化工学报 ›› 2020, Vol. 71 ›› Issue (4): 1781-1790.DOI: 10.11949/0438-1157.20190924
收稿日期:2019-08-12
修回日期:2019-10-27
出版日期:2020-04-05
发布日期:2020-04-05
通讯作者:
毛旭辉
作者简介:刘小艳(1994—),女,硕士研究生,基金资助:
Xiaoyan LIU1( ),Wanxin CAI1,Likun ZHAO1,Xiang ZENG2,Xuhui MAO1(
),Wanxin CAI1,Likun ZHAO1,Xiang ZENG2,Xuhui MAO1( )
)
Received:2019-08-12
Revised:2019-10-27
Online:2020-04-05
Published:2020-04-05
Contact:
Xuhui MAO
摘要:
研究了初始游离氯浓度以及活性炭粒径和投加量等对游离氯去除的影响,并通过Boehm滴定、傅里叶转换红外光谱仪、比表面积分析仪、扫描电子显微镜、光电子能谱等手段分析了反应前后的活性炭,发现活性炭的失效主要是由于其表面还原性官能团的消耗及表面结构的氧化破坏所带来的孔结构和比表面积变化。将失效活性炭在不同气氛(氮气、氢气、氨气)条件下进行热再生,均可使其游离氯去除能力得到恢复,且氨气条件最好,这主要得益于孔结构的提升及还原性官能团的再生。将再生后活性炭进行连续流柱实验,证实其能够长时间有效运行。
中图分类号:
刘小艳, 蔡万欣, 赵立坤, 曾香, 毛旭辉. 活性炭去除游离氯的失效机制及热再生研究[J]. 化工学报, 2020, 71(4): 1781-1790.
Xiaoyan LIU, Wanxin CAI, Likun ZHAO, Xiang ZENG, Xuhui MAO. Failure mechanism and thermal regeneration of activated carbon for free chlorine removal[J]. CIESC Journal, 2020, 71(4): 1781-1790.
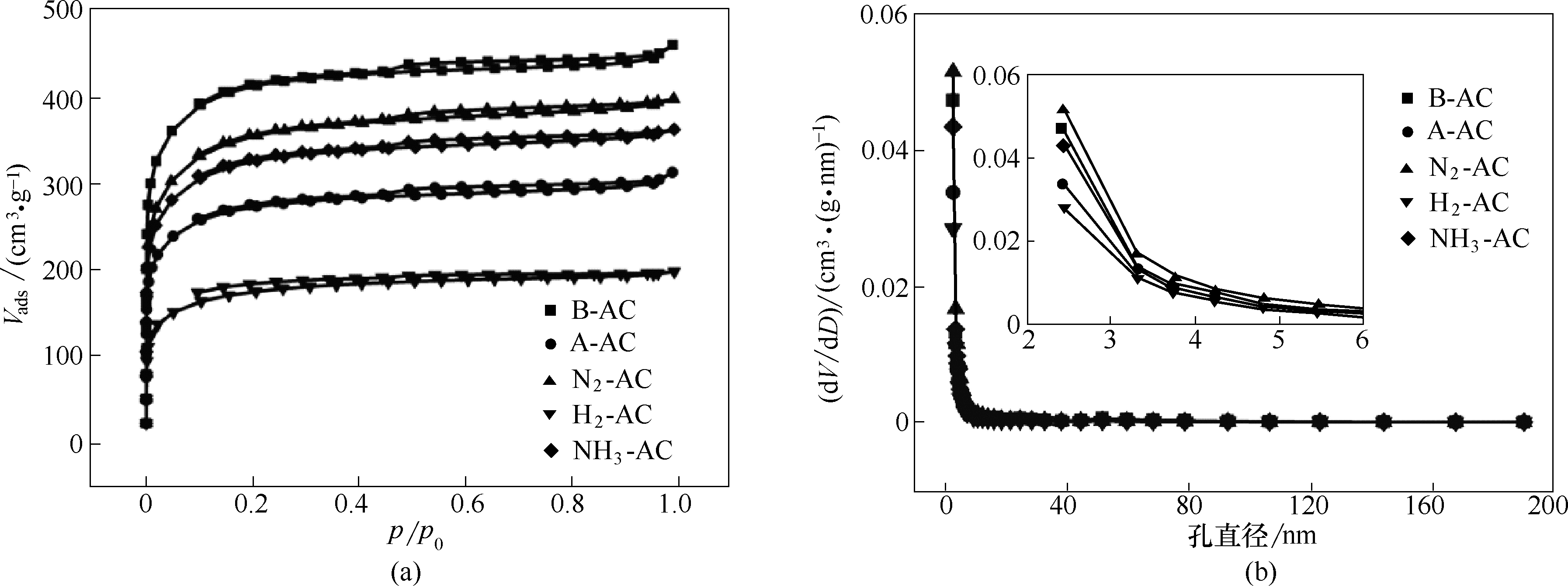
图3 失效及再生前后活性炭的氮气吸脱附曲线(a)及孔径分布(b)
Fig.3 Nitrogen absorption and desorption curves (a) and pore size distribution (b) of activated carbon and the ones after failure and regeneration
| 样品 | 比表面积/ (m2?g-1) | 孔容/(cm3?g-1) | 微孔/(cm3?g-1) | 中孔/(cm3?g-1) | 平均粒径/nm | 元素分析/% | ||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| B-AC | 1512.2 | 0.7128 | 0.665 | 0.0478 | 1.8855 | 73.45 | 1.67 | 0.09 |
| A-AC | 999.1 | 0.4845 | 0.447 | 0.0375 | 1.9399 | 63.60 | 2.11 | 0.05 |
| N2-AC | 1289.8 | 0.616 | 0.588 | 0.028 | 1.9102 | 66.76 | 2.66 | 0.10 |
| H2-AC | 645.93 | 0.3168 | 0.3014 | 0.0154 | 1.9617 | 76.70 | 1.45 | 0.07 |
| NH3-AC | 1186.9 | 0.5608 | 0.5355 | 0.0253 | 1.8899 | 67.88 | 1.98 | 1.35 |
表1 活性炭表面结构特性
Table 1 Surface structure characteristics of activated carbon
| 样品 | 比表面积/ (m2?g-1) | 孔容/(cm3?g-1) | 微孔/(cm3?g-1) | 中孔/(cm3?g-1) | 平均粒径/nm | 元素分析/% | ||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| B-AC | 1512.2 | 0.7128 | 0.665 | 0.0478 | 1.8855 | 73.45 | 1.67 | 0.09 |
| A-AC | 999.1 | 0.4845 | 0.447 | 0.0375 | 1.9399 | 63.60 | 2.11 | 0.05 |
| N2-AC | 1289.8 | 0.616 | 0.588 | 0.028 | 1.9102 | 66.76 | 2.66 | 0.10 |
| H2-AC | 645.93 | 0.3168 | 0.3014 | 0.0154 | 1.9617 | 76.70 | 1.45 | 0.07 |
