-nonanol (C9) | 2.9172 | 4.4871 | 358.14 | 0.26918 | 3398.7 | 13.3660 | 0.57 | 1.05 | 3.84 | 0.48 | 2.12 | 1.77 |
| 1-decanol (C10) | 2.4778 | 4.9442 | 409.06 | 0.28053 | 3457.4 | 14.3970 | 0.83 | 0.66 | 3.29 | 0.77 | 5.51 | 1.98 |
| average | — | — | — | — | — | — | 0.74 | 0.82 | 3.02 | 0.54 | 2.88 | 2.31 |
表1 C6~C10烷醇SAFT-VR Mie状态方程参数回归及热物性预测结果
Table 1 Results of SAFT-VR Mie EoS parameter regression and thermodynamic properties prediction for C6—C10 alcohols
| Substances | m | σ | (ε/k) | r | (εAB/k)/K | λr | AARD/% | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ps | ρs | ΔHvap | ρl | cP | u | |||||||
| 1-hexanol (C6) | 2.4364 | 4.2407 | 315.79 | 0.28852 | 3102.4 | 11.9366 | 0.81 | 1.06 | 1.64 | 0.23 | 1.60 | 2.09 |
| 1-heptanol (C7) | 2.4415 | 4.4413 | 336.05 | 0.26707 | 3404.3 | 12.2372 | 0.81 | 0.73 | 3.11 | 0.57 | 2.00 | 2.12 |
| 1-octanol (C8) | 2.7639 | 4.4240 | 342.37 | 0.29096 | 3180.6 | 12.9708 | 0.67 | 0.61 | 3.21 | 0.64 | 3.17 | 3.61 |
| 1-nonanol (C9) | 2.9172 | 4.4871 | 358.14 | 0.26918 | 3398.7 | 13.3660 | 0.57 | 1.05 | 3.84 | 0.48 | 2.12 | 1.77 |
| 1-decanol (C10) | 2.4778 | 4.9442 | 409.06 | 0.28053 | 3457.4 | 14.3970 | 0.83 | 0.66 | 3.29 | 0.77 | 5.51 | 1.98 |
| average | — | — | — | — | — | — | 0.74 | 0.82 | 3.02 | 0.54 | 2.88 | 2.31 |
| Substances | Vapor-liquid equilibrium | Compressed liquid phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T range/K | T range/K | P range/MPa | |||||||
| Ps | ρs | ΔHvap | ρl | cP | u | ρl | cP | u | |
| 1-hexanol | 340—600 | 340—600 | 280—560 | 278—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—810 |
| 1-heptanol | 320—620 | 320—620 | 280—580 | 278—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—810 |
| 1-octanol | 320—640 | 320—640 | 280—630 | 278—358 | 325—570 | 291—433 | 0.1—60 | 2—30 | 0.1—811 |
| 1-nonanol | 340—640 | 340—640 | 300—640 | 278—358 | 298—318 | 303—393 | 0.1—60 | 0.1—100 | 0.1—506 |
| 1-decanol | 360—660 | 360—660 | 300—660 | 288—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—506 |
