化工学报 ›› 2023, Vol. 74 ›› Issue (8): 3242-3255.DOI: 10.11949/0438-1157.20230426
收稿日期:2023-04-28
修回日期:2023-07-12
出版日期:2023-08-25
发布日期:2023-10-18
通讯作者:
张睿智
作者简介:汪林正(1997—),男,博士研究生,wlz1997@sjtu.edu.cn
基金资助:
Linzheng WANG1( ), Yubing LU2, Ruizhi ZHANG1(
), Yubing LU2, Ruizhi ZHANG1( ), Yonghao LUO1
), Yonghao LUO1
Received:2023-04-28
Revised:2023-07-12
Online:2023-08-25
Published:2023-10-18
Contact:
Ruizhi ZHANG
摘要:
采用反应分子动力学模拟的方法,选择苯、甲苯和苯乙烯作为代表性VOCs组分,分析其在不同温度下发生热解和氧化的反应特性,获得其总包动力学参数,并应用于VOCs在蓄热氧化装置(RTO)中的CFD模拟。芳烃类VOCs的初始热解步骤主要发生脱氢、脱侧链和开环反应,生成对应支链结构的小分子烃类和苯,而氧化过程则直接生成CO、H2O以及少量的烃类。不同VOCs的热解与氧化反应速率存在显著差异,动力学分析表明,使用一级反应假设适用于描述VOCs热解及氧化初始阶段的反应过程。CFD模拟表明,提高入口温度可以显著提升VOCs的转化效率,而在同等VOCs处理量的前提下,提高VOCs浓度、降低进口总流量,对VOCs转化效率的改善程度与提高入口温度相当,这表明VOCs浓缩技术耦合RTO更为高效节能。
中图分类号:
汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255.
Linzheng WANG, Yubing LU, Ruizhi ZHANG, Yonghao LUO. Analysis on thermal oxidation characteristics of VOCs based on molecular dynamics simulation[J]. CIESC Journal, 2023, 74(8): 3242-3255.
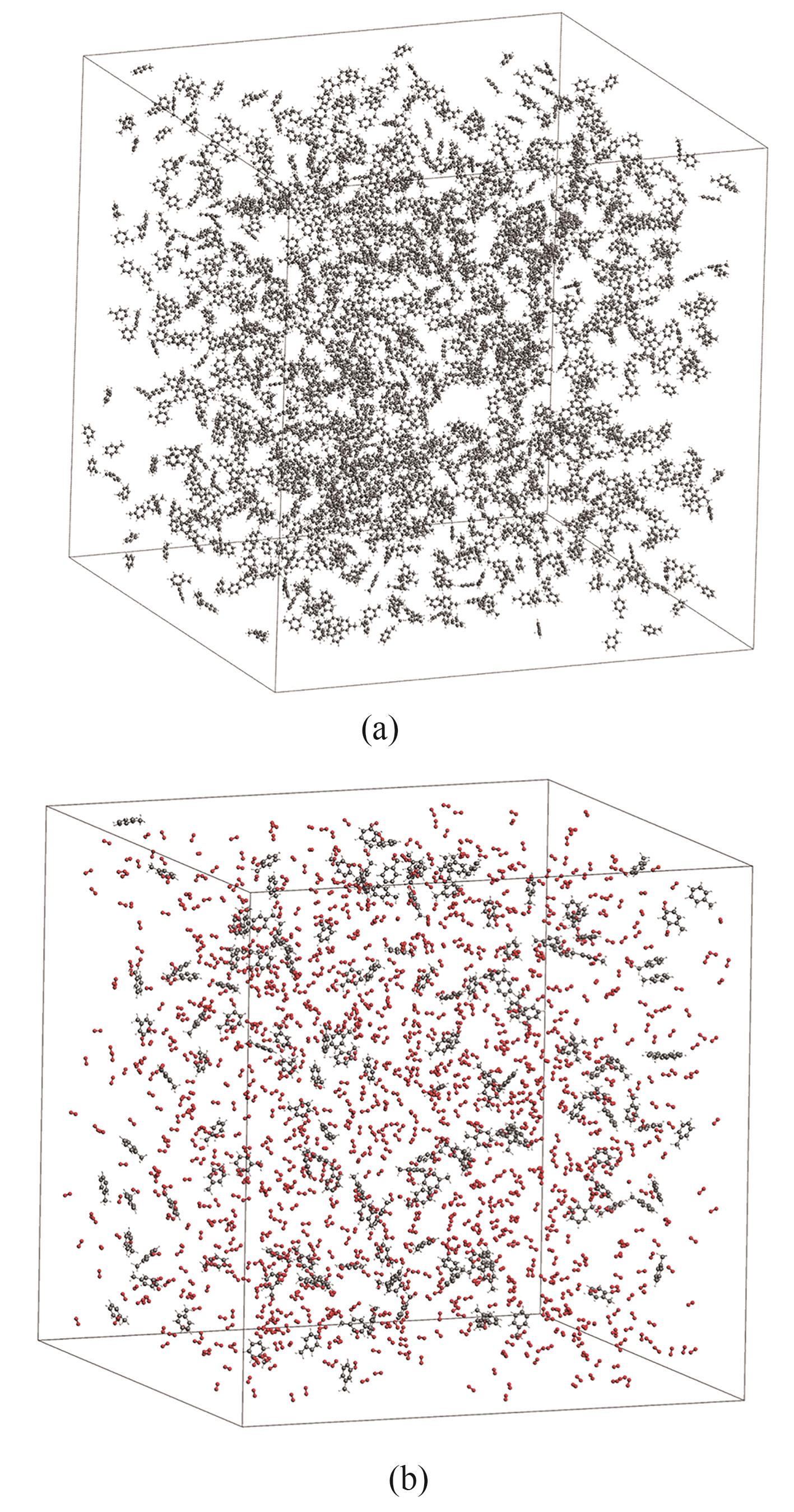
图2 ReaxFF模拟所建立的分子体系示意图(a) 甲苯热解;(b) 甲苯氧化
Fig.2 Schematic of the molecular system for ReaxFF simulation(a) pyrolysis of toluene; (b) oxidation of toluene
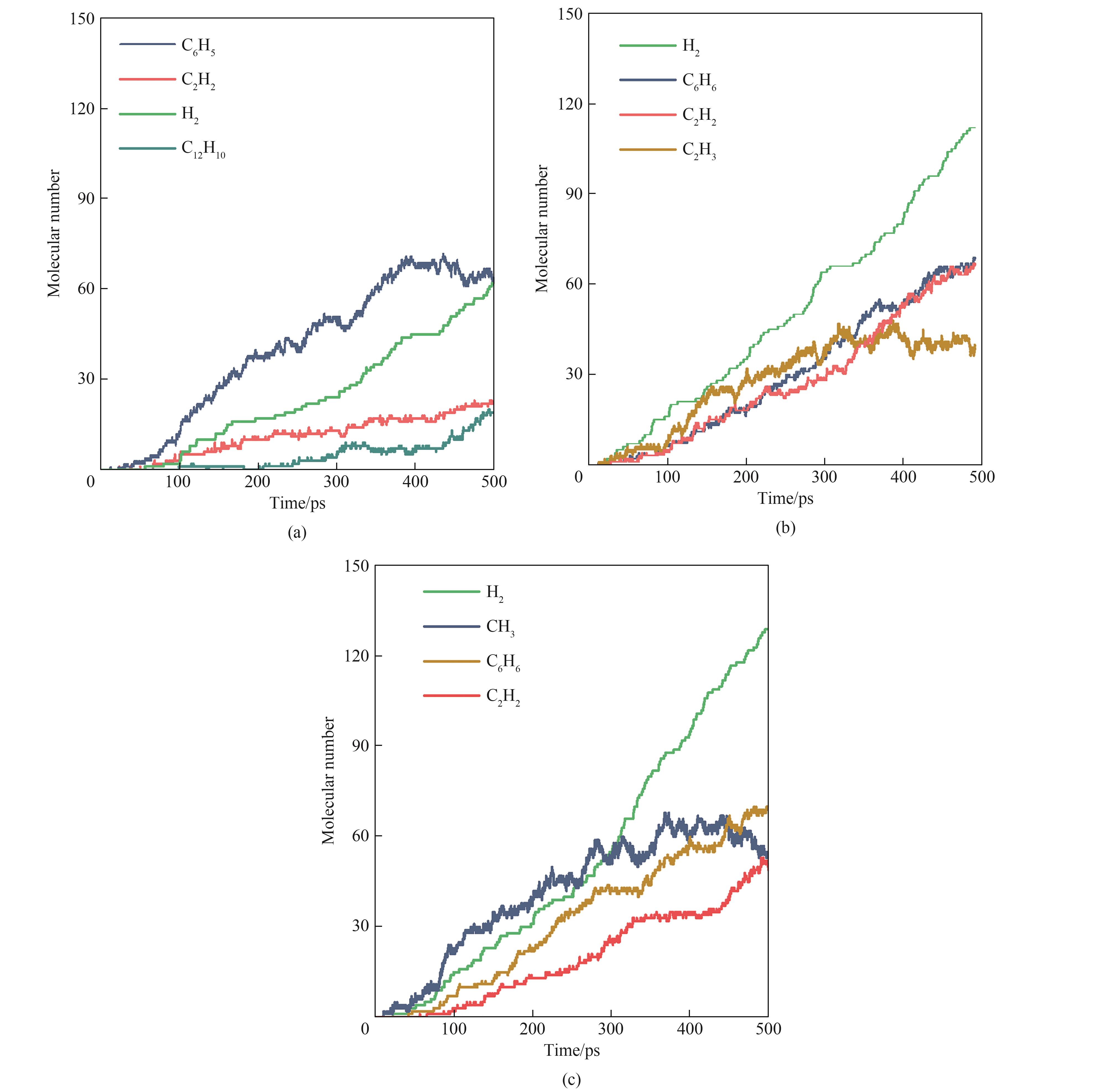
图6 VOCs模型化合物在2500 K下热解反应的主要产物(a) 苯;(b) 甲苯;(c) 苯乙烯
Fig.6 Main products from pyrolysis of VOCs model compounds under 2500 K(a) benzene; (b) toluene; (c) styrene
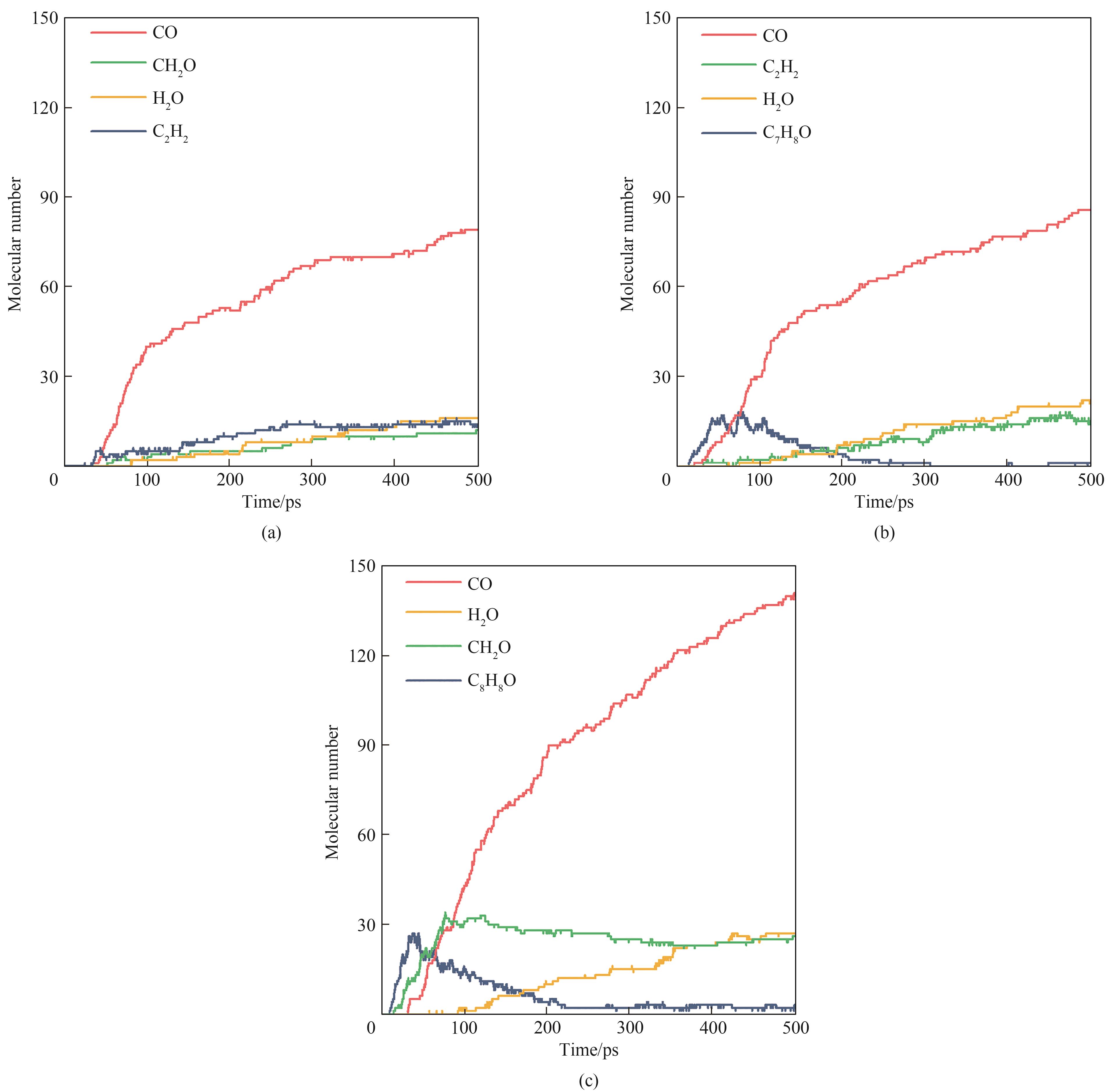
图 7 VOCs模型化合物在2500 K下氧化反应的主要产物(a) 苯;(b) 甲苯;(c) 苯乙烯
Fig. 7 Main products from oxidation of VOCs model compounds under 2500 K(a) benzene; (b) toluene; (c) styrene
| No. | Reaction | A/s-1 | Ea/(kJ/mol) | R2 |
|---|---|---|---|---|
| 1 | 5.170 | 482.014 | 0.964 | |
| 2 | 5.132 | 171.104 | 0.973 | |
| 3 | 1.589 | 390.233 | 0.988 | |
| 4 | 1.266 | 183.197 | 0.957 | |
| 5 | 3.752 | 299.918 | 0.989 | |
| 6 | 2.061 | 134.870 | 0.961 |
表1 MD模拟VOCs转化反应
Table 1 MD simulation VOCs conversion reaction
