化工学报 ›› 2020, Vol. 71 ›› Issue (1): 320-328.DOI: 10.11949/0438-1157.20191133
常苗1( ),刘磊1,阳庆元1,刘大欢1(
),刘磊1,阳庆元1,刘大欢1( ),仲崇立1,2(
),仲崇立1,2( )
)
收稿日期:2019-10-08
修回日期:2019-10-18
出版日期:2020-01-05
发布日期:2020-01-05
通讯作者:
刘大欢,仲崇立
作者简介:常苗(1993—),男,硕士研究生,基金资助:
Miao CHANG1( ),Lei LIU1,Qingyuan YANG1,Dahuan LIU1(
),Lei LIU1,Qingyuan YANG1,Dahuan LIU1( ),Chongli ZHONG1,2(
),Chongli ZHONG1,2( )
)
Received:2019-10-08
Revised:2019-10-18
Online:2020-01-05
Published:2020-01-05
Contact:
Dahuan LIU,Chongli ZHONG
摘要:
SF6/N2混合物的高效分离,对SF6气体的回收与减弱其直接排放所导致的温室效应,具有重要的实际意义。合成了一种具有不同性质孔道结构的金属-有机骨架材料,Cu-MOF-OMe。该材料具有含不饱和配位金属位点和甲氧基双功能化的特征,且表现出良好的水热稳定性和再生性能。该材料表现出优异的综合分离性能,298 K和105 Pa下的分离选择性(361)和吸附剂选择性参数 (SSP) 值 (780)均远高于文献报道值。理论计算发现,亲水性孔道中的不饱和金属位和疏水性孔道中丰富的甲氧基的协同作用,为该MOF材料具有优异SF6/N2分离性能的主要原因。研究结果可为SF6/N2高效分离材料的设计与开发提供参考依据。
中图分类号:
常苗, 刘磊, 阳庆元, 刘大欢, 仲崇立. 水热稳定金属-有机骨架材料用于高效分离SF6/N2混合物的研究[J]. 化工学报, 2020, 71(1): 320-328.
Miao CHANG, Lei LIU, Qingyuan YANG, Dahuan LIU, Chongli ZHONG. Study on efficient separation of SF6/N2 mixture using a hydrothermally stable metal-organic framework[J]. CIESC Journal, 2020, 71(1): 320-328.

图3 Cu-MOF-OMe的77 K N2吸附-脱附等温线(a),FT-IR谱图(b)和SEM图(c)
Fig.3 N2 adsorption- desorption isotherms at 77 K (a), FT-IR curve (b) and SEM image (c) of Cu-MOF-OMe
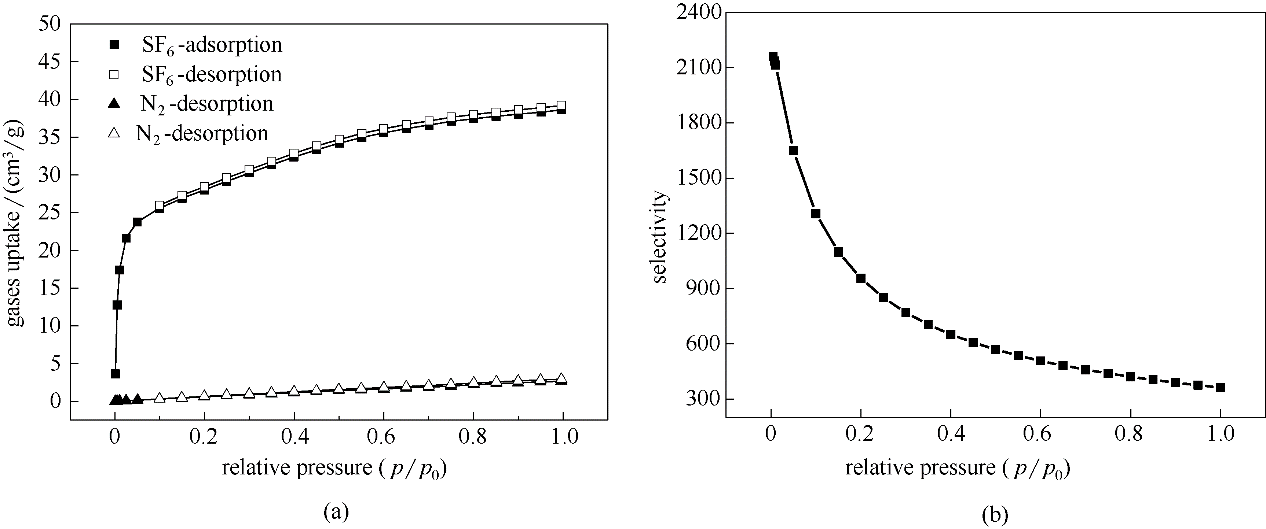
图4 Cu-MOF-OMe在298 K下的 SF6和N2吸附-脱附等温线(a)和IAST理想选择性(b)
Fig.4 Adsorption-desorption isotherms of SF6 and N2 at 298 K (a) and IAST selectivities (b) in Cu-MOF-OMe
| Parameter | SF6 | N2 |
|---|---|---|
| q satA/(cm3/g) | 24.065 | 0.256 |
| b A/Pa-1 | 221729.490 | 1739.130 |
| q satB/(cm3/g) | 30.910 | 25266.260 |
| b B/Pa-1 | 956.938 | 0.0835 |
| R 2 | 0.9992 | 0.9997 |
表1 298 K下Cu-MOF-OMe的SF6和N2吸附数据的拟合参数
Table 1 Fitted parameters of SF6 and N2 sorption data of Cu-MOF-OMe at 298 K
| Parameter | SF6 | N2 |
|---|---|---|
| q satA/(cm3/g) | 24.065 | 0.256 |
| b A/Pa-1 | 221729.490 | 1739.130 |
| q satB/(cm3/g) | 30.910 | 25266.260 |
| b B/Pa-1 | 956.938 | 0.0835 |
| R 2 | 0.9992 | 0.9997 |
| Adsorbents | Selectivity for SF6/N2 mixture (0.1∶0.9) | SF6 uptake at 1.0 bar/(cm3/g) | SSP | Q st for SF6/ (kJ/mol) | Q st for N2/ (kJ/mol) | Ref. |
|---|---|---|---|---|---|---|
| Mg-MOF-74 | 18 | 143.8 | 95 | 32.0 | [ | |
| Co-MOF-74 | 34 | 119.6 | 253 | 40.0 | [ | |
| Zn-MOF-74 | 46 | 82.2 | 447 | 27.0 | [ | |
| Ca-A | 28.5 | 50.4 | 434 | 37.0 | [ | |
| KKUST-1 | 65 | 115.6 | 446 | 25.0 | [ | |