| NH3-AC | 1186.9 | 0.5608 | 0.5355 | 0.0253 | 1.8899 | 67.88 | 1.98 | 1.35 |
| 样品 | 表面官能团/(mmol?g-1) | ||||
|---|---|---|---|---|---|
| —OH | —COOR | —COOH | 酸性 官能团 | 碱性 官能团 | |
| B-AC | 0.07 | 0.22 | 0.07 | 0.36 | 0.55 |
| A-AC | 1.12 | 1.17 | 2.17 | 4.49 | 0.35 |
| N2-AC | 0.37 | 0.07 | — | 0.44 | 0.45 |
| H2-AC | 0.12 | 0.05 | 0.04 | 0.22 | 0.50 |
| NH3-AC | 0.16 | 0.17 | — | 0.34 | 0.71 |
表2 活性炭表面官能团的测定结果
Table 2 Results of surface functional groups of activated carbon
| 样品 | 表面官能团/(mmol?g-1) | ||||
|---|---|---|---|---|---|
| —OH | —COOR | —COOH | 酸性 官能团 | 碱性 官能团 | |
| B-AC | 0.07 | 0.22 | 0.07 | 0.36 | 0.55 |
| A-AC | 1.12 | 1.17 | 2.17 | 4.49 | 0.35 |
| N2-AC | 0.37 | 0.07 | — | 0.44 | 0.45 |
| H2-AC | 0.12 | 0.05 | 0.04 | 0.22 | 0.50 |
| NH3-AC | 0.16 | 0.17 | — | 0.34 | 0.71 |
| 结合能/eV | B-AC | A-AC | N2-AC | H2-AC | NH3-AC | ||
|---|---|---|---|---|---|---|---|
| C 1s | C—C | 284.8 | 71.03 | 56.33 | 60.23 | 61.04 | 57.71 |
| C—O | 286.0 | 16.23 | 14.42 | 17.60 | 8.89 | 11.23 | |
C O O | 287.4 | 0.22 | 1.31 | 0.07 | 3.21 | 6.89 | |
—O—C O O | 288.8 | 2.39 | 7.42 | 3.85 | 2.90 | 2.12 | |
| 总原子C/% | 89.87 | 79.47 | 87.79 | 81.53 | 79.82 | ||
| O 1s | C O O | 531.0~531.9 | 4.81 | 10.92 | 8.16 | 5.65 | 5.8 |
| C—O | 533.0~533.4 | 4.89 | 7.42 | 7.26 | 4.34 | 6.33 | |
| H2O | 535.2~535.4 | 0 | 1.51 | 2.50 | 1.73 | 2.18 | |
| 总原子O/% | 9.70 | 19.86 | 17.92 | 11.73 | 14.31 | ||
| O/C比 | 0.11 | 0.25 | 0.13 | 0.22 | 0.18 | ||
表3 XPS C 1s/O 1s区域拟合的结果(校正为总原子比)
Table 3 Deconvolution of XPS C1s/O1s region (corrected to total oxygen atomic percent)/%(atom)
| 结合能/eV | B-AC | A-AC | N2-AC | H2-AC | NH3-AC | ||
|---|---|---|---|---|---|---|---|
| C 1s | C—C | 284.8 | 71.03 | 56.33 | 60.23 | 61.04 | 57.71 |
| C—O | 286.0 | 16.23 | 14.42 | 17.60 | 8.89 | 11.23 | |
C O O | 287.4 | 0.22 | 1.31 | 0.07 | 3.21 | 6.89 | |
—O—C O O | 288.8 | 2.39 | 7.42 | 3.85 | 2.90 | 2.12 | |
| 总原子C/% | 89.87 | 79.47 | 87.79 | 81.53 | 79.82 | ||
| O 1s | C O O | 531.0~531.9 | 4.81 | 10.92 | 8.16 | 5.65 | 5.8 |
| C—O | 533.0~533.4 | 4.89 | 7.42 | 7.26 | 4.34 | 6.33 | |
| H2O | 535.2~535.4 | 0 | 1.51 | 2.50 | 1.73 | 2.18 | |
