化工学报 ›› 2021, Vol. 72 ›› Issue (6): 3380-3389.DOI: 10.11949/0438-1157.20201653
梁兴唐( ),李凤枝,钟书明,张瑞瑞,焦淑菲,汪双双,尹艳镇(
),李凤枝,钟书明,张瑞瑞,焦淑菲,汪双双,尹艳镇( )
)
收稿日期:2020-11-16
修回日期:2020-12-30
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
尹艳镇
作者简介:梁兴唐(1982—),男,博士,副教授,基金资助:
LIANG Xingtang( ),LI Fengzhi,ZHONG Shuming,ZHANG Ruirui,JIAO Shufei,WANG Shuangshuang,YIN Yanzhen(
),LI Fengzhi,ZHONG Shuming,ZHANG Ruirui,JIAO Shufei,WANG Shuangshuang,YIN Yanzhen( )
)
Received:2020-11-16
Revised:2020-12-30
Online:2021-06-05
Published:2021-06-05
Contact:
YIN Yanzhen
摘要:
为了制备具有胺基利用率高、结构稳定、使用方便的Cr(Ⅵ)吸附材料,以聚乙烯亚胺(PEI)为胺基改性剂,具有类海绵多孔结构的灯芯草(JC)为支撑基材,将吸入JC的PEI通过环氧氯丙烷原位接枝于其纤维表面,获得可整块使用的多孔吸附材料(PEI-JC)。采用元素分析、SEM、FT-IR、XPS表征PEI-JC的组成与结构,分析PEI-JC对Cr(Ⅵ)的吸附机理。考察PEI浓度、溶液的pH、Cr(Ⅵ)浓度与共存化合物等因素对吸附的影响。结果表明,质量分数为10.0%的PEI所制备的PEI10.0-JC,在30℃与pH为2.0的条件下,其Langmuir模型的最大吸附量为474.6 mg·g-1;PEI10.0-JC可将含各种共存化合物溶液中的Cr(Ⅵ)从10 mg·L-1降至排放标准(0.5 mg·L-1)以下;PEI10.0-JC可重复使用,其结构在吸附过程中未发生明显变化;吸附与还原作用是PEI-JC去除水溶液中Cr(Ⅵ)的主要机制。
中图分类号:
梁兴唐, 李凤枝, 钟书明, 张瑞瑞, 焦淑菲, 汪双双, 尹艳镇. 聚乙烯亚胺原位改性多孔灯芯草高效吸附废水中的Cr(Ⅵ)[J]. 化工学报, 2021, 72(6): 3380-3389.
LIANG Xingtang, LI Fengzhi, ZHONG Shuming, ZHANG Ruirui, JIAO Shufei, WANG Shuangshuang, YIN Yanzhen. In-situ modification of porous juncus with polyethyleneimine for efficient capture of Cr(Ⅵ) from wastewater[J]. CIESC Journal, 2021, 72(6): 3380-3389.

图1 JC (a)、PEI5.0-JC (b)、PEI10.0-JC (c)与PEI15.0-JC (d)的SEM照片;PEI10.0-JC吸附Cr(Ⅵ)后的SEM照片(插图)及Cr面扫描能谱 (e);吸附-脱附5次后PEI10.0-JC的SEM照片(f)
Fig.1 SEM images of JC (a), PEI5.0-JC (b), PEI10.0-JC (c) and PEI15.0-JC (d); SEM image(inset) and Cr mapping of PEI10.0-JC after adsorbing Cr(Ⅵ) (e); SEM image of PEI10.0-JC after 5 cycles of adsorption-desorption (f)

图2 JC (a)、PEI10.0-JC (b)、吸附Cr(Ⅵ)后PEI10.0-JC (c)以及吸附-洗脱5次后PEI10.0-JC (d)的FT-IR谱图
Fig.2 FT-IR spectra of JC (a), PEI10.0-JC (b), PEI10.0-JC after the adsorption of Cr(Ⅵ) (c) and the PEI10.0-JC after 5 cycles of adsorption-desorption (d)
| C0 /(mg·L–1) | k /(g·mg–1·min–1) | QE/(mg·g–1) | R2 |
|---|---|---|---|
| 100 | 3.74×10-4 | 106.3 | 0.999 |
| 200 | 1.69×10-4 | 196.1 | 0.998 |
表1 PEI10.0-JC对Cr(Ⅵ)吸附的准二级动力学参数
Table 1 Kinetic parameters of PEI10.0-JC toward Cr(Ⅵ) removal fitted with pseudo-second-order model