| activated carbon | 30 | 54.2 | 120 | 5.0 | [ | |
| CNHs① | 44 | 83.3 | [ | |||
| MIL-100-Fe | 24 | 37.1 | 100 | 21.0 | 13.0 | [ |
| UIO-66-2Br | 220 | 17.9 | 45 | 40.6 | [ | |
| UIO-66 | 74 | 32.5 | 156 | 33.8 | 14.0 | [ |
| Zeolite-13X | 44 | 39.4 | 162 | 29.5 | 28.0 | [ |
| PC-CaCit | 30 | 80.9 | 66 | 30.0 | [ | |
| PC-MgCit | 30 | 74.8 | 133 | 24.6 | [ | |
| CC3α | 74 | 50.4 | 434 | 37.0 | [ | |
| Cu-MOF-OMe | 361 | 38 | 780 | 57.8 | 18.0 | this work |
表2 298 K和1 bar条件下不同材料的选择性、SF6吸附量、SSP数值、SF6和N2吸附热的对比
Table 2 Comparison of IAST selectivity, uptake capacity of SF6, SSP values, adsorption heat of SF6 and N2 in different materials at 298 K and 1 bar
| Adsorbents | Selectivity for SF6/N2 mixture (0.1∶0.9) | SF6 uptake at 1.0 bar/(cm3/g) | SSP | Q st for SF6/ (kJ/mol) | Q st for N2/ (kJ/mol) | Ref. |
|---|---|---|---|---|---|---|
| Mg-MOF-74 | 18 | 143.8 | 95 | 32.0 | [ | |
| Co-MOF-74 | 34 | 119.6 | 253 | 40.0 | [ | |
| Zn-MOF-74 | 46 | 82.2 | 447 | 27.0 | [ | |
| Ca-A | 28.5 | 50.4 | 434 | 37.0 | [ | |
| KKUST-1 | 65 | 115.6 | 446 | 25.0 | [ | |
| activated carbon | 30 | 54.2 | 120 | 5.0 | [ | |
| CNHs① | 44 | 83.3 | [ | |||
| MIL-100-Fe | 24 | 37.1 | 100 | 21.0 | 13.0 | [ |
| UIO-66-2Br | 220 | 17.9 | 45 | 40.6 | [ | |
| UIO-66 | 74 | 32.5 | 156 | 33.8 | 14.0 | [ |
| Zeolite-13X | 44 | 39.4 | 162 | 29.5 | 28.0 | [ |
| PC-CaCit | 30 | 80.9 | 66 | 30.0 | [ | |
| PC-MgCit | 30 | 74.8 | 133 | 24.6 | [ | |
| CC3α | 74 | 50.4 | 434 | 37.0 | [ | |
| Cu-MOF-OMe | 361 | 38 | 780 | 57.8 | 18.0 | this work |
| 吸附分子 | σ/ ? | (ε/k b)/K | 电荷 /e |
|---|---|---|---|
| SF6 | 4.615 | 238.89 | — |
| N2_N | 3.32 | 36.4 | -0.482 |
| N2_com | — | — | 0.964 |
表3 SF6和N2的力场参数
Table 3 Force field information of SF6 and N2
| 吸附分子 | σ/ ? | (ε/k b)/K | 电荷 /e |
|---|---|---|---|
| SF6 | 4.615 | 238.89 | — |
| N2_N | 3.32 | 36.4 | -0.482 |
| N2_com | — | — | 0.964 |
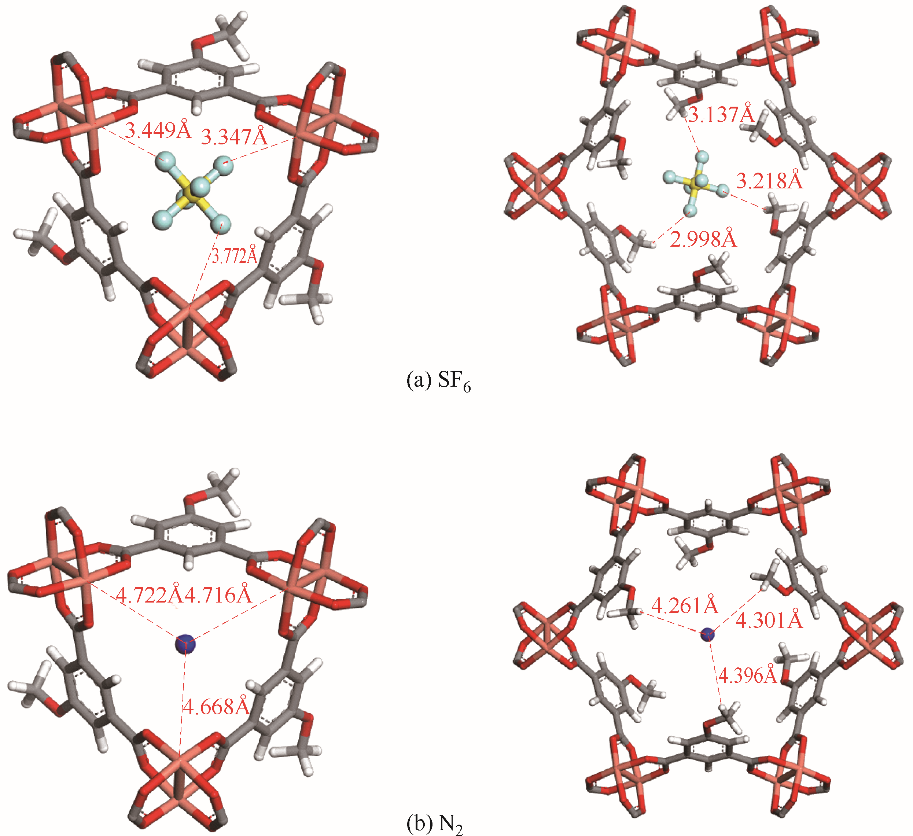
图10 通过DFT计算SF6和N2在Cu - MOF-OMe亲水性孔和疏水性孔中的吸附结合位点
Fig.10 SF6 and N2 adsorption binding sites in hydrophilic and hydrophobic pores of Cu-MOF-OMe by DFT calculation
| 1 | Tsai W T . The decomposition products of sulfur hexafluoride (SF6): reviews of environmental and health risk analysis[J]. Fluorine Chem., 2007, 128: 1345-1352. |
| 2 | Kim M B , Lee S J , Lee C Y , et al . High SF6 selectivities and capacities in isostructural metal-organic frameworks with proper pore sizes and highly dense unsaturated metal sites[J]. Micropor. Mesopor. Mat., 2014, 190: 356-361. |