| No. | Reaction | A/s-1 | Ea/(kJ/mol) | R2 |
|---|---|---|---|---|
| 1 | 5.170 | 482.014 | 0.964 | |
| 2 | 5.132 | 171.104 | 0.973 | |
| 3 | 1.589 | 390.233 | 0.988 | |
| 4 | 1.266 | 183.197 | 0.957 | |
| 5 | 3.752 | 299.918 | 0.989 | |
| 6 | 2.061 | 134.870 | 0.961 |
| Reaction | ReaxFF | Experiment | ||||||
|---|---|---|---|---|---|---|---|---|
| Condition | A/s-1 | Ea/(kcal/mol) | Ref. | Condition | A/s-1 | Ea/(kcal/mol) | Ref. | |
| toluene pyrolysis | 0.2 g/cm3, 2000—2600 K | 2.8×1017 | 95.71 | [ | shock tube, 10 atm | 1.0×1016 | 97.00 | [ |
0.1 g/cm3, 2200—2600 K | 1.589 | 93.27 | this work | shock tube, 1.5 bar | 2.7×1016 | 97.88 | [ | |
| toluene oxidation | 0.2 g/cm3, 2500—3000 K, equivalence ratio: 2 | — | 64.63 | [ | shock tube, 1.95—8.85 atm, 1339—1797 K, toluene: 0.5%—1.5%,O2: 4.48%—13.45% | — | 55.09 | [ |
0.1 g/cm3, 2200—2600 K, O2: 90%(volume ratio) | 1.266 | 43.76 | this work | shock tube, 1.5—5.0 atm, 1400—2000 K, equivalence ratio: 0.5~1.875 | — | 55.24 | [ | |
表2 甲苯热解及氧化的MD模拟及实验得出的动力学参数对比
Table 2 Comparison of kinetic parameters of the pyrolysis and oxidation of toluene from MD modeling and experiments
| Reaction | ReaxFF | Experiment | ||||||
|---|---|---|---|---|---|---|---|---|
| Condition | A/s-1 | Ea/(kcal/mol) | Ref. | Condition | A/s-1 | Ea/(kcal/mol) | Ref. | |
| toluene pyrolysis | 0.2 g/cm3, 2000—2600 K | 2.8×1017 | 95.71 | [ | shock tube, 10 atm | 1.0×1016 | 97.00 | [ |
0.1 g/cm3, 2200—2600 K | 1.589 | 93.27 | this work | shock tube, 1.5 bar | 2.7×1016 | 97.88 | [ | |
| toluene oxidation | 0.2 g/cm3, 2500—3000 K, equivalence ratio: 2 | — | 64.63 | [ | shock tube, 1.95—8.85 atm, 1339—1797 K, toluene: 0.5%—1.5%,O2: 4.48%—13.45% | — | 55.09 | [ |
0.1 g/cm3, 2200—2600 K, O2: 90%(volume ratio) | 1.266 | 43.76 | this work | shock tube, 1.5—5.0 atm, 1400—2000 K, equivalence ratio: 0.5~1.875 | — | 55.24 | [ | |
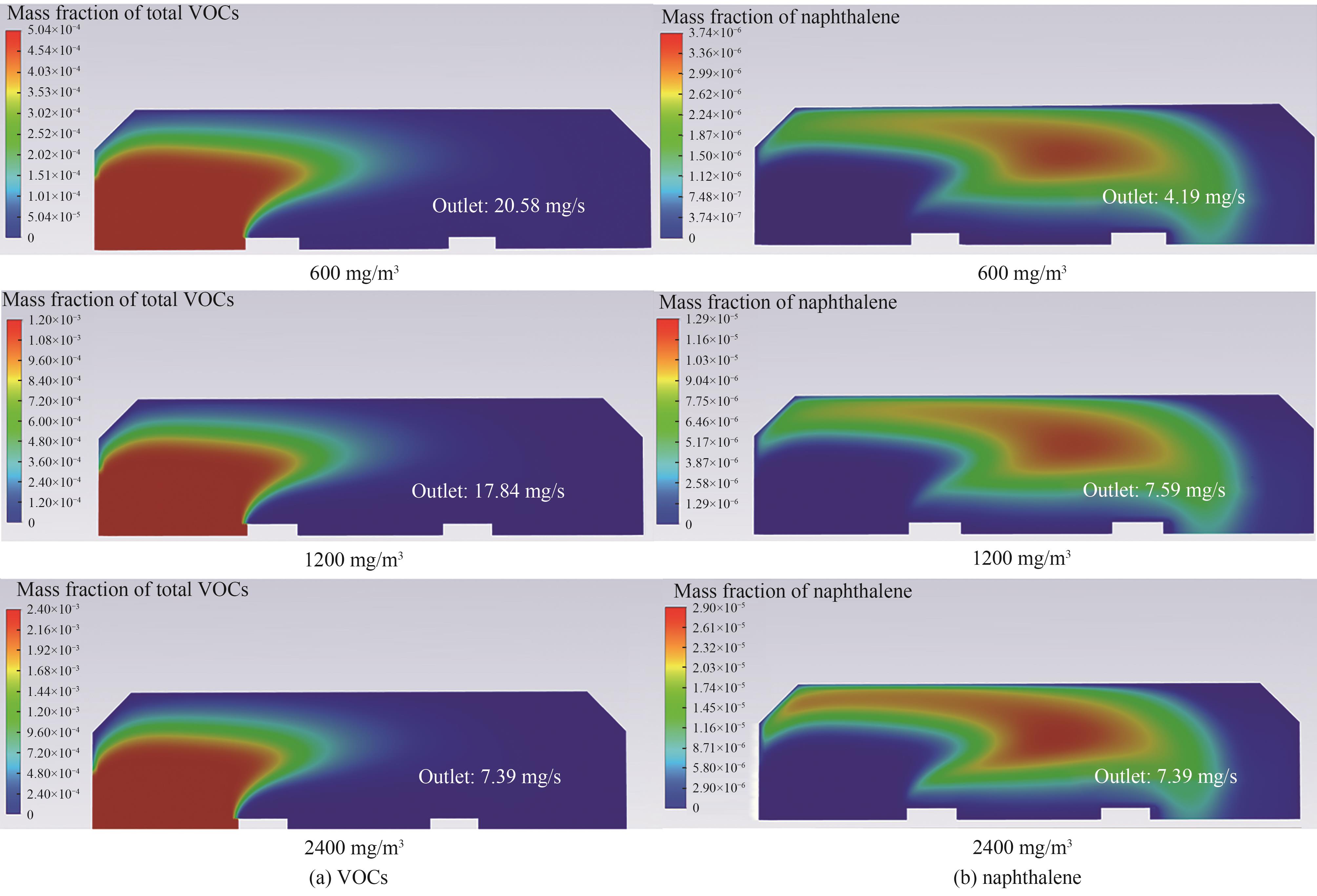
