化工学报 ›› 2020, Vol. 71 ›› Issue (10): 4792-4799.DOI: 10.11949/0438-1157.20200672
陶长元1,2( ),王秀秀1,2,刘作华1,2,刘仁龙1,2,栾进华3
),王秀秀1,2,刘作华1,2,刘仁龙1,2,栾进华3
收稿日期:2020-06-03
修回日期:2020-07-14
出版日期:2020-10-05
发布日期:2020-10-05
通讯作者:
陶长元
作者简介:陶长元(1963—),男,博士,教授,基金资助:
Changyuan TAO1,2( ),Xiuxiu WANG1,2,Zuohua LIU1,2,Renlong LIU1,2,Jinhua LUAN3
),Xiuxiu WANG1,2,Zuohua LIU1,2,Renlong LIU1,2,Jinhua LUAN3
Received:2020-06-03
Revised:2020-07-14
Online:2020-10-05
Published:2020-10-05
Contact:
Changyuan TAO
摘要:
在湿法磷酸浸出过程中由于部分有机物碳化不彻底,使产品磷酸呈现黑色。本文提出了一种新型的催化氧化湿法磷酸净化技术,即在湿法磷酸浸出过程中引入氧化剂(H2O2)及催化剂(MnO2),基于转化过程中形成的
中图分类号:
陶长元, 王秀秀, 刘作华, 刘仁龙, 栾进华. 湿法磷酸浸出强化及有机质去除研究[J]. 化工学报, 2020, 71(10): 4792-4799.
Changyuan TAO, Xiuxiu WANG, Zuohua LIU, Renlong LIU, Jinhua LUAN. Research on leaching rate enhancement and organic matter removal in wet-process phosphoric acid[J]. CIESC Journal, 2020, 71(10): 4792-4799.
| 成分 | 含量/%(mass) |
|---|---|
| Ca | 60.98 |
| P | 20.36 |
| Si | 7.19 |
| F | 4.29 |
| Mg | 2.83 |
| Fe | 2.01 |
| Al | 0.78 |
| K | 0.43 |
| S | 0.37 |
表1 磷矿石的成分
Table1 Composition of phosphate rock
| 成分 | 含量/%(mass) |
|---|---|
| Ca | 60.98 |
| P | 20.36 |
| Si | 7.19 |
| F | 4.29 |
| Mg | 2.83 |
| Fe | 2.01 |
| Al | 0.78 |
| K | 0.43 |
| S | 0.37 |
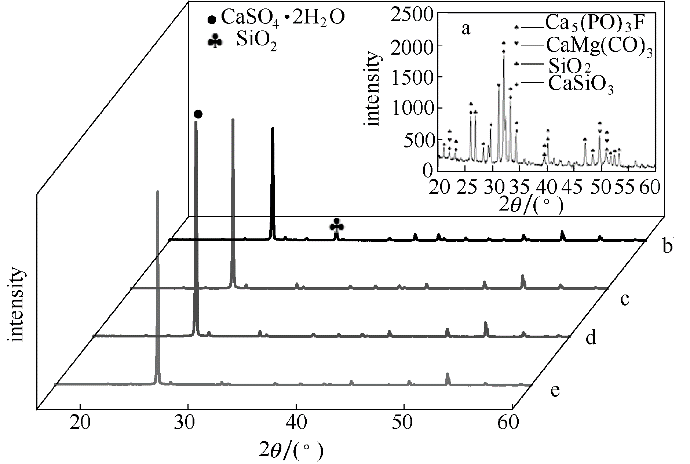
图8 不同浸出条件下磷矿XRD谱图
Fig.8 XRD patterns of phosphate rock under different extraction conditions a—original phosphate rock; b—direct acid extraction; c—H2O2 extraction; d—MnO2 extraction; e—H2O2with MnO2 extraction
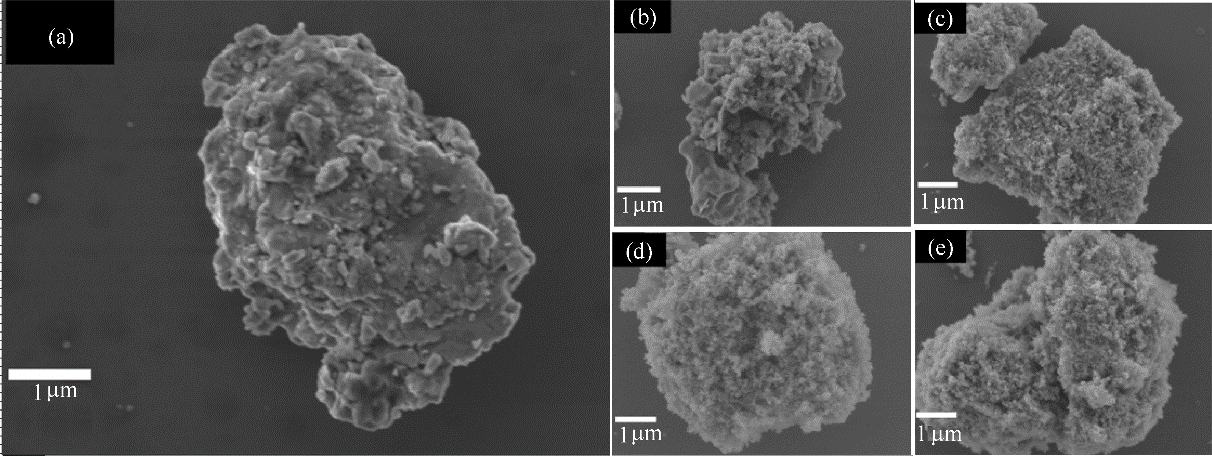
图9 浸出前后磷矿的SEM图(a) 原磷矿; (b) 直接酸浸; (c) MnO2强化浸出; (d) H2O2强化浸出; (e) H2O2与MnO2协同浸出
Fig.9 SEM images of phosphate rock under different extraction conditions
| 1 | Arisht S N, Abdul P M, Liu C M, et al. Biotoxicity assessment and lignocellulosic structural changes of phosphoric acid pre-treated young coconut husk hydrolysate for biohydrogen production[J]. International Journal of Hydrogen Energy, 2019, 44(1): 5830-5843. |
| 2 | Matta S, Stephan K, Stephan J, et al. Phosphoric acid production by attacking phosphate rock with recycled hexafluosilicic acid[J]. International Journal of Mineral Processing, 2017, 161(10): 21-27. |
| 3 | Ma H, Feng X, Zeng B. Self-anticorrosion for the combustion tower of heat recovered thermal process phosphoric acid production[J]. Process Safety and Environmental Protection, 2018, 118(8): 330-347. |
| 4 | 孟详东, 黄群星, 严建华, 等. 磷在污泥热解过程中的迁移转化[J]. 化工学报, 2018, 69(7): 3208-3215. |