| 3 | Christophorou L G , Vanbrunt R J . SF6/N2 mixtures - basic and HV insulation properties[J]. IEEE Trans. Dielectr. Electr. Insul., 1995, 2: 952-1003. |
| 4 | Yamamoto O , Takuma T , Kinouchi M . Recovery of SF6 from N2/SF6 gas mixtures by using a polymer membrane[J]. IEEE Electr. Insul. Mag., 2002, 18: 32-37. |
| 5 | Imai T , Inohara T , Toyoda M . Use of zeolite filter in portable equipment for recovering SF6 in SF6/N2 mixtures[J]. IEEE Trans. Dielectr. Electr. Insul., 2004, 11: 166-173. |
| 6 | Toyoda M , Murase H , Imai T , et al . SF6 reclaimer from SF6/N2 mixtures by gas separation with molecular sieving effect[J]. IEEE Trans. Power Deliv., 2003, 18: 442-448. |
| 7 | Senkovska I , Barea E , Navarro J A R , et al . Adsorptive capturing and storing greenhouse gases such as sulfur hexafluoride and carbon tetrafluoride using metal-organic frameworks[J]. Micropor. Mesopor. Mat., 2012, 156: 115-120. |
| 8 | Lee E K , Lee J D , Lee H J , et al . Pure SF6 and SF6 -N2 mixture gas hydrates equilibrium and kinetic characteristics[J]. Environ. Sci. Technol., 2009, 43: 7723-7727. |
| 9 | Cha I , Lee S , Lee J D , et al . Separation of SF6 from gas mixtures using gas hydrate formation[J]. Environ. Sci. Technol., 2010, 44: 6117-6122. |
| 10 | Skarmoutsos I , Eddaoudi M , Maurin G . Highly tunable sulfur hexafluoride separation by interpenetration control in metal organic frameworks[J]. Micropor. Mesopor. Mat., 2019, 281: 44-49. |
| 11 | Chuah C Y , Goh K L , Bae T H . Hierarchically structured HKUST‑1 nanocrystals for enhanced SF6 capture and recovery[J]. J. Phys. Chem. C, 2017, 121: 6748-6755. |
| 12 | Skarmoutsos I , Tamiolakis G , Froudakis G E . Highly selective separation and adsorption-induced phase transition of SF6 -N2 fluid mixtures in three-dimensional carbon nanotube networks[J]. J. Supercrit. Fluids, 2016, 113: 89-95. |
| 13 | Takase A , Kanoh H , Ohba T . Wide carbon nanopores as efficient sites for the separation of SF6 from N2 [J]. Sci. Rep., 2015, 5: 11994. |
| 14 | Kim P J , You Y W , Park H , et al . Separation of SF6 from SF6/N2 mixture using metal-organic framework MIL-100(Fe) granule[J]. Chem. Eng. J., 2015, 262: 683-690. |
| 15 | Kim M B , Kim K M , Kim T H , et al . Highly selective adsorption of SF6 over N2 in a bromine-functionalized zirconium-based metal-organic framework[J]. Chem. Eng. J., 2018, 339: 223-229. |