图9 不同入口浓度下总VOCs质量分数与PAHs产物(以萘计)质量分数分布
Fig.9 Mass fraction distribution of total VOCs and PAHs (counting naphthalene) at different inlet concentrations
| 1 | 陆思华, 白郁华, 张广山, 等. 大气中挥发性有机化合物(VOCs)的人为来源研究[J]. 环境科学学报, 2006, 26(5): 757-763. |
| Lu S H, Bai Y H, Zhang G S, et al. Source apportionment of anthropogenic emissions of volatile organic compounds[J]. Acta Scientiae Circumstantiae, 2006, 26(5): 757-763. | |
| 2 | 王治民, 孙建薇. 关于VOC废气处理技术的相关思考[J]. 能源与节能, 2014(5): 97-99. |
| Wang Z M, Sun J W. Relevant thinking on the VOC gas treatment technology[J]. Energy and Energy Conservation, 2014(5): 97-99. | |
| 3 | 张小苑. VOC的危害及回收与处理技术[J]. 绿色环保建材, 2017(9): 247-248. |
| Zhang X Y. Harm of VOC and its recovery and treatment technology[J]. Green Environmental Protection Building Materials, 2017(9): 247-248. | |
| 4 | 刘鹏, 周湘梅. VOC的回收与处理技术简介[J]. 石油化工环境保护, 2001(3): 39-42. |
| Liu P, Zhou X M. Recycle of VOC and its treatment technology[J]. Environment Protection in Petrochemical Industry, 2001(3): 39-42. | |
| 5 | 冯正敢, 姚丽, 洪嘉阳, 等. 挥发性有机物(VOCs)治理措施现状与发展趋势[J]. 绿色科技, 2019(2): 35-37, 39. |
| Feng Z G, Yao L, Hong J Y, et al. Present situation and development trend of control measures for volatile organic compounds(VOCs)[J]. Journal of Green Science and Technology, 2019(2): 35-37, 39. | |
| 6 | 李守信, 宋剑飞, 李立清, 等. 挥发性有机化合物处理技术的研究进展[J]. 化工环保, 2008, 28(1): 1-7. |
| Li S X, Song J F, Li L Q, et al. Research progresses in treatment technologies for volatile organic compounds[J]. Environmental Protection of Chemical Industry, 2008, 28(1): 1-7. | |
| 7 | 杨仲卿, 刘显伟, 张力, 等. 挥发性有机废气热氧化技术研究进展[J]. 化工进展, 2017, 36(10): 3866-3875. |
| Yang Z Q, Liu X W, Zhang L, et al. Research progress on the thermal oxidation technologies for volatile organic waste gas[J]. Chemical Industry and Engineering Progress, 2017, 36(10): 3866-3875. | |
| 8 | 殷冬媛. VOCs控制与处理技术综述[J]. 化工管理, 2018(20): 154-155. |
| Yin D Y. Summary of VOCs control and treatment technology[J]. Chemical Enterprise Management, 2018(20): 154-155. | |
