化工学报 ›› 2021, Vol. 72 ›› Issue (6): 3105-3115.DOI: 10.11949/0438-1157.20201727
收稿日期:2020-12-01
修回日期:2021-03-29
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
何利华,赵中伟
作者简介:徐文华(1994—),男,博士研究生, 基金资助:
XU Wenhua( ),LIU Dongfu,HE Lihua(
),LIU Dongfu,HE Lihua( ),LIU Xuheng,ZHAO Zhongwei(
),LIU Xuheng,ZHAO Zhongwei( )
)
Received:2020-12-01
Revised:2021-03-29
Online:2021-06-05
Published:2021-06-05
Contact:
HE Lihua,ZHAO Zhongwei
摘要:
电化学脱嵌法盐湖提锂技术具有选择性好、提取率高、环境友好等优点,但提锂速度慢、效率较低。通过对提锂过程进行动力学研究来探明其控制步骤,为该方法的优化提供理论指导。通过系统研究槽电压、反应温度、锂浓度以及涂覆密度等因素对锂提取速率的影响规律,采用收缩核模型进行了动力学拟合分析。相较于其他因素,槽电压对提锂速率的影响显著,提锂反应速控步骤随着槽电压的升高呈现出由化学反应控制到内扩散控制(溶液向电极内部的传质为反应限制步骤)的转变。高槽电压(0.1 V)时,计算所得反应表观活化能为18.9 kJ/mol,锂浓度反应级数为 0.382,涂覆密度的依赖系数为-1.46。建立了提锂反应动力学方程。
中图分类号:
徐文华, 刘冬福, 何利华, 刘旭恒, 赵中伟. 电化学脱嵌法盐湖提锂电极反应动力学研究[J]. 化工学报, 2021, 72(6): 3105-3115.
XU Wenhua, LIU Dongfu, HE Lihua, LIU Xuheng, ZHAO Zhongwei. Kinetic study on electrochemical intercalation/deintercalation method for lithium extraction from brine[J]. CIESC Journal, 2021, 72(6): 3105-3115.
动力学控制 步骤 | 球形收缩核模型 | 平板收缩核模型 |
|---|---|---|
化学反应 控制 | ||
| 外扩散控制 | ||
内扩散 控制 |
表1 动力学反应模型及动力学方程
Table 1 Kinetic reaction models and kinetic equations
动力学控制 步骤 | 球形收缩核模型 | 平板收缩核模型 |
|---|---|---|
化学反应 控制 | ||
| 外扩散控制 | ||
内扩散 控制 |
温度/ ℃ | 动力学方程 | 锂浓度/ (mol/L) | 动力学方程 | 涂覆密度/ (mg/cm2) | 动力学方程 |
|---|---|---|---|---|---|
| 10 | x2 =1.0×10-3 t | 0.1 | x2 = 0.8×10-3 t | 16 | x2 =3.0×10-3 t |
| 20 | x2 =1.3×10-3 t | 0.2 | x2 = 1.0×10-3 t | 22 | x2 =1.7×10-3 t |
| 30 | x2 =1.7×10-3 t | 0.4 | x2 = 1.3×10-3 t | 30 | x2 =1.2×10-3 t |
| 40 | x2 =2.1×10-3 t | 0.6 | x2 = 1.6×10-3 t | ||
| 50 | x2 =2.8×10-3 t |
表2 不同温度、锂浓度和涂覆密度下所得动力学方程
Table 2 Kinetic equations under different temperature, Li+ concentration and coating density
温度/ ℃ | 动力学方程 | 锂浓度/ (mol/L) | 动力学方程 | 涂覆密度/ (mg/cm2) | 动力学方程 |
|---|---|---|---|---|---|
| 10 | x2 =1.0×10-3 t | 0.1 | x2 = 0.8×10-3 t | 16 | x2 =3.0×10-3 t |
| 20 | x2 =1.3×10-3 t | 0.2 | x2 = 1.0×10-3 t | 22 | x2 =1.7×10-3 t |
| 30 | x2 =1.7×10-3 t | 0.4 | x2 = 1.3×10-3 t | 30 | x2 =1.2×10-3 t |
| 40 | x2 =2.1×10-3 t | 0.6 | x2 = 1.6×10-3 t | ||
| 50 | x2 =2.8×10-3 t |
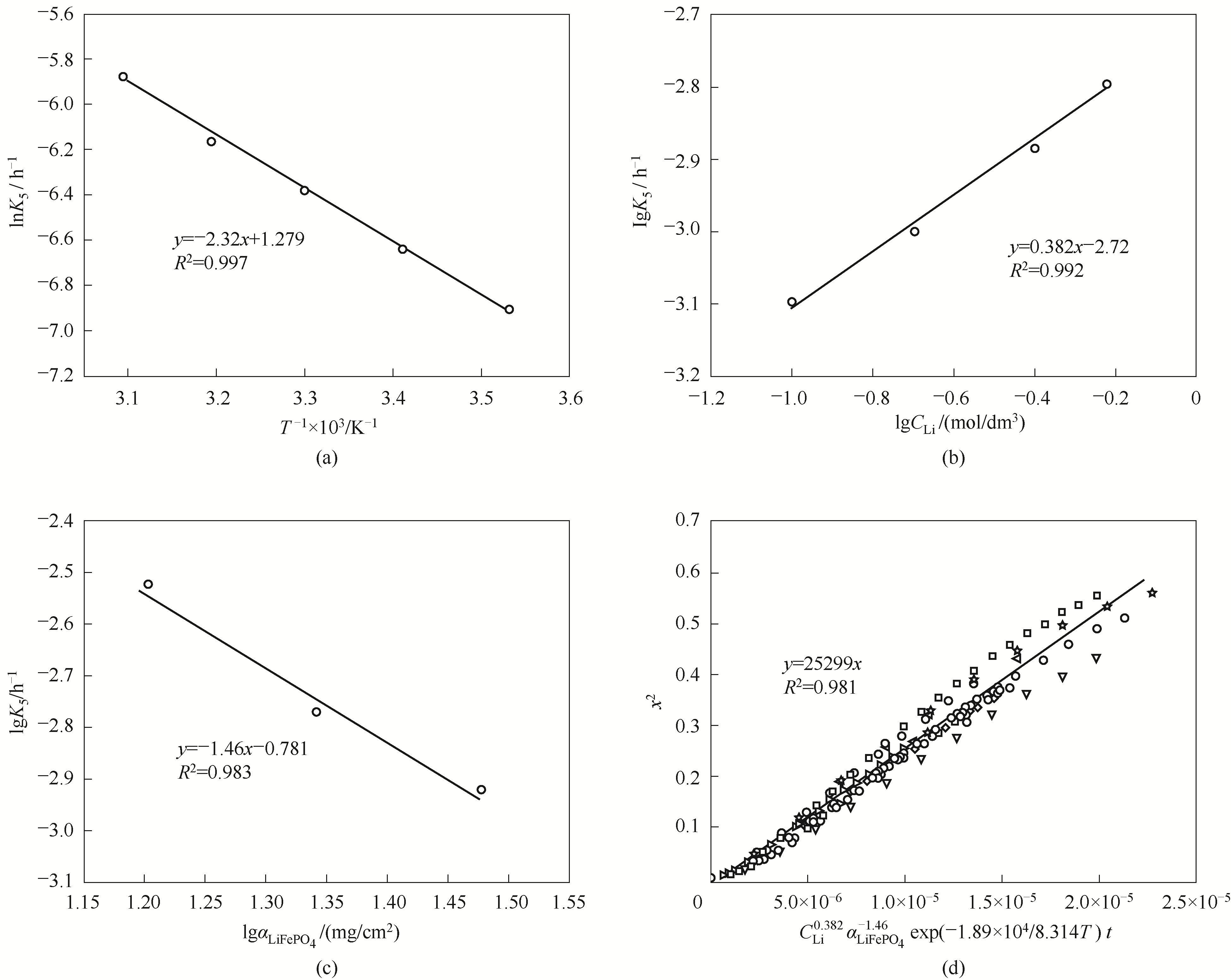
图11 动力学参数修正图: 电化学提锂过程的Arrhenius图(a);综合速率常数lgK5与锂离子浓度lgCLi的关系(b);综合速率常数lgK5与电极材料涂覆密度lgαLiFePO4的关系(c);x2与CLi0.382αLiFePO4-1.46exp?(-1.89×104/8.314?T)t的关系(d)
Fig.11 The correction diagrams of kinetic parameters: Arrhenius diagram of electrochemical lithium extraction reaction (a); The relationship between comprehensive rate constant lgK5 and lithium concentration lgCLi (b); The relationship between comprehensive rate constant lgK5 and coating density lg?αLiFePO4 (c); The relationship between x2 and CLi0.382αLiFePO4-1.46exp(-1.89×104/8.314?T)?t (d)