| C0 /(mg·L–1) | k /(g·mg–1·min–1) | QE/(mg·g–1) | R2 |
|---|---|---|---|
| 100 | 3.74×10-4 | 106.3 | 0.999 |
| 200 | 1.69×10-4 | 196.1 | 0.998 |
| T/℃ | Langmuir | Freundlich | Tempkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm | KL | R2 | KF | n | R2 | KT | b | R2 | |
| 30 | 474.6 | 0.038 | 0.977 | 59.0 | 2.78 | 0.960 | 1.87 | 41.3 | 0.885 |
| 40 | 519.7 | 0.043 | 0.983 | 70.3 | 2.82 | 0.951 | 2.50 | 39.9 | 0.856 |
| 50 | 550.0 | 0.045 | 0.984 | 76.5 | 2.85 | 0.924 | 1.01 | 28.5 | 0.965 |
表2 PEI10.0-JC在不同温度下等温吸附模型的拟合参数
Table 2 Isotherms parameters for the adsorption of PEI10.0-JC toward Cr(Ⅵ) at various temperatures
| T/℃ | Langmuir | Freundlich | Tempkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm | KL | R2 | KF | n | R2 | KT | b | R2 | |
| 30 | 474.6 | 0.038 | 0.977 | 59.0 | 2.78 | 0.960 | 1.87 | 41.3 | 0.885 |
| 40 | 519.7 | 0.043 | 0.983 | 70.3 | 2.82 | 0.951 | 2.50 | 39.9 | 0.856 |
| 50 | 550.0 | 0.045 | 0.984 | 76.5 | 2.85 | 0.924 | 1.01 | 28.5 | 0.965 |
| Sample | pH | T/℃ | Qm/(mg·g-1) | Ref. |
|---|---|---|---|---|
| PEI/氧化石墨 | 2.0 | 35 | 50.3 | [ |
| PEI/纤维素 | 2.0 | 25 | 119.4 | [ |
| PEI/甲壳素 | 2.0 | 25 | 330.4 | [ |
| PEI/boehmite | 3.0 | 25 | 51.1 | [ |
| PEI/pineapple leaf fiber | 2.0 | 30 | 222.4 | [ |
| PEI/PVA | 4.0 | 23 | 150.0 | [ |
| PEI/carbon nanotube | 2.0 | 25 | 40.3 | [ |
| PEI/chitosan | 3.0 | 30 | 331.3 | [ |
| PEI/graphene oxide | 2.0 | 30 | 436.2 | [ |
| PEI10.0-JC | 2.0 | 30 | 474.6 | 本研究 |
表3 基于PEI改性吸附剂的性能比较
Table 2 Comparison of the performance for the PEI modified adsorbents
| Sample | pH | T/℃ | Qm/(mg·g-1) | Ref. |
|---|---|---|---|---|
| PEI/氧化石墨 | 2.0 | 35 | 50.3 | [ |
| PEI/纤维素 | 2.0 | 25 | 119.4 | [ |
| PEI/甲壳素 | 2.0 | 25 | 330.4 | [ |
| PEI/boehmite | 3.0 | 25 | 51.1 | [ |
| PEI/pineapple leaf fiber | 2.0 | 30 | 222.4 | [ |
| PEI/PVA | 4.0 | 23 | 150.0 | [ |
| PEI/carbon nanotube | 2.0 | 25 | 40.3 | [ |
| PEI/chitosan | 3.0 | 30 | 331.3 | [ |
| PEI/graphene oxide | 2.0 | 30 | 436.2 | [ |
| PEI10.0-JC | 2.0 | 30 | 474.6 | 本研究 |
| T/K | ΔG/(kJ·mol-1) | ΔS/(kJ·(mol·K)-1) | ΔH/(kJ·mol-1) |
|---|---|---|---|
| 303 | -19.11 | ||
| 313 | -20.05 | 0.086 | 6.91 |
| 323 | -20.83 |