表2 热物性实验数据选取的温度和压力范围[7,31-34]
Table 2 The temperature and pressure range selected for the thermodynamic properties experimental data[7,31-34]
| Substances | Vapor-liquid equilibrium | Compressed liquid phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T range/K | T range/K | P range/MPa | |||||||
| Ps | ρs | ΔHvap | ρl | cP | u | ρl | cP | u | |
| 1-hexanol | 340—600 | 340—600 | 280—560 | 278—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—810 |
| 1-heptanol | 320—620 | 320—620 | 280—580 | 278—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—810 |
| 1-octanol | 320—640 | 320—640 | 280—630 | 278—358 | 325—570 | 291—433 | 0.1—60 | 2—30 | 0.1—811 |
| 1-nonanol | 340—640 | 340—640 | 300—640 | 278—358 | 298—318 | 303—393 | 0.1—60 | 0.1—100 | 0.1—506 |
| 1-decanol | 360—660 | 360—660 | 300—660 | 288—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—506 |
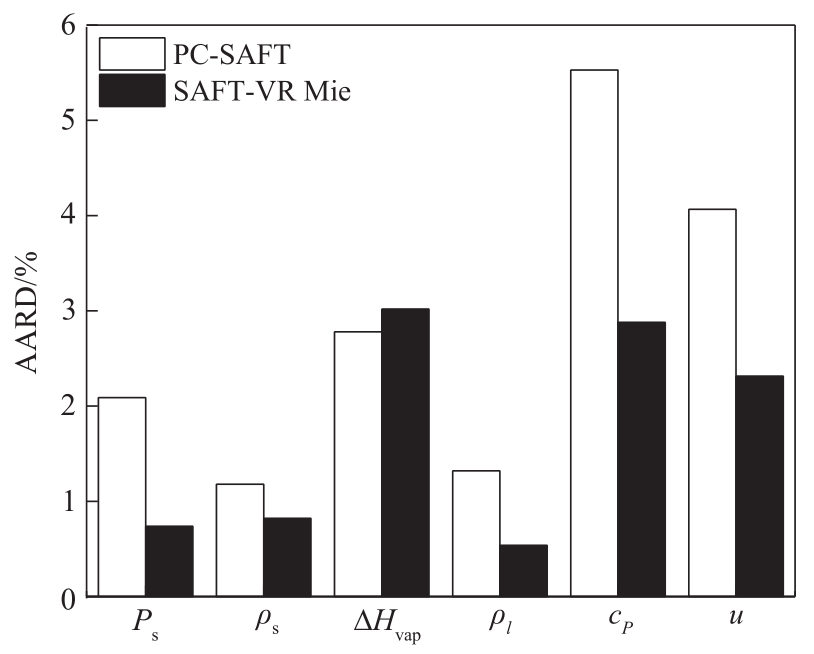
图2 SAFT-VR Mie和PC-SAFT状态方程对C6~C10烷醇热物性平均预测偏差比较
Fig.2 Comparison of the deviations of SAFT-VR Mie and PC-SAFT EoS for predicting the thermodynamic properties of C6—C10 alcohols
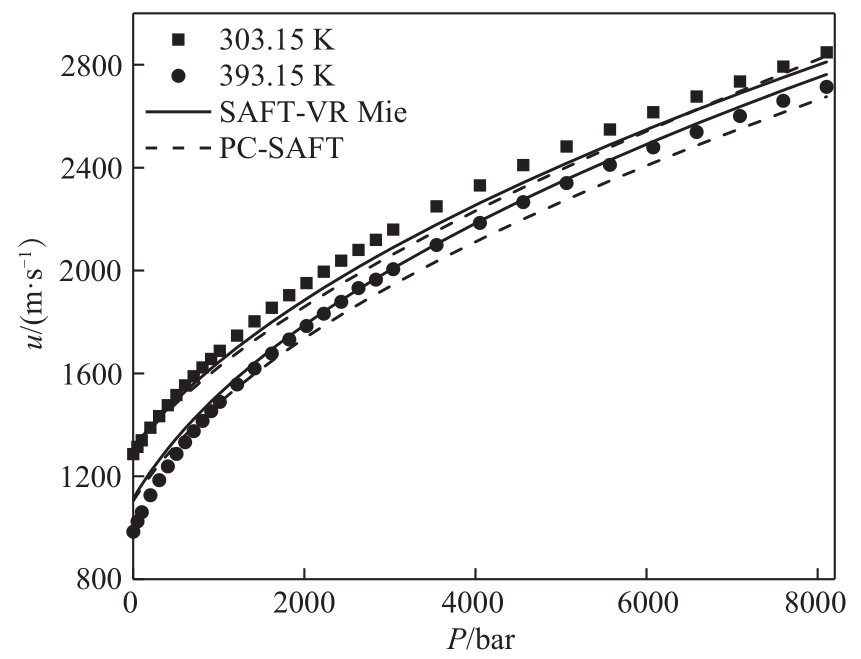
图3 SAFT-VR Mie和PC-SAFT状态方程对正己醇(C6)声速预测结果比较(1 bar=105 Pa)
Fig.3 Comparison of the results of SAFT-VR Mie and PC-SAFT EoS for predicting the speed of sound of 1-hexanol (C6)
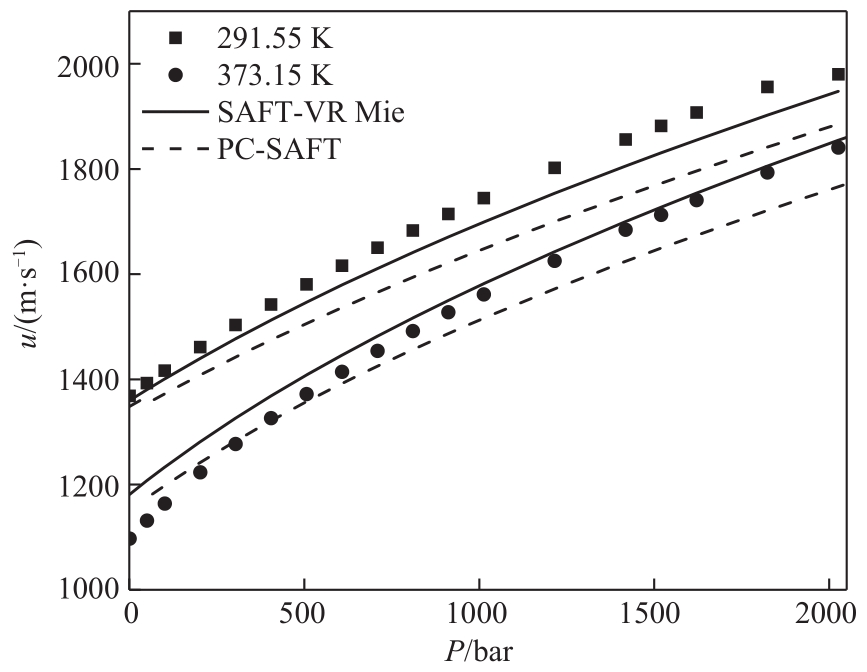
图4 SAFT-VR Mie和PC-SAFT状态方程对正辛醇(C8)声速预测结果比较
Fig.4 Comparison of the results of SAFT-VR Mie and PC-SAFT EoS for predicting the speed of sound of 1-octanol (C8)
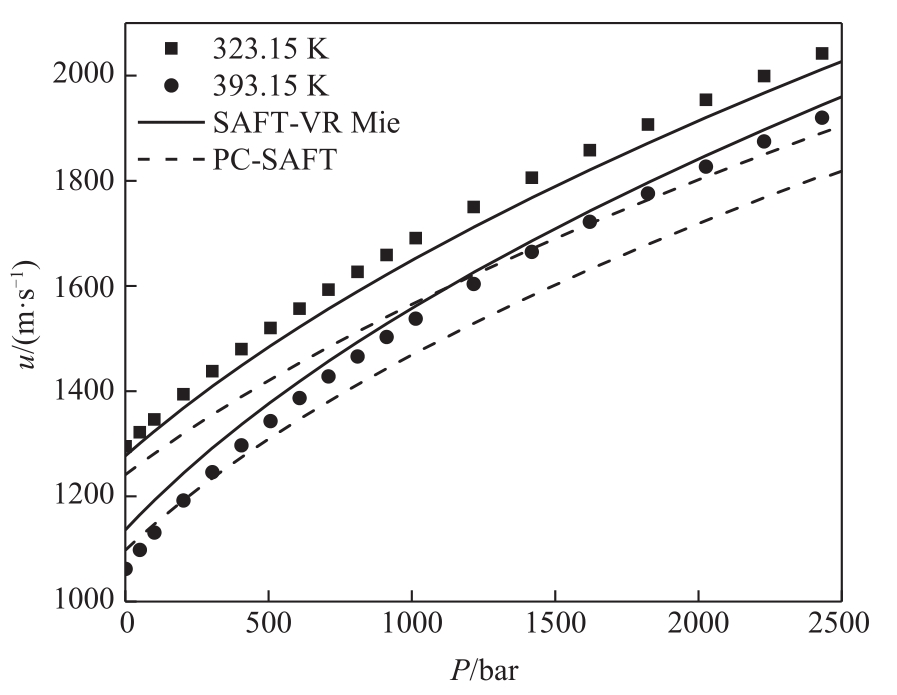
图5 SAFT-VR Mie和PC-SAFT状态方程对正癸醇(C10)声速预测结果比较
Fig.5 Comparison of the results of SAFT-VR Mie and PC-SAFT EoS for predicting the speed of sound of 1-decanol (C10)
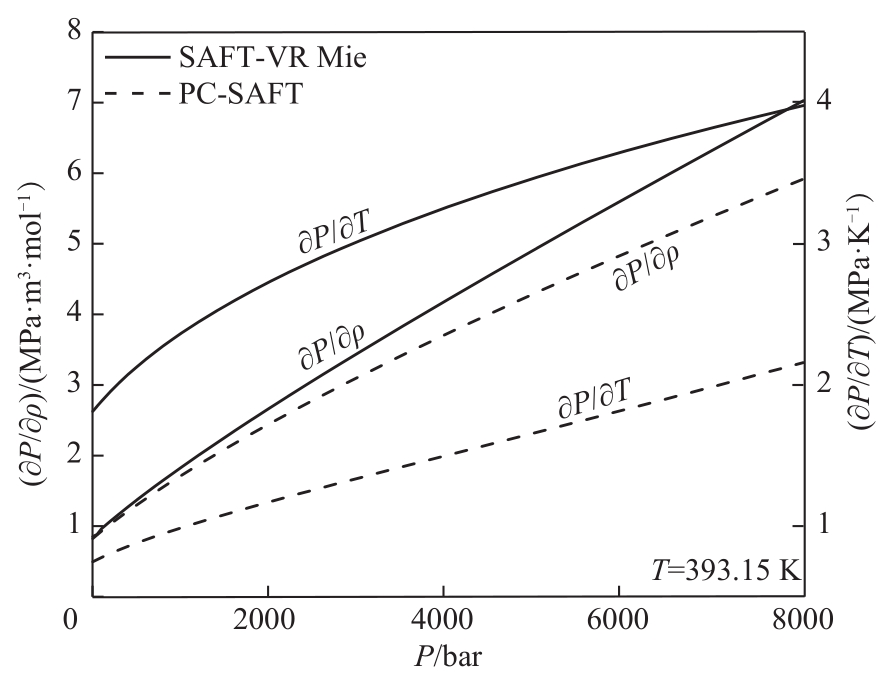
图6 SAFT-VR Mie和PC-SAFT状态方程对正己醇(C6)压力-密度和压力-温度导数预测结果比较
Fig.6 Comparison of the results of SAFT-VR Mie and PC-SAFT EoS for predicting the pressure-density and pressure-temperature derivatives of 1-hexanol (C6)