| Meng X D, Huang Q X, Yan J H, et al. Migration and transformation of phosphorus during pyrolysis process of sewage sludge[J]. CIESC Journal, 2018, 69(7): 3208-3215. | |
| 5 | 张海燕, 邹密, 杨劲, 等. 中低品位磷矿直接制湿法磷酸技术研究[J]. 化工矿物与加工, 2015, 44(1): 17-20. |
| Zhang H Y, Zhou M, Yang J, et al.Study on the direct preparation of wet phosphoric acid from medium and low grade phosphate[J]. Industrial Minerals and Processing, 2015, 44(1): 17-20. | |
| 6 | Han Y, Cui X, Lv X, et al. Preparation and characterization of geopolymers based on a phosphoric-acid-activated electrolytic manganese dioxide residue[J]. Journal of Cleaner Production, 2018, 205(20): 488-498. |
| 7 | Li G, Zhou Q, Zhu Z, et al. Selective leaching of nickel and cobalt from limonitic laterite using phosphoric acid: an alternative for value-added processing of laterite[J]. Journal of Cleaner Production, 2018, 189(10): 620-626. |
| 8 | Chen H, Li W, Wang J, et al. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: selective adsorption and influence of dissolved organic matter[J]. Bioresource Technology, 2019, 292: 12-19. |
| 9 | Khoualdia B, Loungou M, Elaloui E. Adsorption of organic matter from industrial phosphoric acid (H3PO4) onto activated bentonite[J]. Arabian Journal of Chemistry, 2017, 10: S1073-S1080. |
| 10 | Zermane S, Meniai A H. Experimental study of competitive adsorption of heavy metals and organic matter for the phosphoric acid purification[J]. Energy Procedia, 2012, 18: 888-895. |
| 11 | 李天祥, 李白玉, 刘飞, 等. 湿法磷酸中有机物的脱除方法[J]. 无机盐工业, 2008, 40(12): 44-46. |
| Li T X, Li B Y, Liu F, et al. Method for removal of organic matter from wet phosphoric acid[J]. Inorganic Chemicals Industry, 2008, 40(12): 44-46. | |
| 12 | 李燕凤, 李军, 任永胜, 等. 预分散溶剂萃取用于浓缩湿法磷酸脱色[J]. 无机盐工业, 2007,5 (7): 33-35. |
| Li Y F, Li J, Ren Y S, et al. Predispersed solvent extraction is used to concentrate wet phosphoric acid for decolorization[J]. Inorganic Chemicals Industry, 2007,5 (7): 33-35. | |
| 13 | 林倩, 耿建铭, 江燕斌, 等. 超纯过氧化氢制备中有机杂质的吸附净化技术进展 [J]. 化工进展, 2006, 67(9): 1031-1035. |
| Lin Q, Geng J M, Jiang Y B, et al. Advances in the adsorption and purification of organic impurities in the preparation of ultrapure hydrogen peroxide[J]. Chemical industry and Engineering Progress, 2006, 67(9): 1031-1035. | |