| 9 | 毕贵芹. 催化燃烧技术在石化企业VOCs处理中的进展[J]. 广东化工, 2013, 40(23): 104-105. |
| Bi G Q. Advance for catalytic combustion of volatile organic compounds in petro-chemical enterprise[J]. Guangdong Chemical Industry, 2013, 40(23): 104-105. | |
| 10 | 崔龙哲, 蔡俊雄, 申哲昊, 等. 旋转浓缩-蓄热氧化法处理涂装废气的中试研究[J]. 现代化工, 2009, 29(12): 75-78. |
| Cui L Z, Cai J X, Shin C H, et al. Pilot study on treatment of painting exhaust-gas by RC-RTO system[J]. Modern Chemical Industry, 2009, 29(12): 75-78. | |
| 11 | Giuntini L, Bertei A, Tortorelli S, et al. Coupled CFD and 1-D dynamic modeling for the analysis of industrial regenerative thermal oxidizers[J]. Chemical Engineering and Processing-Process Intensification, 2020, 157: 108117. |
| 12 | 陆超, 姜治芳, 王涛. 利用缩比模型CFD数值模拟计算舰船舰面空气流场相似准数的影响探讨[J]. 中国舰船研究, 2008, 3(6): 45-48. |
| Lu C, Jiang Z F, Wang T. Discussion on comparability of scaled models for CFD numerical simulation for ship airwake[J]. Chinese Journal of Ship Research, 2008, 3(6): 45-48. | |
| 13 | 刘胜. 基于CFD的三体船水动力性能计算[D]. 大连: 大连理工大学, 2016. |
| Liu S. Hydrodynamic calculation of trimaran based on CFD method[D]. Dalian: Dalian University of Technology, 2016. | |
| 14 | 张丹娜. 大气对流层中几种VOCs反应机理及动力学研究[D]. 济南: 山东大学, 2020. |
| Zhang D N. Theoretical investigation on the reaction mechanism and kinetics of several VOCs in the troposphere[D]. Jinan: Shandong University, 2020. | |
| 15 | 王瑜. 挥发性含氯有机废气(CVOCs)氧化消除的高效催化剂研究[D]. 金华: 浙江师范大学, 2015. |
| Wang Y. Highly efficient catalysts for chlorinated volatile organic compounds (CVOCs) combustion[D]. Jinhua: Zhejiang Normal University, 2015. | |
| 16 | Marín P, Díez F V, Ordóñez S. A new method for controlling the ignition state of a regenerative combustor using a heat storage device[J]. Applied Energy, 2014, 116: 322-332. |
| 17 | Hao X W, Li R X, Wang J A, et al. Numerical simulation of a regenerative thermal oxidizer for volatile organic compounds treatment[J]. Environmental Engineering Research, 2018, 23(4): 397-405. |