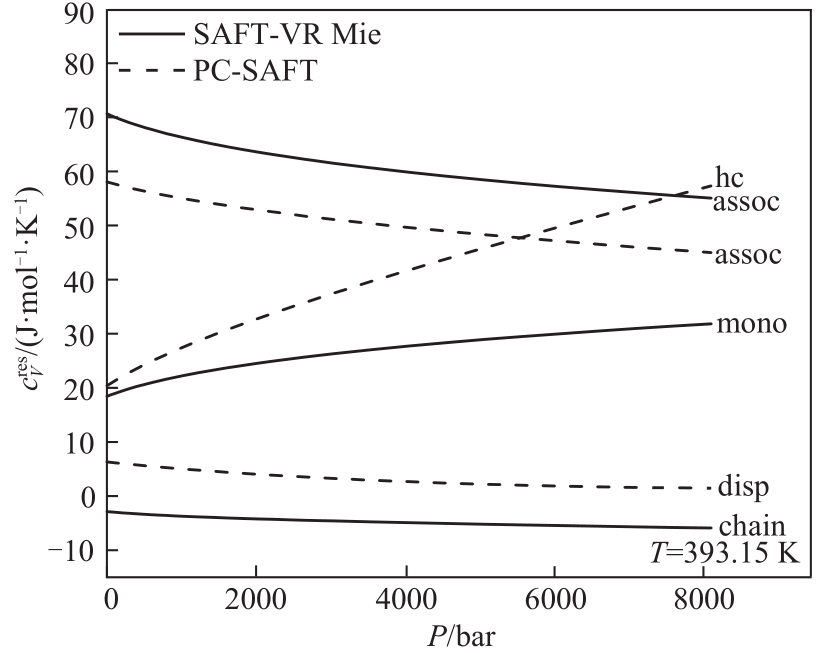
图7 SAFT-VR Mie和PC-SAFT状态方程对正己醇(C6)剩余比定容热容不同微观贡献预测结果比较
Fig.7 Comparison of different microscopic contributions of SAFT-VR Mie and PC-SAFT EoS for predicting residual isochoric specific heat capacity of 1-hexanol (C6)

图8 SAFT-VR Mie和PC-SAFT状态方程对正己醇(C6)二阶温度导数不同微观贡献预测结果比较
Fig.8 Comparison of different microscopic contributions of SAFT-VR Mie and PC-SAFT EoS for predicting second-order temperature derivative of 1-hexanol (C6)
| 1 | Hayer H, Haghbakhsh R, Keshtkari S, et al. Support vector machine and CPA EoS for the prediction of high-pressure liquid densities of normal alkanols[J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(6): 2888-2898. |
| 2 | Menegazzo T A S, Soares Junior A M, Mota B T, et al. Application of an equation of state incorporating association to alcohols up to decanol[J]. Fluid Phase Equilibria, 2019, 482: 24-37. |
| 3 | Pokorný V, Štejfa V, Klajmon M, et al. Vapor pressures and thermophysical properties of 1-heptanol, 1-octanol, 1-nonanol, and 1-decanol: data reconciliation and PC-SAFT modeling[J]. Journal of Chemical & Engineering Data, 2021, 66(1): 805-821. |
| 4 | Schwarz C E. High pressure phase behavior of the homologous series CO2+1-alcohols[J]. Journal of Chemical & Engineering Data, 2018, 63(7): 2451-2466. |
| 5 | Gunasekara S N, Martin V, Chiu J N. Phase equilibrium in the design of phase change materials for thermal energy storage: state-of-the-art[J]. Renewable and Sustainable Energy Reviews, 2017, 73: 558-581. |
| 6 | Pakravesh A, Zarei F, Zarei H. PρT parameterization of SAFT equation of state: developing a new parameterization method for equations of state[J]. Fluid Phase Equilibria, 2021, 538: 113024. |
| 7 | Dávila M J, Alcalde R, Atilhan M, et al. PρT measurements and derived properties of liquid 1-alkanols[J]. The Journal of Chemical Thermodynamics, 2012, 47: 241-259. |
| 8 | Schubert T. Production routes of advanced renewable C1 to C4 alcohols as biofuel components—a review[J]. Biofuels, Bioproducts and Biorefining, 2020, 14(4): 845-878. |