| 1 | Opitz A, Badami P, Shen L, et al. Can Li-ion batteries be the panacea for automotive applications?[J]. Renewable and Sustainable Energy Reviews, 2017, 68: 685-692. |
| 2 | Gröger O, Gasteiger H A, Suchsland J P. Review—electromobility: batteries or fuel cells?[J]. Journal of the Electrochemical Society, 2015, 162(14): A2605-A2622. |
| 3 | Dunn B, Kamath H, Tarascon J M. Electrical energy storage for the grid: a battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 4 | 曹佳宁, 高翔, 罗英武, 等. 一种用于磷酸铁锂电极的水性黏结剂制备与性能研究[J]. 化工学报, 2021, 72(2): 1169-1180. |
| Cao J N, Gao X, Luo Y W, et al. Study on preparation and performance of aqueous binder for lithium iron phosphate electrodes in lithium-ion battery[J]. CIESC Journal, 2021, 72(2): 1169-1180. | |
| 5 | 戈海文, 王怀有, 王敏. 碳酸锂在碳酸钠溶液中的溶解度与热力学[J]. 化工学报, 2019, 70(11): 4123-4130. |
| Ge H W, Wang H Y, Wang M. Solubility and thermodynamics of lithium carbonate in sodium carbonate solution[J]. CIESC Journal, 2019, 70(11): 4123-4130. | |
| 6 | 刘东帆, 孙淑英, 于建国. 盐湖卤水提锂技术研究与发展[J]. 化工学报, 2018, 69(1): 141-155. |
| Liu D F, Sun S Y, Yu J G. Research and development on technique of lithium recovery from salt lake brine[J]. CIESC Journal, 2018, 69(1): 141-155. | |
| 7 | U.S.Geological. Survey. Mineral Commodity Summaries 2018[R]. 2018, doi:10.3133/70194932. |
| 8 | Vikström H, Davidsson S, Höök M. Lithium availability and future production outlooks[J]. Applied Energy, 2013, 110: 252-266. |
| 9 | Flexer V, Baspineiro C F, Galli C I. Lithium recovery from brines: a vital raw material for green energies with a potential environmental impact in its mining and processing[J]. Science of the Total Environment, 2018, 639: 1188-1204. |
| 10 | 郭佳明, 刘明言, 吴强, 等. 硝酸锂改性钛系离子筛的制备及其吸附性能[J]. 化工学报, 2020, 71(2): 879-888. |
| Guo J M, Liu M Y, Wu Q, et al. Preparation and adsorption performance of titanium based lithium ion sieve improved by LiNO3[J]. CIESC Journal, 2020, 71(2): 879-888. | |
| 11 | Li X W, Chao Y H, Chen L L, et al. Taming wettability of lithium ion sieve via different TiO2 precursors for effective Li recovery from aqueous lithium resources[J]. Chemical Engineering Journal, 2020, 392: 123731. |
| 12 | Wang H S, Cui J J, Li M L, et al. Selective recovery of lithium from geothermal water by EGDE cross-linked spherical CTS/LMO[J]. Chemical Engineering Journal, 2020, 389: 124410. |