表4 PEI10.0-JC对Cr(Ⅵ)的吸附的热力学参数
Table 4 Thermodynamic parameters for the adsorption of Cr(Ⅵ) by PEI10.0-JC
| T/K | ΔG/(kJ·mol-1) | ΔS/(kJ·(mol·K)-1) | ΔH/(kJ·mol-1) |
|---|---|---|---|
| 303 | -19.11 | ||
| 313 | -20.05 | 0.086 | 6.91 |
| 323 | -20.83 |
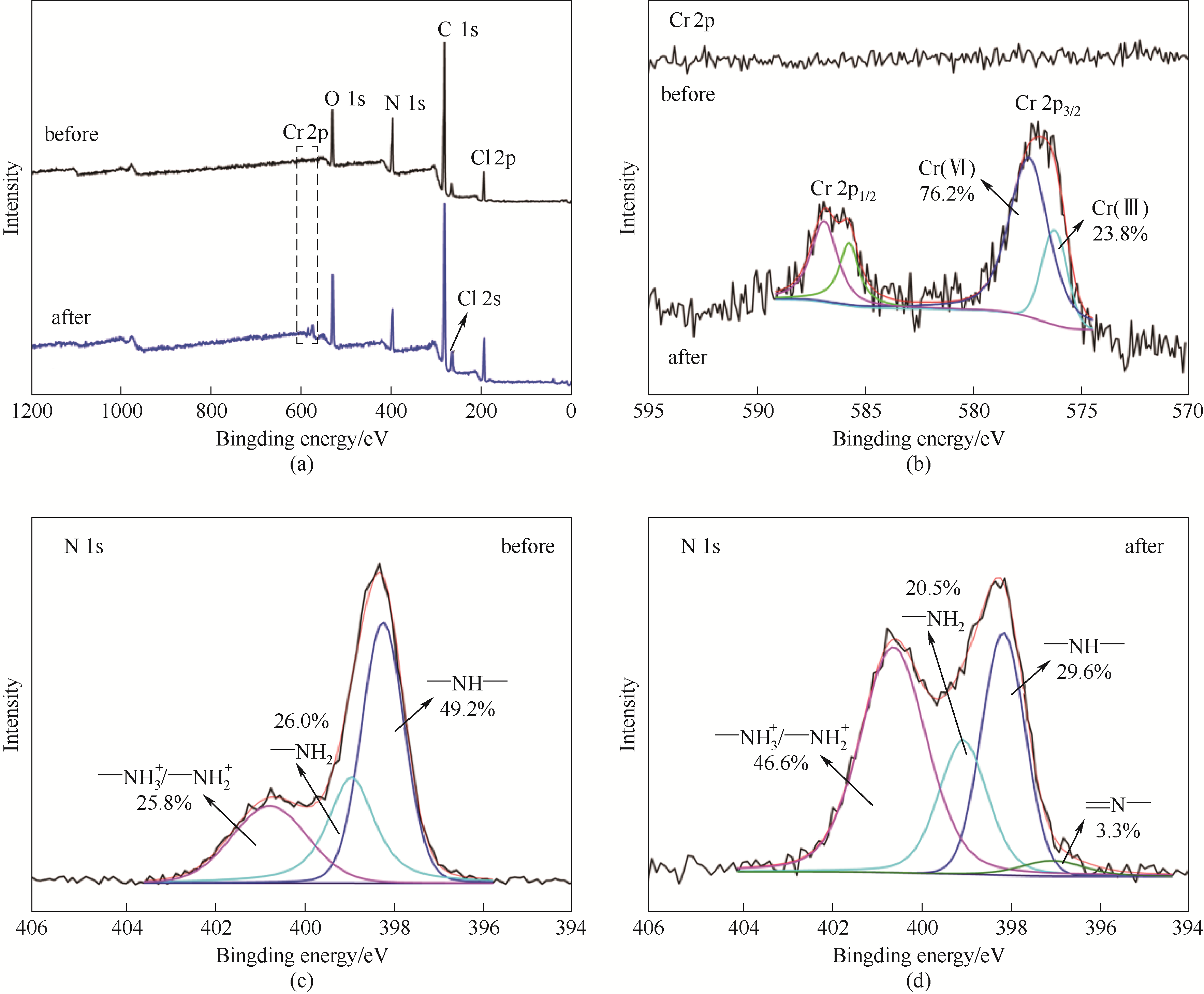
图10 Cr(Ⅵ)吸附前、后PEI10.0-JC的全范围(a)、Cr 2p (b)和N 1s[(c)、(d)]的XPS谱图
Fig.10 Full range (a), Cr 2p (b) and N 1s [(c),(d)] XPS spectra of PEI10.0-JC before and after Cr(Ⅵ) adsorption
| 1 | 余淦申, 郭茂新, 黄进勇. 工业废水处理及再生利用[M]. 北京: 化学工业出版社, 2012: 266-268. |
| Yu G S, Guo M X, Huang J Y. Industrial Wastewater Treatment and Reuse[M]. Beijing: Chemical Industry Press, 2012: 266-268. | |
| 2 | Jobby R, Jha P, Yadav A K, et al. Biosorption and biotransformation of hexavalent chromium [Cr(Ⅵ)]: a comprehensive review[J]. Chemosphere, 2018, 207: 255-266. |
| 3 | Peng H, Guo J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review[J]. Environmental Chemistry Letters, 2020, 18: 2055-2068. |
| 4 | Hosseini S M, Sohrabnejad S, Nabiyouni G, et al. Magnetic cation exchange membrane incorporated with cobalt ferrite nanoparticles for chromium ions removal via electrodialysis[J]. Journal of Membrane Science, 2019, 583: 292-300. |
| 5 | 王子帅, 王耀强, 肖刚, 等. 磁性纳米Fe3O4@TiO2可见光下光催化还原Cr(Ⅵ)[J]. 化工学报, 2019, 70(10): 4062-4071. |
| Wang Z S, Wang Y Q, Xiao G, et al. Photocatalytic reduction of Cr(Ⅵ) by magnetic nanomaterial Fe3O4@TiO2 under visible light[J]. CIESC Journal, 2019, 70(10): 4062-4071. | |
| 6 | 伍清新, 刘杰, 游少鸿, 等. 李氏禾湿地系统净化Cr(Ⅵ)污染水体的机理研究[J]. 环境科学学报, 2014, 34(9): 2306-2312. |
| Wu Q X, Liu J, You S H, et al. Decontamination mechanism of Cr(Ⅵ)-polluted water in constructed wetland planted with Leersia hexandra Swartz[J]. Acta Scientiae Circumstantiae, 2014, 34(9): 2306-2312. | |
| 7 | Zhang Y, Zhu C, Liu F, et al. Effects of ionic strength on removal of toxic pollutants from aqueous media with multifarious adsorbents: a review[J]. Science of the Total Environment, 2019, 646: 265-279. |
| 8 | Zhang H, Li P, Wang Z, et al. Sustainable disposal of Cr(Ⅵ): adsorption-reduction strategy for treating textile wastewaters with amino-functionalized boehmite hazardous solid wastes[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(5): 6811-6819. |
