化工学报 ›› 2021, Vol. 72 ›› Issue (6): 3022-3030.DOI: 10.11949/0438-1157.20201728
收稿日期:2020-12-01
修回日期:2021-02-09
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
成怀刚
作者简介:高姣丽(1996—),女,硕士研究生,基金资助:
GAO Jiaoli( ),LI Enze,KANG Jin,CHENG Huaigang(
),LI Enze,KANG Jin,CHENG Huaigang( )
)
Received:2020-12-01
Revised:2021-02-09
Online:2021-06-05
Published:2021-06-05
Contact:
CHENG Huaigang
摘要:
盐湖老卤中镁资源储量丰富,现阶段的初级利用形式主要是提取结晶氯化镁。为提高过程效益,提出了兑卤及变温直接分离高纯结晶硫酸镁的方案,并分别考察MgSO4饱和卤水、MgSO4-NaCl共饱和卤水、MgSO4-NaCl-MgCl2共饱和卤水在非平衡动态降温过程中硫酸镁晶体微观形貌的变化以及降温速率对晶体形貌的影响。结果表明,MgSO4饱和卤水非平衡动态降温过程中晶体形成的速率与降温速率有关,在-1.02℃/min的降温速率下,MgSO4饱和卤水降温30 min后即可形成径向约1300 μm的MgSO4·7H2O晶体;但是,当卤水中存在NaCl时,最终的晶体形式为MgSO4·6H2O,且多为不规则的块状;卤水中的MgCl2能促使晶体形成棱柱状的MgSO4·6H2O。在NaCl和MgCl2的作用下,MgSO4-NaCl-MgCl2共饱和卤水降温15 h后可以析出完整晶型的高纯结晶硫酸镁。
中图分类号:
高姣丽, 李恩泽, 康锦, 成怀刚. 盐湖老卤动态变温分离高纯结晶硫酸镁[J]. 化工学报, 2021, 72(6): 3022-3030.
GAO Jiaoli, LI Enze, KANG Jin, CHENG Huaigang. Separation of high purity magnesium sulfate hydrates from salt lake tail brine in a dynamic temperature-changing process[J]. CIESC Journal, 2021, 72(6): 3022-3030.

图4 MgSO4-NaCl-MgCl2共饱和卤水非平衡动态降温过程中晶体形貌变化(显微镜,放大倍数为4倍)
Fig.4 Crystal morphology changes during non-equilibrium dynamic cooling of MgSO4-NaCl-MgCl2 saturated brine (microscope, magnification 4 times)
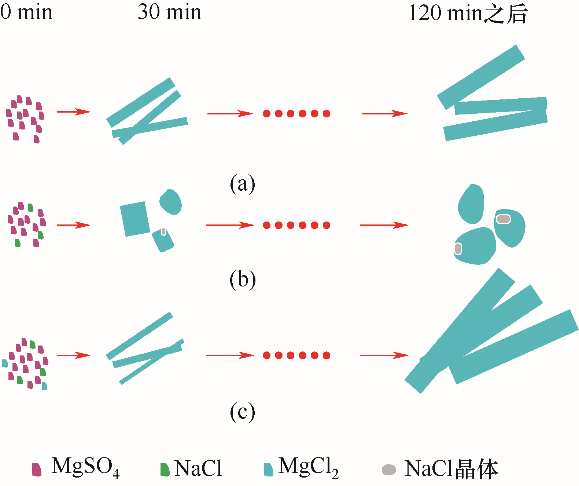
图5 不同饱和卤水非平衡动态降温过程中晶体形貌变化示意图(a) MgSO4饱和卤水非平衡动态降温过程;(b) MgSO4-NaCl共饱和卤水非平衡动态降温过程;(c) MgSO4-NaCl-MgCl2共饱和卤水非平衡动态降温过程
Fig.5 Schematic diagram of crystal morphology changes during non-equilibrium dynamic cooling of different saturated brines

图6 三种不同饱和卤水降温后晶体XRD谱图a—MgSO4饱和卤水非平衡动态降温120 min;b—MgSO4-NaCl共饱和卤水非平衡动态降温120 min;c—MgSO4-NaCl-MgCl2共饱和卤水非平衡动态降温15 h
Fig.6 XRD patterns of crystal after cooling of three different saturated brine
| 1 | 王孟雪, 余晓平, 郭亚飞, 等. 卤水中镁的分离提取研究进展[J]. 盐科学与化工, 2017, 46(7): 4-8. |
| Wang M X, Yu X P, Guo Y F, et al. Progresses on the separation and extraction of magnesium from brine[J]. Journal of Salt Science and Chemical Industry, 2017, 46(7): 4-8. | |
| 2 | 李东星. 盐湖老卤开发利用进展[J]. 盐科学与化工, 2019, 48(8): 6-10. |
| Li D X. The advances in the exploitation and utilization of saline lake halogen[J]. Journal of Salt Science and Chemical Industry, 2019, 48(8): 6-10. | |
| 3 | 熊增华, 王石军. 察尔汗盐湖资源开发利用现状及关键技术进展[J]. 化工矿物与加工, 2021, 50(1): 33-37. |
| Xiong Z H, Wang S J. Development and utilization of Qarhan Salt Lake resources and the progress of key technologies[J]. Chemical Minerals and Processing, 2021, 50(1): 33-37. | |
| 4 | 马广超, 狄跃忠, 彭建平, 等. 青海盐湖水氯镁石利用技术现状[J]. 矿产保护与利用, 2019, 39(3): 160-166. |
| Ma G C, Di Y Z, Peng J P, et al. Utilization technical status of bischofite in Qinghai salt lake[J]. Conservation and Utilization of Mineral Resources, 2019, 39(3): 160-166. | |
| 5 | Pan X J, Dou Z H, Zhang T A, et al. Separation of metal ions and resource utilization of magnesium from saline lake brine by membrane electrolysis[J]. Separation and Purification Technology, 2020, 251: 117316. |
| 6 | 魏廷祥, 李丽, 刘素芹. 利用老卤兑卤生产硫酸镁试验研究[J]. 盐业与化工, 2016, 45(1): 27-28. |