| 18 | 刘健, 李晓霞, 郭力, 等. 反应分子动力学(ReaxFF MD)模拟结果分析工具VARxMD[J]. 计算机与应用化学, 2014, 31(6): 641-647. |
| Liu J, Li X X, Guo L, et al. VARxMD, a new tool for reaction analysis of ReaxFF MD simulations[J]. Computers and Applied Chemistry, 2014, 31(6): 641-647. | |
| 19 | 刘捷, 范俊辉, 卢文强. 常见VOCs在PVC板中扩散的分子动力学模拟[J]. 工程热物理学报, 2014, 35(6): 1181-1184. |
| Liu J, Fan J H, Lu W Q. Molecular dynamics simulation of the diffusion of VOCs in PVC board[J]. Journal of Engineering Thermophysics, 2014, 35(6): 1181-1184. | |
| 20 | 谷雪贤, 谢彩铃. 计算机分子模拟在活性炭吸附VOCs行为研究中的应用[J]. 广东化工, 2015, 42(15): 158-159. |
| Gu X X, Xie C L. Application of molecular simulation in the behavior of adsorption of VOCs in activated carbon[J]. Guangdong Chemical Industry, 2015, 42(15): 158-159. | |
| 21 | Liu Q, Liu S X, Lv Y D, et al. Atomic-scale insight into the pyrolysis of polycarbonate by ReaxFF-based reactive molecular dynamics simulation[J]. Fuel, 2021, 287: 119484. |
| 22 | 韩君易, 李晓霞, 郭力, 等. ReaxFF MD模拟的物种和化学反应自动分类及可视化[J]. 计算机与应用化学, 2015, 32(5): 519-526. |
| Han J Y, Li X X, Guo L, et al. Automatic classification and visualization of species and reactions obtained from ReaxFF MD simulations[J]. Computers and Applied Chemistry, 2015, 32(5): 519-526. | |
| 23 | Chen T, Ku X K, Li T, et al. High-temperature pyrolysis modeling of a thermally thick biomass particle based on an MD-derived tar cracking model[J]. Chemical Engineering Journal, 2021, 417: 127923. |
| 24 | Ashraf C, Shabnam S, Jain A, et al. Pyrolysis of binary fuel mixtures at supercritical conditions: a ReaxFF molecular dynamics study[J]. Fuel, 2019, 235: 194-207. |
| 25 | Li X X, Zheng M, Ren C X, et al. ReaxFF molecular dynamics simulations of thermal reactivity of various fuels in pyrolysis and combustion[J]. Energy & Fuels, 2021, 35(15): 11707-11739. |
| 26 | 范红玉, 李小松, 刘艳霞, 等. 循环的存储-放电等离子体催化新过程脱除室内空气中甲苯[J]. 化工学报, 2011, 62(7): 1922-1926. |