| 总原子O/% | 9.70 | 19.86 | 17.92 | 11.73 | 14.31 | ||
| O/C比 | 0.11 | 0.25 | 0.13 | 0.22 | 0.18 | ||
| 时间/d | 游离氯/活性炭/ (mg?g-1) | B-AC 出水游离氯/ (mg?L-1) | N2-AC出水游离氯/ (mg?L-1) | NH3-AC出水游离氯/(mg?L-1) |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 3 | 172.8 | 0.6 | 0.2 | 0.4 |
| 6 | 345.2 | 1.4 | 0 | 0 |
| 7 | 403.2 | 0.4 | 2.4 | 0.2 |
| 10 | 576 | 0.4 | 2.8 | 0.4 |
| 11 | 633.6 | 0.2 | 4.2 | 1.2 |
| 14 | 806.4 | 0.6 | 6.8 | 2 |
| 15 | 864 | 8.6 | — | — |
| 17 | 979.2 | 29.2 | 6.7 | 2.8 |
表4 原始活性炭及热再生活性炭柱实验结果
Table 4 Column experimental results of original activated carbon and thermal-regenerated activated carbon
| 时间/d | 游离氯/活性炭/ (mg?g-1) | B-AC 出水游离氯/ (mg?L-1) | N2-AC出水游离氯/ (mg?L-1) | NH3-AC出水游离氯/(mg?L-1) |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 3 | 172.8 | 0.6 | 0.2 | 0.4 |
| 6 | 345.2 | 1.4 | 0 | 0 |
| 7 | 403.2 | 0.4 | 2.4 | 0.2 |
| 10 | 576 | 0.4 | 2.8 | 0.4 |
| 11 | 633.6 | 0.2 | 4.2 | 1.2 |
| 14 | 806.4 | 0.6 | 6.8 | 2 |
| 15 | 864 | 8.6 | — | — |
| 17 | 979.2 | 29.2 | 6.7 | 2.8 |
| 20 | Perrard A, Retailleau L, Berjoan R, et al. Liquid phase oxidation kinetics of an ex-cellulose activated carbon cloth by NaOCl[J]. Carbon, 2012, 50(6): 2226-2234. |
| 21 | Guedidi H, Reinert L, Leveque J M, et al. The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen[J]. Carbon, 2013, 54: 432-443. |
| 22 | Pereira M F R, Soares S F, Orfao J J M, et al. Adsorption of dyes on activated carbons: influence of surface chemical groups[J]. Carbon, 2003, 41(4): 811-821. |
| 23 | Menendez J A, Phillips J, Xia B, et al. On the modification and characterization of chemical surface properties of activated carbon: in the search of carbons with stable basic properties[J]. Langmuir, 1996, 12(18): 4404-4410. |
| 24 | Mangun C L, Benak K R, Economy J, et al. Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia[J]. Carbon, 2001, 39(12): 1809-1820. |
| 25 | 李霞, 陈思莉, 卓琼芳, 等. 热改性活性炭吸附甲萘威的性能[J]. 安全与环境学报, 2017, 17(5): 1915-1921. |
| Li X, Chen S L, Zhuo Q F, et al. On the adsorptive performance of carbaryl onto the activated carbons with the thermal treatment[J]. Journal of Safety and Environment, 2017, 17(5): 1915-1921. | |