| 13 | Xiao J L, Sun S Y, Wang J, et al. Synthesis and adsorption properties of Li1.6Mn1.6O4 spinel[J]. Industrial & Engineering Chemistry Research, 2013, 52(34): 11967-11973. |
| 14 | 冯文贤. 电渗析法分离卤水中镁锂的研究[D]. 天津: 河北工业大学, 2016. |
| Feng W X. The research on separation of magnesium and lithium from brine by electrodialysis[D]. Tianjin: Hebei University of Technology, 2016. | |
| 15 | 陈青柏. 面向卤水/海水锂资源提取的电渗析法分离纯化锂的研究[D]. 天津: 河北工业大学, 2017. |
| Chen Q B. The research on separation and purification of lithium from brine/seawater by electrodialysis[D]. Tianjin: Hebei University of Technology, 2017. | |
| 16 | Onorato C, Banasiak L J, Schäfer A I. Inorganic trace contaminant removal from real brackish groundwater using electrodialysis[J]. Separation and Purification Technology, 2017, 187: 426-435. |
| 17 | 张秀峰, 谭秀民, 张利珍. 纳滤膜分离技术应用于盐湖卤水提锂的研究进展[J]. 无机盐工业, 2017, 49(1): 1-5. |
| Zhang X F, Tan X M, Zhang L Z. Research progress in lithium extraction from salt lake brine by nanofiltration membrane separation technology[J]. Inorganic Chemicals Industry, 2017, 49(1): 1-5. | |
| 18 | Li W, Shi C, Zhou A, et al. A positively charged composite nanofiltration membrane modified by EDTA for LiCl/MgCl2 separation[J]. Separation and Purification Technology, 2017, 186: 233-242. |
| 19 | Song J F, Huang T, Qiu H B, et al. Recovery of lithium from salt lake brine of high Mg/Li ratio using Na[FeCl4·2TBP] as extractant: thermodynamics, kinetics and processes[J]. Hydrometallurgy, 2017, 173: 63-70. |
| 20 | Çelebi E E, Öncel M S, Kobya M, et al. Extraction of lithium from wastewaters using a synergistic solvent extraction system consisting of Mextral EOL and Cyanex 923[J]. Hydrometallurgy, 2019, 185: 46-54. |
| 21 | He L H, Xu W H, Song Y F, et al. New insights into the application of lithium-ion battery materials: selective extraction of lithium from brines via a rocking-chair lithium-ion battery system[J]. Global Challenges, 2018, 2(2): 1700079. |
| 22 | Liu X H, Chen X Y, Zhao Z W, et al. Effect of Na+ on Li extraction from brine using LiFePO4/FePO4 electrodes[J]. Hydrometallurgy, 2014, 146: 24-28. |
| 23 | Zhao Z W, Si X F, Liu X H, et al. Li extraction from high Mg/Li ratio brine with LiFePO4/FePO4 as electrode materials[J]. Hydrometallurgy, 2013, 133: 75-83. |
| 24 | Liu X H, Chen X Y, He L H, et al. Study on extraction of lithium from salt lake brine by membrane electrolysis[J]. Desalination, 2015, 376: 35-40. |