| Wei T X, Li L, Liu S Q. Experimental study on production of magnesium sulfate by mixing the old brine[J]. Journal of Salt and Chemical Industry, 2016, 45(1): 27-28. | |
| 7 | 王玉萍, 贺春宝, 郭向东. 盐湖苦卤直接制取精制硫酸镁的研究[J]. 无机盐工业, 2004, 36(3): 35-37. |
| Wang Y P, He C B, Guo X D. Study on the direct purified magnesium sulfate from bittern in salt lake[J]. Inorganic Chemicals Industry, 2004, 36(3): 35-37. | |
| 8 | 贺春宝. 盐湖苦卤自然冷冻直接制取硫酸镁的研究[J]. 海湖盐与化工, 2005, 34(2): 10-11. |
| He C B. Research on extraction of magnesium sulphate directly from salt lake bittern by freezing process[J]. Sea-Lake Salt and Chemical Industry, 2005, 34(2): 10-11. | |
| 9 | 侯殿保, 杨海云, 陈育刚, 等. 硫酸盐型盐湖盐田泻利盐矿硫酸镁浸取结晶工艺条件研究[J]. 盐湖研究, 2020, 28(2): 71-78. |
| Hou D B, Yang H Y, Chen Y G, et al. Study on the leaching and crystallization conditions of magnesium sulfate leaching from EPS mine of sulfate salt lake[J]. Journal of Salt Lake Research, 2020, 28(2): 71-78. | |
| 10 | 王朝乾, 张朝彬, 邵凯. 兑卤法冷冻硫酸镁生产工艺研究[J]. 盐业与化工, 2013, 42(6): 25-27. |
| Wang C Q, Zhang C B, Shao K. Research on the process of magnesium sulfate production by the method of mixing brine and frozen[J]. Journal of Salt and Chemical Industry, 2013, 42(6): 25-27. | |
| 11 | 吴辉, 侯殿保, 李海民, 等. 用泻利盐矿和卤水制备硫酸镁工艺探究[J]. 盐湖研究, 2015, 23(3): 58-62. |
| Wu H, Hou D B, Li H M, et al. Study on the preparation of magnesium sulfate heptahydrate by using the brine and epsomite mineral[J]. Journal of Salt Lake Research, 2015, 23(3): 58-62. | |
| 12 | 成怀刚, 程芳琴, 赵静, 等. 一种从卤水中提取高纯六水硫酸镁的方法: 106395867A[P]. 2017-02-15. |
| Cheng H G, Cheng F Q, Zhao J, et al. Method used for extracting high purity magnesium sulfate hexahydrate from brine: 106395867A[P]. 2017-02-15. | |
| 13 | Cheng H G, He Y Y, Zhao J, et al. Pilot test and cost-based feasibility study of solar-assisted evaporation for direct preparation of high-purity magnesium sulfate hydrates from metastable Na+, Mg2+//Cl-, SO42--H2O salt-water system[J]. Hydrometallurgy, 2019, 189: 105140. |
| 14 | 刘立新, 杜卫民, 朱晓燕, 等. MoO3纳米材料的形貌控制合成及其可见光催化性能研究[J]. 化工新型材料, 2015, 43(6): 184-187. |
| Liu L X, Du W M, Zhu X Y, et al. Shape-controlled synthesis and visible-light photocatalytic property of MoO3 nanomaterial[J]. New Chemical Materials, 2015, 43(6): 184-187. | |
| 15 | 王丽丽, 李嘉荣, 唐定中. 矿化剂氧化铝的形貌对二氧化硅基陶瓷型芯性能的影响[J]. 航空材料学报, 2015, 35(1): 8-12. |
| Wang L L, Li J R, Tang D Z. Effects of alumina particles morphology on properties of silica-based ceramic cores[J]. Journal of Aeronautical Materials, 2015, 35(1): 8-12. | |
| 16 | 赵晓丽, 方莉, 赵泽森, 等. 硫酸铝铵-氯化氢反应制备六水氯化铝过程中晶体形貌的研究[J]. 无机盐工业, 2019, 51(5): 28-32. |
| Zhao X L, Fang L, Zhao Z S, et al. Study on crystal morphology in preparation of aluminum chloride hexahydrate by ammonium aluminum sulfate-hydrogen chloride reaction[J]. Inorganic Chemicals Industry, 2019, 51(5): 28-32. | |
| 17 | 仇满德, 崔炎龙, 商梦莉, 等. CTAB辅助制备环状MoS2晶体及微结构调控[J]. 人工晶体学报, 2019, 48(3): 398-404, 417. |
| Qiu M D, Cui Y L, Shang M L, et al. CTAB-assisted preparation and microstructure regulation of ring-MoS2 crystals[J]. Journal of Synthetic Crystals, 2019, 48(3): 398-404, 417. | |