| 26 | Torrellas S A, Lovera R G, Escalona N, et al. Chemical-activated carbons from peach stones for the adsorption of emerging contaminants in aqueous solutions[J]. Chemical Engineering Journal, 2015, 279: 788-798. |
| 27 | Vassallo A M, Attalla M I. Studies of thermal transformations of fulvic acids using Fourier transform-infrared emission spectroscopy[J]. Journal of Analytical and Applied Pyrolysis, 1992, 23(1): 73-85. |
| 28 | Lua A C, Yang T. Effects of vacuum pyrolysis conditions on the characteristics of activated carbons derived from pistachio-nut shells[J]. Journal of Colloid and Interface Science, 2004, 276(2): 364-372. |
| 29 | Yang S Y, Li L, Xiao T, et al. Role of surface chemistry in modified ACF (activated carbon fiber)-catalyzed peroxymonosulfate oxidation[J]. Applied Surface Science, 2016, 383: 142-150. |
| 30 | Pradhan B K, Sandle N K. Effect of different oxidizing agent treatments on the surface properties of activated carbons[J]. Carbon, 1999, 37(8): 1323-1332. |
| 1 | 张怀旭, 刘婉冬, 李冰璟, 等. 活性炭去除水中余氯的研究[J]. 环境污染与防治, 2008, 30(5): 63-68. |
| Zhang H X, Liu W D, Li B J, et al. Activated carbon treatment for removing residual free chlorine in water[J]. Environmental Pollution and Control, 2008, 30(5): 63-68. | |
| 31 | Aviles F, Cauich-Rodriguez J V, Moo-Tah L, et al. Evaluation of mild acid oxidation treatments for MWCNT functionalization[J]. Carbon, 2009, 47(13): 2970-2975. |
| 32 | Kabel K I, Farag A A, Elnaggar E M, et al. Removal of oxidation fragments from multi-walled carbon nanotubes oxide using high and low concentrations of sodium hydroxide[J]. Arabian Journal for Science and Engineering, 2016, 41(6): 2211-2220. |
| 2 | Gopal K, Tripathy S S, Bersillon J L, et al. Chlorination byproducts, their toxicodynamics and removal from drinking water[J]. Journal of Hazardous Materials, 2007, 140(1/2): 1-6. |
| 3 | Simate G S, Iyuke S E, Ndlovu S, et al. Human health effects of residual carbon nanotubes and traditional water treatment chemicals in drinking water[J]. Environment International, 2012, 39(1): 38-49. |
| 4 | Jaguaribe E F, Medeiros L L, Barreto M C S, et al. The performance of activated carbons from sugarcane bagasse, babassu, and coconut shells in removing residual chlorine[J]. Brazilian Journal of Chemical Engineering, 2005, 22(1): 41-47. |