| 9 | Yang Z, Gong M Q, Zhou Y, et al. Vapor-liquid equilibria of CH4, CO2 and their binary system CH4+CO2: a comparison between the molecular simulation and equation of state[J]. Science China Technological Sciences, 2015, 58(4): 650-658. |
| 10 | Diamantonis N I, Boulougouris G C, Mansoor E, et al. Evaluation of cubic, SAFT, and PC-SAFT equations of state for the vapor-liquid equilibrium modeling of CO2 mixtures with other gases[J]. Industrial & Engineering Chemistry Research, 2013, 52(10): 3933-3942. |
| 11 | Redlich O, Kwong J N S. On the thermodynamics of solutions (Ⅴ): An equation of state. Fugacities of gaseous solutions[J]. Chemical Reviews, 1949, 44(1): 233-244. |
| 12 | Soave G. Equilibrium constants from a modified Redlich-Kwong equation of state[J]. Chemical Engineering Science, 1972, 27(6): 1197-1203. |
| 13 | Peng D Y, Robinson D B. A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals, 1976, 15(1): 59-64. |
| 14 | Kontogeorgis G M, Folas G K. Thermodynamic Models for Industrial Applications[M]. United Kingdom: John Wiley & Sons Ltd., 2009. |
| 15 | 吴瑕, 贾文龙, 李长俊, 等. 基于CPA状态方程计算天然气-甲醇-水气液相平衡[J]. 化学工程, 2018, 46(6): 37-41. |
| Wu X, Jia W L, Li C J, et al. Phase equilibrium of natural gas/methanol/water mixtures by use of CPA EoS[J]. Chemical Engineering (China), 2018, 46(6): 37-41. | |
| 16 | De Villiers A J. Evaluation and improvement of the sPC-SAFT equation of state for complex mixtures[D]. Stellenbosch: Stellenbosch University, 2011. |
| 17 | Al-Saifi N M, Hamad E Z, Englezos P. Prediction of vapor-liquid equilibrium in water-alcohol-hydrocarbon systems with the dipolar perturbed-chain SAFT equation of state[J]. Fluid Phase Equilibria, 2008, 271(1/2): 82-93. |
| 18 | Chapman W G, Gubbins K E, Jackson G, et al. New reference equation of state for associating liquids[J]. Industrial & Engineering Chemistry Research, 1990, 29(8): 1709-1721. |