| Fan H Y, Li X S, Liu Y X, et al. Cycled storage-discharge plasma catalytic process for toluene removal from indoor air[J]. CIESC Journal, 2011, 62(7): 1922-1926. | |
| 27 | 李津津, 陈扉然, 马修卫, 等. 燃煤有机污染物排放及其控制技术研究展望[J]. 化工进展, 2019, 38(12): 5539-5547. |
| Li J J, Chen F R, Ma X W, et al. Emission of coal-fired VOCs and prospect of control technology[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5539-5547. | |
| 28 | Saggese C, Frassoldati A, Cuoci A, et al. A wide range kinetic modeling study of pyrolysis and oxidation of benzene[J]. Combustion and Flame, 2013, 160(7): 1168-1190. |
| 29 | 李玉阳. 芳烃燃料低压预混火焰的实验和动力学模型研究[D]. 合肥: 中国科学技术大学, 2010. |
| Li Y Y. Experimental and kinetic modeling study of premixed aromatic hydrocarbon flames at low pressure[D]. Hefei: University of Science and Technology of China, 2010. | |
| 30 | 刘慧利, 胡建杭, 王华, 等. 松木屑低温热解液形成机理的实验研究[J]. 太阳能学报, 2014, 35(3): 396-401. |
| Liu H L, Hu J H, Wang H, et al. Experimental study of pyrolysis liquids formation mechanism from pine sawdust pyrolysis at low temperatures[J]. Acta Energiae Solaris Sinica, 2014, 35(3): 396-401. | |
| 31 | Simmie J M. Detailed chemical kinetic models for the combustion of hydrocarbon fuels[J]. Progress in Energy and Combustion Science, 2003, 29(6): 599-634. |
| 32 | 曾文, 马洪安, 解茂昭. 正庚烷部分预混燃烧下多环芳烃生成的简化机理[J]. 燃烧科学与技术, 2011, 17(4): 313-320. |
| Zeng W, Ma H A, Xie M Z. A reduced mechanism of PAHs formation in n-heptane/air partially premixed combustion[J]. Journal of Combustion Science and Technology, 2011, 17(4): 313-320. | |
| 33 | 吴文睿, 周兵. 关于RTO焚烧炉技术的简单探讨[J]. 资源节约与环保, 2016(1): 32. |
| Wu W R, Zhou B. Simple discussion on RTO incinerator technology[J]. Resources Economization & Environmental Protection, 2016(1): 32. | |
| 34 | Wang Q D, Wang J B, Li J Q, et al. Reactive molecular dynamics simulation and chemical kinetic modeling of pyrolysis and combustion of n-dodecane[J]. Combustion and Flame, 2011, 158(2): 217-226. |