| 5 | 王丽萍, 徐斌, 钱灏. 净水用颗粒活性炭对水中余氯去除的动力学原理效能[J]. 净水技术, 2018, 39(1): 45-52. |
| Wang L P, Xu B, Qian H. Principle and efficiency of residual chlorine removal by granular activated carbon in drinking water[J]. Water Purification Technology, 2018, 39(1): 47-52. | |
| 6 | Martin R J, Shackleton R C. Comparison of two partially activated carbon fabrics for the removal of chlorine and other impurities from water[J]. Water Research, 1990, 24(2): 474-484. |
| 7 | Asada T, Okazaki A, Kawata K, et al. Influence of pore properties and solution pH on removal of free chlorine and combined chlorine by porous carbon[J]. Journal of Health Science, 2009, 55(4): 649-656. |
| 8 | Ogata F, Tominaga H, Ueda A, et al. Application of activated carbons from coal and coconut shell for removing free residual chlorine[J]. Journal of Oleo Science, 2013, 62(4): 241-244. |
| 9 | 邹萍, 隋贤栋, 黄肖容. 铜锌改性活性炭的制备及对水中余氯的去除[J]. 材料开发与应用, 2009, 24(4): 48-50. |
| Zou P, Sui X D, Huang X R. Cu-Zn modified activated carbon: preparation and function in removing of chlorine residue[J]. Development and Application of Materials, 2009, 24(4): 48-50. | |
| 10 | Suidan M T, Cross W H, Chacey K A. Extended dechlorination studies with granular activated carbon filters[J]. Journal Water Pollution Control Federation, 1980, 52(11): 2634-2646. |
| 11 | Salvador F, Martin-Sanchez N, Sanchez-Hernandez R, et al. Regeneration of carbonaceous adsorbents(I): Thermal regeneration[J]. Microporous and Mesoporous Materials, 2015, 202: 259-276. |
| 12 | 吉中伟. 几种活性炭再生技术的比较[J]. 科技技术创新, 2017, (36): 200-201. |
| Ji Z W. Comparison of several activated carbon regeneration technologies[J]. Scientific and Technological Innovation, 2017, (36): 200-201. | |
| 13 | 李立清, 顾庆伟, 石瑞, 等. 热改性活性炭吸附有机气体的性能[J]. 化工学报, 2012, 63(6): 1749-1756. |
| Li L Q, Gu Q W, Shi R, et al. Adsorption of VOCs onto activated carbons with thermal treatment[J]. CIESC Journal, 2012, 63(6): 1749-1756. | |
| 14 | Przepiorski J. Enhanced adsorption of phenol from water by ammonia-treated activated carbon[J]. Journal of Hazardous Materials, 2006, 135(1/2/3): 453-456. |