| 9 | Wang J, Sun T, Saleem A, et al. Enhanced adsorptive removal of Cr(Ⅵ) in aqueous solution by polyethyleneimine modified palygorskite[J]. Chinese Journal of Chemical Engineering, 2020, 28(10): 2650-2657. |
| 10 | Yan Y, An Q, Xiao Z, et al. Flexible core-shell/bead-like alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(Ⅵ)[J]. Chemical Engineering Journal, 2017, 313: 475-486. |
| 11 | 王家宏, 尹小龙, 吉艳芬. 聚乙烯亚胺改性氧化石墨对水中Cr(Ⅵ)的吸附[J]. 无机化学学报, 2015, 31(6): 1185-1193. |
| Wang J H, Yin X L, Ji Y F. Cr(Ⅵ) adsorption on polyethyleneimine modified graphite oxide[J]. Chinese Journal of Inorganic Chemistry, 2015, 31(6): 1185-1193. | |
| 12 | Tangtubtim S, Saikrasun S. Adsorption behavior of polyethyleneimine-carbamate linked pineapple leaf fiber for Cr(Ⅵ) removal[J]. Applied Surface Science, 2019, 467: 596-607. |
| 13 | Fayazi M, Ghanbarian M. One-pot hydrothermal synthesis of polyethylenimine functionalized magnetic clay for efficient removal of noxious Cr(Ⅵ) from aqueous solutions[J]. Silicon, 2020, 12: 125-134. |
| 14 | Setyono D, Valiyaveettil S. Functionalized paper—a readily accessible adsorbent for removal of dissolved heavy metal salts and nanoparticles from water[J]. Journal of Hazardous Materials, 2016, 302: 120-128. |
| 15 | Zhang S, Shi Q, Korfiatis G, et al. Chromate removal by electrospun PVA/PEI nanofibers: adsorption, reduction, and effects of co-existing ions[J]. Chemical Engineering Journal, 2020, 387: 124179. |
| 16 | Qiu B, Guo J, Zhang X, et al. Polyethylenimine facilitated ethyl cellulose for hexavalent chromium removal with a wide pH range[J]. ACS Applied Materials & Interfaces, 2014, 6: 19816-19824. |
| 17 | Song L, Liu F, Zhu C, et al. Facile one-step fabrication of carboxymethyl cellulose based hydrogel for highly efficient removal of Cr(Ⅵ) under mild acidic condition[J]. Chemical Engineering Journal, 2019, 369: 641-651. |
| 18 | Liang X, Liang B, Wei J, et al. A cellulose-based adsorbent with pendant groups of quaternary ammonium and amino for enhanced capture of aqueous Cr(Ⅵ)[J]. International Journal of Biological Macromolecules, 2020, 148: 802-810. |