| 14 | Ulu F, Gengec E, Kobya M. Removal of natural organic matter from Lake Terkos by EC process: studying on removal mechanism by floc size and zeta potential measurement and characterization by HPSEC method[J]. Journal of Water Process Engineering, 2019, 17: 31-35. |
| 15 | Särkkä H, Vepsäläinen M, Sillanpää M. Natural organic matter (NOM) removal by electrochemical methods — a review[J]. Journal of Electroanalytical Chemistry, 2015, 755(1): 100-108. |
| 16 | Vepsäläinen M, Ghiasvand M, Selin J, et al. Investigations of the effects of temperature and initial sample pH on natural organic matter (NOM) removal with electrocoagulation using response surface method (RSM)[J]. Separation and Purification Technology, 2009, 69(15): 255-261. |
| 17 | Ma L, He M, Fu P, et al. Adsorption of volatile organic compounds on modified spherical activated carbon in a new cyclonic fluidized bed[J]. Separation and Purification Technology, 2020, 235: 116146. |
| 18 | 李兵, 杨义, 刘作华, 等. 湿法磷酸固-液体系混沌混合与浸出强化行为[J]. 化工学报, 2019, 70(5): 1742-1749. |
| Li B, Yang Y, Liu Z H, et al. Olid-liquid chaotic mixing and leaching enhancement performance in phosphoric acid leaching process[J]. CIESC Journal, 2019, 70(5): 1742-1749. | |
| 19 | Guillossou R, Le Roux J, Mailler R, et al. Influence of dissolved organic matter on the removal of 12 organic micropollutants from wastewater effluent by powdered activated carbon adsorption[J]. Water Research, 2020, 172: 115487. |
| 20 | 陈亮, 李军, 钟本和. 浓缩湿法磷酸脱色研究 [J]. 无机盐工业, 2005, 37(7): 21-22. |
| Chen L, Li J, Zhong B H. Study on decolorization of concentrated wet phosphoric acid[J]. Inorganic Chemicals Industry, 2005, 37(7): 21-22. | |
| 21 | Ike I A, Karanfil T, Cho J, et al. Oxidation byproducts from the degradation of dissolved organic matter by advanced oxidation processes — a critical review[J]. Water Research, 2019, 164: 114929. |
| 22 | Wang H, Quan B, Bo G, et al. Advanced oxidation treatment of dissolved organic matter from wastewater treatment plant secondary effluent using scattering electrical reactor[J]. Journal of Cleaner Production, 2020, 267: 122258. |
| 23 | Fu L, Wu C, Zhou Y, et al. Ozonation reactivity characteristics of dissolved organic matter in secondary petrochemical wastewater by single ozone, ozone/H2O2, and ozone/catalyst[J]. Chemosphere, 2019, 233: 34-43. |
| 24 | Razaviarani V, Zazo J A, Casas J A, et al. Coupled fenton-denitrification process for the removal of organic matter and total nitrogen from coke plant wastewater[J]. Chemosphere, 2019, 224: 653-657. |