| 15 | Mangun C L, Benak K R, Economy J, et al. Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia[J]. Carbon, 2001, 39: 1809-1820. |
| 16 | Boehm H P. Surface oxides on carbon and their analysis: a critical assessment[J]. Carbon, 2002, 40(2): 145-149. |
| 17 | Boehm H P. Some aspects of the surface-chemistry of carbon-blacks and other carbons[J]. Carbon, 1994, 32(5): 759-769. |
| 18 | Meng F K, Li G P, Zhang B B, et al. Chemical kinetics and particle size effects of activated carbon for free chlorine removal from drinking water[J]. Water Practice and Technology, 2018, 14: 19-26. |
| 19 | Wang Z W, Shirley M D, Meikle S T, et al. The surface acidity of acid oxidised multi-walled carbon nanotubes and the influence of in-situ generated fulvic acids on their stability in aqueous dispersions[J]. Carbon, 2009, 47(1): 73-79. |
| [1] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [2] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [3] | 吕龙义, 及文博, 韩沐达, 李伟光, 高文芳, 刘晓阳, 孙丽, 王鹏飞, 任芝军, 张光明. 铁基导电材料强化厌氧去除卤代有机污染物:研究进展及未来展望[J]. 化工学报, 2023, 74(8): 3193-3202. |
| [4] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [5] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [6] | 卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638. |
| [7] | 周继鹏, 何文军, 李涛. 异形催化剂上乙烯催化氧化失活动力学反应工程计算[J]. 化工学报, 2023, 74(6): 2416-2426. |
| [8] | 王承泽, 顾凯丽, 张晋华, 石建轩, 刘艺娓, 李锦祥. 硫化协同老化零价铁增效去除水中Cr(Ⅵ)的作用机制[J]. 化工学报, 2023, 74(5): 2197-2206. |
| [9] | 陈瑞哲, 程磊磊, 顾菁, 袁浩然, 陈勇. 纤维增强树脂复合材料化学回收技术研究进展[J]. 化工学报, 2023, 74(3): 981-994. |
| [10] | 潘煜, 王子航, 王佳韵, 王如竹, 张华. 基于可得然-氯化锂复合吸附剂的除湿换热器热湿性能研究[J]. 化工学报, 2023, 74(3): 1352-1359. |
| [11] | 黄宽, 马永德, 蔡镇平, 曹彦宁, 江莉龙. 油脂催化加氢转化制备第二代生物柴油研究进展[J]. 化工学报, 2023, 74(1): 380-396. |
| [12] | 鲁文静, 李先锋. 液流电池多孔离子传导膜研究进展[J]. 化工学报, 2023, 74(1): 192-204. |
| [13] | 李彬, 宋文明, 杨坤龙, 姜爽, 张天永. 水系有机液流电池活性材料的分子工程研究进展[J]. 化工学报, 2022, 73(7): 2806-2818. |
| [14] | 陈永安, 周安宁, 李云龙, 石智伟, 贺新福, 焦卫红. 磁性MgFe2O4及其核壳催化剂制备与煤热解性能研究[J]. 化工学报, 2022, 73(7): 3026-3037. |
| [15] | 李丽媛, 王建强, 陈奕, 郭友娣, 周健, 刘志成, 王仰东, 谢在库. 甲醇制丙烯反应中ZSM-5分子筛催化剂积炭失活介尺度机制研究[J]. 化工学报, 2022, 73(6): 2669-2676. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号