| 19 | Huang S H, Radosz M. Equation of state for small, large, polydisperse, and associating molecules[J]. Industrial & Engineering Chemistry Research, 1990, 29(11): 2284-2294. |
| 20 | Gross J, Sadowski G. Perturbed-chain SAFT: an equation of state based on a perturbation theory for chain molecules[J]. Industrial & Engineering Chemistry Research, 2001, 40(4): 1244-1260. |
| 21 | Lafitte T, Apostolakou A, Avendaño C, et al. Accurate statistical associating fluid theory for chain molecules formed from Mie segments[J]. The Journal of Chemical Physics, 2013, 139(15): 154504. |
| 22 | Jamali A, Behnejad H. Observations regarding the first and second order thermodynamic derivative properties of non-polar and light polar fluids by perturbed chain-SAFT equations of state[J]. Cryogenics, 2019, 99: 78-86. |
| 23 | 杨智, 公茂琼, 李会亚, 等. 基于不同的SAFT类状态方程研究甲烷的热物性[J]. 低温工程, 2015(3): 6-12. |
| Yang Z, Gong M Q, Li H Y, et al. Thermophysical properties of methane studied by different SAFT-type equation of state[J]. Cryogenics, 2015(3): 6-12. | |
| 24 | 屈绍广, 王昶昊, 施云海, 等. 基团贡献状态方程的开发与热力学模型参数的理论预测[J]. 化工学报, 2020, 71(1): 200-208. |
| Qu S G, Wang C H, Shi Y H, et al. Development of group-contribution equation of state and theoretical prediction of thermodynamic model parameters[J]. CIESC Journal, 2020, 71(1): 200-208. | |
| 25 | Hurter R M. Comparing the group-contribution SAFT-γ Mie equation of state with SAFT-VR Mie[D]. Stellenbosch: Stellenbosch University, 2019. |
| 26 | Ramírez-Vélez N, Piña-Martinez A, Jaubert J N, et al. Parameterization of SAFT models: analysis of different parameter estimation strategies and application to the development of a comprehensive database of PC-SAFT molecular parameters[J]. Journal of Chemical & Engineering Data, 2020, 65(12): 5920-5932. |
| 27 | Anoune I, Mimoune Z, Madani H, et al. New modified PC-SAFT pure component parameters for accurate VLE and critical phenomena description[J]. Fluid Phase Equilibria, 2021, 532: 112916. |