| 25 | Zhang Y, Liu H, Dai X, et al. The release of organic matter, nitrogen, phosphorus and heavy metals from erythromycin fermentation residue under heat-activated persulfate oxidation conditioning[J]. Science of the Total Environment, 2020, 724: 138349. |
| 26 | Ernst M, Lurot F, Schrotter J C. Catalytic ozonation of refractory organic model compounds in aqueous solution by aluminum oxide[J]. Applied Catalysis B: Environmental, 2004, 47(8): 15-25. |
| 27 | Tang Y, Zheng S, Xu Y, et al. Advanced batteries based on manganese dioxide and its composites[J]. Energy Storage Materials, 2018, 12: 284-309. |
| 28 | 国家技术监督局, 化工部化工矿山设计研究院. 磷矿石和磷精矿中五氧化二磷含量的测定磷钼酸喹啉重量法和容量法: GB/T 1871.1—1995[S]. 北京: 中国标准出版社, 1995. |
| The State Bureau of Quality and Technical Supervision, Chemical Mine Design and Research Institute, Ministry of Chemical Industry, Standardization Administration of The People's Republic of China. Phosphate rock and concentrate—determination of phosphrous pentoxide content—quinoline phosphomolybdate gravimetric and volumetric methods: GB/T 1871.1—1995[S]. Beijing: Standards Press of China, 1995. | |
| 29 | 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 循环冷却水中总有机碳(TOC)的测定: GB/T 32116—2015[S]. 北京: 中国标准出版社, 2015. |
| General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of the People's Republic of China. Determination of total organic carbon (TOC) in industrial circulating cooling water: GB/T 32116-2015 [S]. Beijing: Standards Press of China, 2015. | |
| 30 | Shu J, Liu R, Liu Z, et al. Enhanced extraction of manganese from electrolytic manganese residue by electrochemical[J]. Journal of Electroanalytical Chemistry, 2016, 780(1): 32-37. |
| 31 | Vega E, Valdés H. New evidence of the effect of the chemical structure of activated carbon on the activity to promote radical generation in an advanced oxidation process using hydrogen peroxide[J]. Microporous and Mesoporous Materials, 2018, 259(15): 1-8. |
| 32 | Hao Z, Wang J, Yin Y, et al. Abiotic formation of organoiodine compounds by manganese dioxide induced iodination of dissolved organic matter[J]. Environment Pollution, 2018, 236: 672-679. |
| 33 | Shu J, Liu R, Liu Z, et al. Manganese recovery and ammonia nitrogen removal from simulation wastewater by pulse electrolysis[J]. Separation and Purification Technology, 2016, 168(10): 107-113. |