| 19 | Rizzo C, Andrews J L, Steed J W, et al. Carbohydrate-supramolecular gels: adsorbents for chromium (Ⅵ) removal from wastewater[J]. Journal of Colloid and Interface Science, 2019, 548: 184-196. |
| 20 | Luo T, Tian X, Yang C, et al. Polyethylenimine-functionalized corn bract, an agricultural waste material, for efficient removal and recovery of Cr(Ⅵ) from aqueous solution[J]. Journal of Agricultural and Food Chemistry, 2017, 65: 7153-7158. |
| 21 | 韩业钜, 张耿崚, 王小琴, 等. 表面活性剂强化超声波-离子液体预处理对水葫芦酶解的影响[J]. 环境科学学报, 2017, 37(7) : 2699-2706 |
| Han Y J, Chang K L, Wang X Q, et al. Surfactant assisted ultrasound-ionic liquid pretreatment process for enzymatic hydrolysis of water hyacinth[J]. Acta Scientiae Circumstantiae, 2017, 37(7): 2699-2706. | |
| 22 | Liang X, Fan X, Li R, et al. Efficient removal of Cr(Ⅵ) from water by quaternized chitin/branched polyethylenimine biosorbent with hierarchical pore structure[J]. Bioresource Technology, 2018, 250: 178-184. |
| 23 | 陈豪宇, 张胜利, 凯橙橙, 等. 聚乙烯亚胺改性纤维素纤维对Cr(Ⅵ)的吸附研究[J]. 环境科学学报, 2018, 38(8): 3090-3098. |
| Chen H Y, Zhang S L, Kai C C, et al. Polyethyleneimine modified cellulose fiber for Cr(Ⅵ) removal from aqueous solution[J]. Acta Scientiae Circumstantiae, 2018, 38(8): 3090-3098. | |
| 24 | 梁兴唐, 钟书明, 刘子杰, 等. 甲壳素/聚乙烯亚胺复合物对水溶液中Cr(Ⅵ)的吸附[J]. 化工学报, 2018, 69(5): 2255-2262. |
| Liang X T, Zhong S M, Liu Z J, et al. Chitin/polyethyleneimine composite as an adsorbent of aqueous Cr(Ⅵ)[J]. CIESC Journal, 2018, 69(5): 2255-2262. | |
| 25 | Polowczyk I, Urbano B, Rivas B L, et al. Equilibrium and kinetic study of chromium sorption on resins with quaternary ammonium and N-methyl-D-glucamine groups[J]. Chemical Engineering Journal, 2016, 284: 395-404. |
| 26 | Al-Ghouti M A, Da'ana D A. Guidelines for the use and interpretation of adsorption isotherm models: a review[J]. Journal of Hazardous Materials, 2020, 393: 122383. |
| 27 | Sambaza S, Masheane M L, Malinga S, et al. Polyethyleneimine-carbon nanotube polymeric nanocomposite adsorbents for the removal of Cr6+ from water[J]. Physics and Chemistry of the Earth, 2017, 100: 236-246. |
| 28 | Zhu W J, Dang Q F, Liu C S, et al. Cr(Ⅵ) and Pb(Ⅱ) capture on pH-responsive polyethyleneimine and chloroacetic acid functionalized chitosan microspheres[J]. Carbohydrate Polymers, 2019, 219: 353-367. |