| 16 | Kim M B , Yoon T U , Hong D Y , et al . High SF6/N2 selectivity in a hydrothermally stable zirconium-based metal-organic framework[J]. Chem. Eng. J., 2015, 276: 315-321. |
| 17 | Sun R , Tai C W , Strømme M , et al . Hierarchical porous carbon synthesized from novel porous amorphous calcium or magnesium citrate with enhanced SF6 uptake and SF6/N2 selectivity[J]. ACS Appl. Nano Mater., 2019, 2: 778-789. |
| 18 | Hasell T , Miklitz M , Stephenson A , et al . Porous organic cages for sulfur hexafluoride separation[J]. J. Am. Chem. Soc., 2016, 138: 1653-1659. |
| 19 | Fang X K , Hu X , Maenhout G J , et al . Sulfur hexafluoride (SF6) emission estimates for china: an inventory for 1990—2010 and a projection to 2020[J]. Environ. Sci. Technol., 2013, 47: 3848-3855. |
| 20 | Chiang Y C , Wu P Y . Adsorption equilibrium of sulfur hexafluoride on multi-walled carbon nanotubes[J]. J. Hazard. Mater., 2010, 178: 729-738. |
| 21 | Mohindra V , Chae H , Sawin H H , et al . Abatement of perfluorocompounds (PFC’s) in a microwave tubular reactor using O as an additive gas[J]. IEEE Trans. Plasma Sci., 1997, 10: 399-407. |
| 22 | Ravishankara A R , Solomon S , Turnipseed A A , et al . Atmospheric lifetimes of long-lived halogenated species[J]. Science, 1993, 259:194-199. |
| 23 | Builes S , Roussel T , Vega L F . Optimization of the separation of sulfur hexafluoride and nitrogen by selective adsorption using Monte Carlo simulations[J]. AIChE J. 2011, 57: 962-974. |
| 24 | Kim D H , Ko Y H , Kim T H , et al . Separation of N2/SF6 binary mixtures using polyethersulfone (PESf) hollow fiber membrane[J]. Korean J. Chem. Eng., 2012, 29: 1081-1085. |
| 25 | Cho W S , Lee K H , Chang H J , et al . Evaluation of pressure-temperature swing adsorption for sulfur hexafluoride (SF6) recovery from SF6 and N2 gas mixture[J]. Korean J. Chem. Eng., 2011, 28: 2196-2201. |
| 26 | Ho M T , Allinson G W , Wiley D E . Reducing the cost of CO2 capture from flue gases using pressure swing adsorption[J]. Ind. Eng. Chem. Res., 2008, 47: 4883-4890. |
| 27 | Wiersum A D , Chang J S , Serre C , et al . An adsorbent performance indicator as a first step evaluation of novel sorbents for gas separations: application to metal-organic frameworks[J]. Langmuir, 2013, 29: 3301-3309. |
| 28 | Tong M M , Lan Y S , Yang Q Y , et al . Exploring the structure-property relationships of covalent organic frameworks for noble gas separations[J]. Chem. Eng. Sci., 2017, 168: 456-464. |