| 34 | Zhang X, Wang J, Dong X X, et al. Functionalized metal-organic frameworks for photocatalytic degradation of organic pollutants in environment[J]. Chemosphere, 2019, 242: 125144. |
| 35 | Filipovic M R, Koppenol W H. The Haber-Weiss reaction — the latest revival[J]. Free Radical Biology Medicine, 2019, 145: 221-222. |
| 36 | Baldikova E, Pospiskova K, Safarikova M, et al. Non-woven fabric supported manganese dioxide microparticles as a low-cost, easily recoverable catalyst for hydrogen peroxide decomposition[J]. Materials Chemistry and Physics, 2018, 203(1): 280-283. |
| 37 | Zhang Y, Wang F, Ou P, et al. High efficiency and rapid degradation of bisphenol A by the synergy between adsorption and oxidization on the MnO2@nano hollow carbon sphere[J]. Journal of Hazardous Materials, 2018, 360(15): 223-232. |
| 38 | Qu F, Yan Z, Liu W, et al. Effects of manganese dioxides on the ultrafiltration membrane fouling by algal extracellular organic matter[J]. Separation and Purification Technology, 2015, 153(16): 29-36. |
| [1] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [2] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [3] | 王辰, 史秀锋, 武鲜凤, 魏方佳, 张昊虹, 车寅, 吴旭. 氧化还原法制备Mn3O4催化剂及其甲苯催化氧化性能与机理研究[J]. 化工学报, 2023, 74(6): 2447-2457. |
| [4] | 苏晓丹, 朱干宇, 李会泉, 郑光明, 孟子衡, 李防, 杨云瑞, 习本军, 崔玉. 湿法磷酸半水工艺考察与石膏结晶过程研究[J]. 化工学报, 2023, 74(4): 1805-1817. |
| [5] | 蹇建, 张嘉明, 佘祥, 周虎, 游奎一, 罗和安. V4+和V5+比例对钒磷氧催化NO2氧化环己烷性能的影响[J]. 化工学报, 2023, 74(4): 1570-1577. |
| [6] | 赵涛岩, 曹江涛, 李平, 冯琳, 商瑀. 区间二型模糊免疫PID在环己烷无催化氧化温度控制系统中的应用[J]. 化工学报, 2022, 73(7): 3166-3173. |
| [7] | 叶凯, 刘香华, 姜月, 于颖, 赵亚飞, 庄烨, 郑进保, 陈秉辉. 低温等离子体协同CeO2/13X催化降解甲苯[J]. 化工学报, 2021, 72(7): 3706-3715. |
| [8] | 孙静, 董一霖, 李法齐, 李文翔, 马晓玲, 王文龙. Co3O4改性USY分子筛吸附和催化氧化甲苯特性研究[J]. 化工学报, 2021, 72(6): 3306-3315. |
| [9] | 梁文俊, 朱玉雪, 石秀娟, 孙慧频, 任思达. Ce掺杂对Ru/TiO2催化氯苯性能的影响[J]. 化工学报, 2020, 71(8): 3585-3593. |
| [10] | 李兵, 杨义, 刘作华, 陶长元, 谷德银, 许传林, 王运东. 湿法磷酸固-液体系混沌混合与浸出强化行为[J]. 化工学报, 2019, 70(5): 1742-1749. |
| [11] | 王超, 李长明, 皇甫林, 李萍, 杨运泉, 高士秋, 余剑, 许光文. 赤泥催化剂的制备及其对模拟烟气中微量氨的脱除性能[J]. 化工学报, 2019, 70(3): 1056-1064. |
| [12] | 赫帅, 郭凤, 康国俊, 余剑, 任雪峰, 许光文. 络合-溶剂热法制备钯基催化剂及其催化氧化间二甲苯性能[J]. 化工学报, 2019, 70(3): 937-943. |
| [13] | 芮泽宝, 杨晓庆, 陈俊妃, 纪红兵. 光热协同催化净化挥发性有机物的研究进展及展望[J]. 化工学报, 2018, 69(12): 4947-4958. |
| [14] | 张娟, 胡颜荟, 任腾杰, 李未康, 赵地顺. Ti-MCM-41负载酞菁铁光催化氧化脱硫[J]. 化工学报, 2015, 66(9): 3437-3443. |
| [15] | 徐希化, 费兆阳, 陈献, 汤吉海, 崔咪芬, 乔旭. 气凝胶骨架镶嵌的CeO2纳米团簇催化氧化HCl制Cl2[J]. 化工学报, 2015, 66(9): 3421-3427. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号