| 35 | Cheng X M, Wang Q D, Li J Q, et al. ReaxFF molecular dynamics simulations of oxidation of toluene at high temperatures[J]. The Journal of Physical Chemistry A, 2012, 116(40): 9811-9818. |
| 36 | Chenoweth K, van Duin A C T, Goddard W A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation[J]. The Journal of Physical Chemistry A, 2008, 112(5): 1040-1053. |
| 37 | Liu H, Liang J H, He R N, et al. Overall mechanism of JP-10 pyrolysis unraveled by large-scale reactive molecular dynamics simulation[J]. Combustion and Flame, 2022, 237: 111865. |
| 38 | Liu J, Guo X. ReaxFF molecular dynamics simulation of pyrolysis and combustion of pyridine[J]. Fuel Processing Technology, 2017, 161: 107-115. |
| 39 | Ashraf C, van Duin A C T. Extension of the ReaxFF combustion force field toward syngas combustion and initial oxidation kinetics[J]. The Journal of Physical Chemistry A, 2017, 121(5): 1051-1068. |
| 40 | Liu Y, Wei X, Sun W Z, et al. ReaxFF MD investigation of the high-temperature combustion of six octane isomers[J]. Energy & Fuels, 2021, 35(20): 16778-16790. |
| 41 | te Velde G, Bickelhaupt F M, Baerends E J, et al. Chemistry with ADF[J]. Journal of Computational Chemistry, 2001, 22(9): 931-967. |
| 42 | Ali Masmoudi M, Halouani K, Sahraoui M. Comprehensive experimental investigation and numerical modeling of the combined partial oxidation-gasification zone in a pilot downdraft air-blown gasifier[J]. Energy Conversion and Management, 2017, 144: 34-52. |
| 43 | Nikitin V F, Mikhalchenko E V. Safety of a rotating detonation engine fed by acetylene-oxygen mixture launching stage[J]. Acta Astronautica, 2022, 194: 496-503. |
| 44 | Zhao P, Han S, Li X X, et al. Comparison of RP-3 pyrolysis reactions between surrogates and 45-component model by ReaxFF molecular dynamics simulations[J]. Energy & Fuels, 2019, 33(8): 7176-7187. |