| 28 | Rehner P, Gross J. Multiobjective optimization of PCP-SAFT parameters for water and alcohols using surface tension data[J]. Journal of Chemical & Engineering Data, 2020, 65(12): 5698-5707. |
| 29 | Dufal S, Lafitte T, Galindo A, et al. Developing intermolecular-potential models for use with the SAFT-VR Mie equation of state[J]. AIChE Journal, 2015, 61(9): 2891-2912. |
| 30 | Cripwell J, Smith S A M, Schwarz C E, et al. SAFT-VR Mie: application to phase equilibria of alcohols in mixtures with n-alkanes and water[J]. Industrial & Engineering Chemistry Research, 2018, 57(29): 9693-9706. |
| 31 | Cibulka I. Saturated liquid densities of 1-alkanols from C1 to C10 and n-alkanes from C5 to C16: a critical evaluation of experimental data[J]. Fluid Phase Equilibria, 1993, 89(1): 1-18. |
| 32 | Fulem M, Růžička K, Růžička V. Heat capacities of alkanols. Part I. Selected 1-alkanols C2 to C10 at elevated temperatures and pressures[J]. Thermochimica Acta, 2002, 382(1): 119-128. |
| 33 | Postnikov E B, Goncharov A L, Cohen N, et al. Estimating the liquid properties of 1-alkanols from C5 to C12 by FT-EoS and CP-PC-SAFT: simplicity versus complexity[J]. The Journal of Supercritical Fluids, 2015, 104: 193-203. |
| 34 | Green D W, Southard M Z. Perry’s Chemical Engineers’ Handbook[M]. New York: McGraw-Hill Education, 2019. |
| 35 | Lee L L. Molecular Thermodynamics of Nonideal Fluids[M]. Amsterdam: Elsevier, 1988: 373-393. |
| 36 | Barker J A, Henderson D. Perturbation theory and equation of state for fluids (Ⅱ): A successful theory of liquids[J]. The Journal of Chemical Physics, 1967, 47(11): 4714-4721. |
| 37 | Barker J A, Henderson D. What is “liquid”? Understanding the states of matter[J]. Reviews of Modern Physics, 1976, 48(4): 587-671. |
| 38 | Wertheim M S. Fluids of dimerizing hard spheres, and fluid mixtures of hard spheres and dispheres[J]. The Journal of Chemical Physics, 1986, 85(5): 2929-2936. |
| 39 | Chapman W G, Gubbins K E, Jackson G, et al. SAFT: equation-of-state solution model for associating fluids[J]. Fluid Phase Equilibria, 1989, 52: 31-38. |