| 25 | Xu W H, Liu D F, He L H, et al. A comprehensive membrane process for preparing lithium carbonate from high Mg/Li brine[J]. Membranes, 2020, 10(12): 371. |
| 26 | Zhao Z W, Liu X H. Method and device for extracting and enriching lithium: US9062385[P]. 2015-06-23. |
| 27 | Xu W H, He L H, Zhao Z W. Lithium extraction from high Mg/Li brine via electrochemical intercalation/de-intercalation system using LiMn2O4 materials[J]. Desalination, 2021, 503: 114935. |
| 28 | Liu C, Li Y B, Lin D C, et al. Lithium extraction from seawater through pulsed electrochemical intercalation[J]. Joule, 2020, 4(7): 1459-1469. |
| 29 | Sun S, Yu X P, Li M L, et al. Green recovery of lithium from geothermal water based on a novel lithium iron phosphate electrochemical technique[J]. Journal of Cleaner Production, 2020, 247: 119178. |
| 30 | Zhao M Y, Ji Z Y, Zhang Y G, et al. Study on lithium extraction from brines based on LiMn2O4/Li1-xMn2O4 by electrochemical method[J]. Electrochimica Acta, 2017, 252: 350-361. |
| 31 | Liu D F, Sun S Y, Yu J G. Electrochemical and adsorption behaviour of Li+, Na+, K+, Ca2+, and Mg2+ in LiMn2O4/λ-MnO2 structures[J]. The Canadian Journal of Chemical Engineering, 2018, 97: 1589-1595. |
| 32 | 何利华. 基于“摇椅式”锂离子电池原理的盐湖提锂新工艺及其理论研究[D]. 长沙: 中南大学, 2015. |
| He L H. Theoretical and technical study on the novel process for lithium extraction from salt lake brine basing on the principles of “Rocking-chair” lithium ion batteries [D]. Changsha: Central South University, 2015. | |
| 33 | Zhou J F, Zhao J H, Yang F, et al. Leaching kinetics of potassium and aluminum from phosphorus-potassium associated ore in HCl-CaF2 system[J]. Separation and Purification Technology, 2020, 253: 117528. |
| 34 | Li L, Bian Y F, Zhang X X, et al. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching[J]. Waste Management, 2018, 71: 362-371. |
| [1] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [2] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [5] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [6] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [7] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [8] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [9] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [10] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [11] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [12] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [13] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [14] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [15] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号