| 45 | Yuan W H, Li Y Y, Dagaut P, et al. Experimental and kinetic modeling study of styrene combustion[J]. Combustion and Flame, 2015, 162(5): 1868-1883. |
| 46 | Colket M B, Seery D J. Reaction mechanisms for toluene pyrolysis[J]. Symposium (International) on Combustion, 1994, 25(1): 883-891. |
| 47 | Oehlschlaeger M A, Davidson D F, Hanson R K. Thermal decomposition of toluene: overall rate and branching ratio[J]. Proceedings of the Combustion Institute, 2007, 31(1): 211-219. |
| 48 | Burcat A, Snyder C, Brabbs T A. Ignition Delay Times of Benzene and Toluene with Oxygen in Argon Mixtures[M]. Washington, DC: National Aeronautics and Space Administration, 1985. |
| 49 | Vasudevan V, Davidson D F, Hanson R K. Shock tube measurements of toluene ignition times and OH concentration time histories[J]. Proceedings of the Combustion Institute, 2005, 30(1): 1155-1163. |
| [1] | 张思雨, 殷勇高, 贾鹏琦, 叶威. 双U型地埋管群跨季节蓄热特性研究[J]. 化工学报, 2023, 74(S1): 295-301. |
| [2] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [3] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [4] | 温凯杰, 郭力, 夏诏杰, 陈建华. 一种耦合CFD与深度学习的气固快速模拟方法[J]. 化工学报, 2023, 74(9): 3775-3785. |
| [5] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [6] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [7] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [8] | 岳林静, 廖艺涵, 薛源, 李雪洁, 李玉星, 刘翠伟. 凹坑缺陷对厚孔板喉部空化流动特性影响研究[J]. 化工学报, 2023, 74(8): 3292-3308. |
| [9] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [10] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [11] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [12] | 何晓崐, 刘锐, 薛园, 左然. MOCVD生长AlN单晶薄膜的气相和表面化学反应综述[J]. 化工学报, 2023, 74(7): 2800-2813. |
| [13] | 董明, 徐进良, 刘广林. 超临界水非均质特性分子动力学研究[J]. 化工学报, 2023, 74(7): 2836-2847. |
| [14] | 牛超, 沈胜强, 杨艳, 潘泊年, 李熠桥. 甲烷BOG喷射器流动过程计算与性能分析[J]. 化工学报, 2023, 74(7): 2858-2868. |
| [15] | 刘道银, 陈柄岐, 张祖扬, 吴琰. 颗粒聚团结构对曳力特性影响的数值模拟[J]. 化工学报, 2023, 74(6): 2351-2362. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号