| 40 | Lafitte T, Piñeiro M M, Daridon J L, et al. A comprehensive description of chemical association effects on second derivative properties of alcohols through a SAFT-VR approach[J]. The Journal of Physical Chemistry. B, 2007, 111(13): 3447-3461. |
| 41 | Gross J, Sadowski G. Application of the perturbed-chain SAFT equation of state to associating systems[J]. Industrial & Engineering Chemistry Research, 2002, 41(22): 5510-5515. |
| 42 | Zheng K, Wu H S, Geng C Y, et al. A comparative study of the perturbed-chain statistical associating fluid theory equation of state and activity coefficient models in phase equilibria calculations for mixtures containing associating and polar components[J]. Industrial & Engineering Chemistry Research, 2018, 57(8): 3014-3030. |
| [1] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [2] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [3] | 李贵贤, 曹阿波, 孟文亮, 王东亮, 杨勇, 周怀荣. 耦合固体氧化物电解槽的CO2制甲醇过程设计与评价研究[J]. 化工学报, 2023, 74(7): 2999-3009. |
| [4] | 李振, 张博, 王丽伟. PEG-EG固-固相变材料的制备和性能研究[J]. 化工学报, 2023, 74(6): 2680-2688. |
| [5] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [6] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [7] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [8] | 张雪婷, 胡激江, 赵晶, 李伯耿. 高分子量聚丙二醇在微通道反应器中的制备[J]. 化工学报, 2023, 74(3): 1343-1351. |
| [9] | 何金峰, 李秀珍, 寇建耀, 陶庭杰, 余灿, 刘欢, 陈永元, 赵豪健, 江大好, 李小年. 乙醇制高级醇有序介孔氧化铝负载铜基催化剂研究[J]. 化工学报, 2023, 74(3): 1082-1091. |
| [10] | 王帅, 杨富凯, 徐新宇. 阻燃型全生物基多元醇聚氨酯泡沫的制备及性能研究[J]. 化工学报, 2023, 74(3): 1399-1408. |
| [11] | 项望凯, 刘园园, 郑映, 潘鹏举. 基于熔融/固相缩聚制备中高分子量聚乙醇酸[J]. 化工学报, 2023, 74(2): 933-940. |
| [12] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [13] | 沈辰阳, 孙楷航, 张月萍, 刘昌俊. 二氧化碳加氢合成甲醇氧化铟及其负载金属催化剂研究进展[J]. 化工学报, 2023, 74(1): 145-156. |
| [14] | 刘佳宁, 马嘉浩, 张军营, 程珏. 顺序双重热固化的硫醇-丙烯酸酯-环氧树脂三维网络的构建及性能[J]. 化工学报, 2022, 73(9): 4173-4186. |
| [15] | 杨松涛, 李东洋, 牛玉清, 李鑫钢, 康绍辉, 李洪, 叶开凯, 周志全, 高鑫. 氟化物势能函数和热力学性质的分子模拟研究进展[J]. 化工学报, 2022, 73(9): 3828-3840. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号