| 29 | Cui X L , Chen K J , Xing H B , et al . Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene[J]. Science, 2016, 353:141-144. |
| 30 | Li B , Cui X L , O’Nolan D , et al . An ideal molecular sieve for acetylene removal from ethylene with record selectivity and productivity[J]. Adv. Mater., 2017, 29: 1704210. |
| 31 | Yang L F , Cui X L , Yang Q W , et al . A single-molecule propyne trap: highly efficient removal of propyne from propylene with anion-pillared ultramicroporous materials[J]. Adv. Mater., 2018, 30: 1705374. |
| 32 | Li L B , Wen H M , He C H , et al . A metal-organic framework with suitable pore size and specific functional sites for the removal of trace propyne from propylene[J]. Angew. Chem. Int. Ed., 2018, 57: 15183-15188. |
| 33 | Furukawa H , Cordova K E , O’Keeffe M , et al . The chemistry and application of metal-organic frameworks[J]. Science, 2013, 341: 974-986. |
| 34 | Mueller U , Schubert M , Teich F , et al . Metal-organic frameworks-prospective industrial applications[J]. J. Mater. Chem., 2006, 16: 626-636. |
| 35 | Bae Y S , Snurr R Q . Development and evaluation of porous materials for carbon dioxide separation and capture[J]. Angew. Chem. Int. Ed., 2011, 50: 11586-11596. |
| 36 | Biswas S , Vanpoucke D E P , Verstraelen T , et al . New functionalized metal-organic frameworks MIL-47-X (X = —Cl, —Br, —CH3, —CF3, —OH, —OCH3): synthesis, characterization, and CO2 adsorption properties[J]. J. Phys. Chem. C, 2013, 117: 22784-22796. |
| 37 | Au V K M , Nakayashiki K , Huang H B , et al . Stepwise expansion of layered metal-organic frameworks for nonstochastic exfoliation into porous nanosheets[J]. J. Am. Chem. Soc., 2019, 141: 53-57. |
| 38 | Bachman J E , Reed D A , Kapelewski M T , et al . Enabling alternative ethylene production through its selective adsorption in the metal-organic framework Mn2(m-dobdc)[J]. Energy Environ. Sci., 2018, 11: 2423-2431. |
| 39 | Wang K K , Huang H L , Liu D H , et al . Covalent triazine-based frameworks with ultramicropores and high nitrogen contents for highly selective CO2 capture[J]. Environ. Sci. Technol., 2016, 50; 4869-4876. |
| 40 | Breneman C M , Wiberg K B . Determining atom-centered monopoles from molecular electrostatic potentials: the need for high sampling density in formamide conformational analysis[J]. J. Comput. Chem., 1990, 11: 361-373. |