| 29 | Geng J, Yin Y, Liang Q, et al. Polyethyleneimine cross-linked graphene oxide for removing hazardous hexavalent chromium: adsorption performance and mechanism[J]. Chemical Engineering Journal, 2019, 361: 1497-1510. |
| 30 | Niazi L, Lashanizadegan A, Sharififard H. Chestnut oak shells activated carbon: preparation, characterization and application for Cr(Ⅵ) removal from dilute aqueous solutions[J]. Journal of Cleaner Production, 2018, 185: 554-561. |
| 31 | Iftime M M, Marin L. Chiral betulin-imino-chitosan hydrogels by dynamic covalent sonochemistry[J]. Ultrasonics Sonochemistry, 2018, 45: 238-247. |
| 32 | Kwak H W, Lee K H. Polyethylenimine-functionalized silk sericin beads for high-performance remediation of hexavalent chromium from aqueous solution[J]. Chemosphere, 2018, 207: 507-516. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [3] | 张艳梅, 袁涛, 李江, 刘亚洁, 孙占学. 高效SRB混合菌群构建及其在酸胁迫条件下的性能研究[J]. 化工学报, 2023, 74(6): 2599-2610. |
| [4] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [5] | 闫新龙, 黄志刚, 胡清勋, 张新, 胡晓燕. Cu/Co掺杂多孔炭活化过硫酸盐降解水中硝基酚研究[J]. 化工学报, 2023, 74(3): 1102-1112. |
| [6] | 李承威, 骆华勇, 张铭轩, 廖鹏, 方茜, 荣宏伟, 王竞茵. 氢氧化镧交联壳聚糖微球的微流控制备及其除磷性能[J]. 化工学报, 2022, 73(9): 3929-3939. |
| [7] | 赵希强, 张健, 孙爽, 王文龙, 毛岩鹏, 孙静, 刘景龙, 宋占龙. 生物质炭改性微球去除化工废水中无机磷的性能研究[J]. 化工学报, 2022, 73(5): 2158-2173. |
| [8] | 贾艳萍, 丁雪, 刚健, 佟泽为, 张海丰, 张兰河. Mn强化Fe/C微电解工艺条件优化及降解油墨废水机理[J]. 化工学报, 2022, 73(5): 2183-2193. |
| [9] | 王祺, 房阔, 贺聪慧, 王凯军. 流动电极电容去离子技术综述:研究进展与未来挑战[J]. 化工学报, 2022, 73(3): 975-989. |
| [10] | 毛恒, 王月, 王森, 刘伟民, 吕静, 陈甫雪, 赵之平. APTES改性ZIF-L/PEBA混合基质膜强化渗透汽化分离苯酚研究[J]. 化工学报, 2022, 73(3): 1389-1402. |
| [11] | 王洒, 温怡静, 郭丹煜, 周欣, 李忠. 锆基MOF次级结构单元调控及轻烃吸附分离性能增强[J]. 化工学报, 2022, 73(2): 730-738. |
| [12] | 郑喜, 王涛, 任永胜, 赵珍珍, 王雪琪, 赵之平. 聚间苯二甲酰间苯二胺平板膜的制备及其性能研究[J]. 化工学报, 2022, 73(10): 4707-4721. |
| [13] | 张兰河, 汪露, 李梓萌, 唐宏, 郭静波, 贾艳萍, 张明爽. 电极超滤膜生物反应器处理阴离子表面活性剂废水[J]. 化工学报, 2022, 73(10): 4679-4691. |
| [14] | 付鹏波,田金乙,吕文杰,黄渊,刘毅,卢浩,杨强,修光利,汪华林. 物理法水处理技术[J]. 化工学报, 2022, 73(1): 59-72. |
| [15] | 黄莉婷, 韩昫身, 金艳, 马强, 于建国. 煤化工反渗透浓水的高效降解菌株筛选、鉴定及应用研究[J]. 化工学报, 2021, 72(9): 4881-4891. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号