| 18 | Cao X P, Zhao H, Liu X Y, et al. Preparation of petal-like magnesium hydroxide particles by adding sulfate ions[J]. Journal of Crystal Growth, 2020, 550: 125841. |
| 19 | 田继. 哈达贺休盐湖镁资源的综合利用[J]. 盐科学与化工, 2018, 47(3): 35-36. |
| Tian J. Comprehensive utilization of magnesium resources in Hadahexiu salt lake[J]. Journal of Salt Science and Chemical Industry, 2018, 47(3): 35-36. | |
| 20 | 李海民, 雷光远, 陈育刚, 等. 硫酸钾镁肥浮选尾矿热浸-冷结晶法提取七水硫酸镁工艺研究[J]. 盐湖研究, 2012, 20(2): 44-51. |
| Li H M, Lei G Y, Chen Y G, et al. Synthesis technology of MgSO4·7H2O produced with flotation tailing of potassium-magnesium sulphate fertilizer through hot leaching and cooling crystallization[J]. Journal of Salt Lake Research, 2012, 20(2): 44-51. | |
| 21 | 高文远, 谢超, 季荣, 等. 热溶结晶法生产七水硫酸镁工艺实验研究[J]. 无机盐工业, 2013, 45(3): 45-47. |
| Gao W Y, Xie C, Ji R, et al. Research on production process of MgSO4·7H2O by hot melt crystallization method[J]. Inorganic Chemicals Industry, 2013, 45(3): 45-47. | |
| 22 | Bian S J, Li D D, Gao D D, et al. Hydrometallurgical processing of lithium, potassium, and boron for the comprehensive utilization of Da Qaidam lake brine via natural evaporation and freezing[J]. Hydrometallurgy, 2017, 173: 80-83. |
| 23 | 米登峰, 张瑞, 张璇, 等. 球形化TKX-50的制备及性能表征[J]. 火炸药学报, 2020, 43(1): 64-68. |
| Mi D F, Zhang R, Zhang X, et al. Preparation and characterization of spheroidized TKX-50[J]. Chinese Journal of Explosives & Propellants, 2020, 43(1): 64-68. | |
| 24 | 贾楠, 田昌, 苏明旭. 无水醋酸钠结晶过程中析晶温度和颗粒粒径在线测量[J]. 化工学报, 2019, 70(12): 4664-4672. |
| Jia N, Tian C, Su M X. In situ measurement of crystallization temperature and particle size distribution during crystallization of sodium acetate[J]. CIESC Journal, 2019, 70(12): 4664-4672. | |
| 25 | Jin M M, Frohberg P, Sun Y Z, et al. Study on metastable zone width and crystal growth of a ternary system: case study MgCl2·6H2O·1, 4-dioxane[J]. Chemical Engineering Science, 2015, 133: 181-189. |
| 26 | 裴滨, 詹光, 陈攀泽, 等. 硫酸钾的转化法制备及其晶体生长过程原位观察[J]. 工程科学学报, 2016, 38(3): 391-400. |
| Pei B, Zhan G, Chen P Z, et al. Preparation of K2SO4 using convention method and in situ observation of its crystal growth[J]. Chinese Journal of Engineering, 2016, 38(3): 391-400. | |
| 27 | 郑绵平. 论中国盐湖[J]. 矿床地质, 2001, 20(2): 181-189, 128. |
| Zheng M P. On saline lakes of China[J]. Mineral Deposits, 2001, 20(2): 181-189, 128. | |
| 28 | 许小静, 张圆圆, 郑鹏艳, 等. 氨法脱硫中杂质对硫酸铵结晶的影响[J]. 煤炭转化, 2020, 43(6): 75-83. |
| Xu X J, Zhang Y Y, Zheng P Y, et al. Effects of impurities on ammonium sulfate crystallization in ammonia desulfurization[J]. Coal Conversion, 2020, 43(6): 75-83. | |
| 29 | 牛韩根. 中温转化-冷却结晶法制备硫酸镁原理及工艺研究[D]. 青海: 中国科学院青海盐湖研究所, 2016. |
| Niu H G. Study on the process of preparation magnesium sulfate by medium transformation and low temperature freezing crystation[D]. Qinghai: Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, 2016. | |
| 30 | 闫平科, 田海山, 高玉娟, 等. 反应物浓度对三水碳酸镁晶体生长形貌的影响研究[J]. 硅酸盐通报, 2013, 32(12): 2568-2571, 2577. |