| 41 | Dellis D , Samios J . Molecular force field investigation for sulfur hexafluoride: a computer simulation study[J]. Fluid Phase Equilibria, 2010, 291: 81-89. |
| 42 | Potoff J J , Siepmann J I . Vapor-liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen[J]. AIChE J., 2001, 47: 1676-1682. |
| 43 | Rappé A K , Casewit C J , Colwell K S , et al . UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations[J]. J. Am. Chem. Soc., 1992, 114: 10024-10035. |
| 44 | Vlugt T J H , García P E , Dubbeldam D , et al . Computing the heat of adsorption using molecular simulations: the effect of strong coulombic interactions[J]. J. Chem. Theory Comput., 2008, 4: 1107-1118. |
| 45 | Chen Y J , Li P , Modica J A , et al . Acid-resistant mesoporous metal-organic framework toward oral insulin delivery: protein encapsulation, protection, and release[J]. J. Am. Chem. Soc., 2018, 140: 5678-5681 |
| 46 | Li L Y , Yang L F , Wang J W , et al . Highly efficient separation of methane from nitrogen on a squarate-based metal-organic framework[J]. AIChE J., 2018, 64: 3681-3689. |
| 47 | Hu J L , Sun T J , Liu X W , et al . Separation of CH4 /N2 mixtures in metal-organic frameworks with 1D micro-channels[J]. RSC Adv., 2016, 6: 64039-64046. |
| 48 | Lin R B , Wu H , Li L B , et al . Boosting ethane/ethylene separation within isoreticular ultramicroporous metal-organic frameworks[J]. J. Am. Chem. Soc., 2018, 140: 12940-12946. |
| [1] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [2] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [3] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [4] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [7] | 高金明, 郭玉娇, 鄂承林, 卢春喜. 一种封闭罩内顺流多旋臂气液分离器的分离特性研究[J]. 化工学报, 2023, 74(7): 2957-2966. |
| [8] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [9] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| [10] | 朱兴驰, 郭志远, 纪志永, 汪婧, 张盼盼, 刘杰, 赵颖颖, 袁俊生. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| [11] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [12] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [13] | 孙永尧, 高秋英, 曾文广, 王佳铭, 陈艺飞, 周永哲, 贺高红, 阮雪华. 面向含氮油田伴生气提质利用的膜耦合分离工艺设计优化[J]. 化工学报, 2023, 74(5): 2034-2045. |
| [14] | 张正, 何永平, 孙海东, 张荣子, 孙正平, 陈金兰, 郑一璇, 杜晓, 郝晓刚. 蛇形流场电控离子交换装置用于选择性提锂[J]. 化工学报, 2023, 74(5): 2022-2033. |
| [15] | 王荣, 王永洪, 张新儒, 李晋平. 6FDA型聚酰亚胺炭分子筛气体分离膜的构筑及其应用[J]. 化工学报, 2023, 74(4): 1433-1445. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号