| Yan P K, Tian H S, Gao Y J, et al. Effects of reactant concentration on the growth morphology of nesquehonite crystal[J]. Bulletin of the Chinese Ceramic Society, 2013, 32(12): 2568-2571, 2577. | |
| 31 | Cheng H G, Zhang Y Y, Wang X, et al. Theoretical and experimental investigation of time-varying properties in the coagulation of kaolinite containing wastewater by gypsum[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 71: 253-259. |
| 32 | 邓天龙. 水盐体系相图及应用[M]. 北京: 化学工业出版社, 2013: 292,316. |
| Deng T L. Phase Diagram of Water-salt System and Its Application [M]. Beijing: Chemical Industry Press, 2013: 292,316. | |
| 33 | 周涛, 陈芳, 李军, 等. TKX-50在甲酸/水混合溶剂中生长形貌的分子动力学模拟[J]. 含能材料, 2020, 28(9): 865-873. |
| Zhou T, Chen F, Li J, et al. Growth morphology of TKX-50 in formic acid/water mixed solvent by molecular dynamics simulation[J]. Chinese Journal of Energetic Materials, 2020, 28(9): 865-873. | |
| 34 | 杨静, 陈明洋, 许史杰, 等. 无机盐晶体形貌调控研究进展[J]. 无机盐工业, 2018, 50(8): 11-15. |
| Yang J, Chen M Y, Xu S J, et al. Progress on crystal shape control of inorganic salts[J]. Inorganic Chemicals Industry, 2018, 50(8): 11-15. | |
| 35 | 陈贵兰, 宋兴福, 于建国. 杂质离子对MgCl2和CO2反应-萃取-醇析耦合过程的影响[J]. 化工学报, 2017, 68(2): 702-707. |
| Chen G L, Song X F, Yu J G. Effects of impurity ions on coupled reaction-extraction-alcohol precipitation process of MgCl2 and CO2[J]. CIESC Journal, 2017, 68(2): 702-707. | |
| 36 | Fang C H, Fang Y, Zhou Y Q, et al. Recent progress on structure of aqueous polyborate solutions[J]. Journal of Salt Lake Research, 2019, 27(2): 11-39, 2. |
| 37 | 吴敬礼, 陈世祥, 王智勇. 高温盐制取七水硫酸镁的研究[J]. 天津化工, 2018, 32(3): 18-19. |
| Wu J L, Chen S X, Wang Z Y. Study on the preparation of magnesium sulfite by the by-product higt-temperature salt[J]. Tianjin Chemical Industry, 2018, 32(3): 18-19. | |
| 38 | 房春晖, 房艳, 郭亚梅, 等. 过饱和MgSO4溶液结构的X射线衍射研究[J]. 化学学报, 2004, 62(3): 268-273. |
| Fang C H, Fang Y, Guo Y M, et al. An X-ray diffraction study on the structure of the supersaturated MgSO4 solutions[J]. Acta Chimica Sinica, 2004, 62(3): 268-273. |
| [1] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [2] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [3] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [4] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [5] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [6] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [7] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [8] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [9] | 高金明, 郭玉娇, 鄂承林, 卢春喜. 一种封闭罩内顺流多旋臂气液分离器的分离特性研究[J]. 化工学报, 2023, 74(7): 2957-2966. |
| [10] | 刘春雨, 周桓宇, 马跃, 岳长涛. CaO调质含油污泥干燥特性及数学模型[J]. 化工学报, 2023, 74(7): 3018-3027. |
| [11] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [12] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| [13] | 朱兴驰, 郭志远, 纪志永, 汪婧, 张盼盼, 刘杰, 赵颖颖, 袁俊生. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| [14] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [15] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号