化工学报 ›› 2022, Vol. 73 ›› Issue (2): 663-671.DOI: 10.11949/0438-1157.20210916
收稿日期:2021-07-02
修回日期:2021-11-05
出版日期:2022-02-05
发布日期:2022-02-18
通讯作者:
徐震原
作者简介:王洁冰(1998—),女,硕士研究生,基金资助:
Jiebing WANG( ),Jintong GAO,Zhenyuan XU(
),Jintong GAO,Zhenyuan XU( )
)
Received:2021-07-02
Revised:2021-11-05
Online:2022-02-05
Published:2022-02-18
Contact:
Zhenyuan XU
摘要:
太阳能界面蒸发可实现高效太阳能海水淡化和蒸发式污水处理,但目前的研究大多局限于纯水或NaCl溶液。实际脱盐或废水处理中溶质会与此不同,导致溶液蒸气压变化并影响蒸发性能。本文首先分析了溶液表面蒸气压曲线类型,将其分为上凸型、下凹型和直线型。针对这几种蒸气压曲线进一步选取[EMIM][OTf]、[EMIM][Ac]和NaCl水溶液作为代表溶液,在不同辐照强度和浓度下进行了实验研究,并与纯水的蒸发作对比。实验结果表明:低浓度下[EMIM][OTf]水溶液展现出了良好的蒸发性能, 主要原因是由于其蒸气压处于上凸区间;当溶液浓度升高或辐照强度提升时,[EMIM][OTf]溶液的蒸发速率提升较NaCl水溶液小,主要原因在于蒸发过程的浓度极化导致气液界面处的[EMIM][OTf]浓度升高,蒸气压相差较小;不同工况下[EMIM][Ac]水溶液的蒸发速率均较慢,纯水的蒸发速率最快,体现了蒸气压对蒸发性能的关键影响,原因是低蒸气压导致高蒸发温度,并带来更多的能量损失。
中图分类号:
王洁冰, 高金彤, 徐震原. 基于不同类型溶液蒸气压特性的太阳能界面蒸发实验研究[J]. 化工学报, 2022, 73(2): 663-671.
Jiebing WANG, Jintong GAO, Zhenyuan XU. Experimental study on solar interfacial evaporation based on vapor pressure characteristics of different solutions[J]. CIESC Journal, 2022, 73(2): 663-671.
| 测量类型 | 不确定度 |
|---|---|
| 温度(UNC,K型热电偶)/K | 0.2 |
温度(DAQ)/K 质量(UNC,分析天平)/g 质量(DAQ)/g 长度(UNC,游标卡尺)/mm 光照强度(UNC,辐照仪)/(W/m2) | 0 0.02 0 0.02 0.1% |
表1 仪器 (UNC) 和数据采集(DAQ)的测量不确定度
Table 1 Measurement uncertainty from sensor (UNC) and data acquisition (DAQ)
| 测量类型 | 不确定度 |
|---|---|
| 温度(UNC,K型热电偶)/K | 0.2 |
温度(DAQ)/K 质量(UNC,分析天平)/g 质量(DAQ)/g 长度(UNC,游标卡尺)/mm 光照强度(UNC,辐照仪)/(W/m2) | 0 0.02 0 0.02 0.1% |
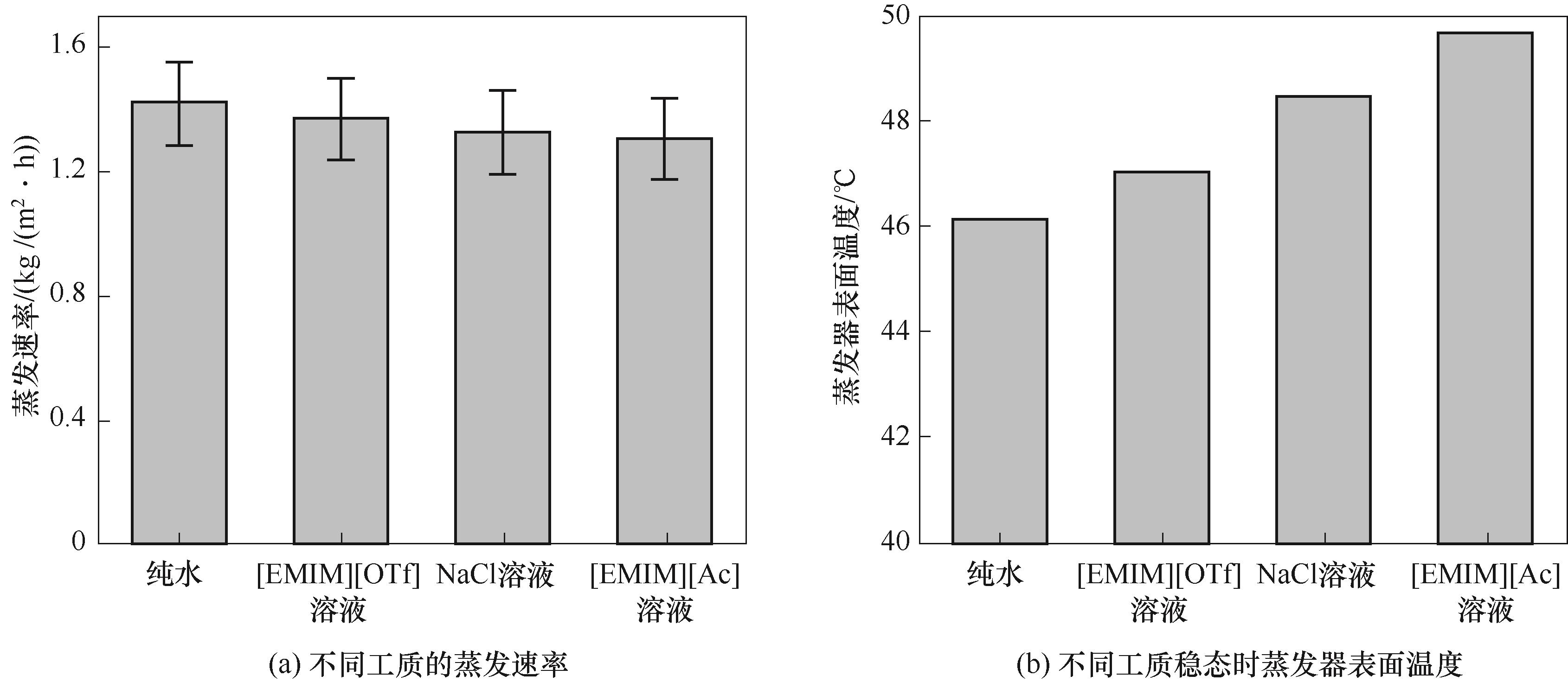
图6 1个光照强度下质量分数为5%的不同工质蒸发速率和蒸发器表面温度
Fig.6 The evaporation rate and temperature of different working fluids with a mass fraction of 5% under a sunlight intensity
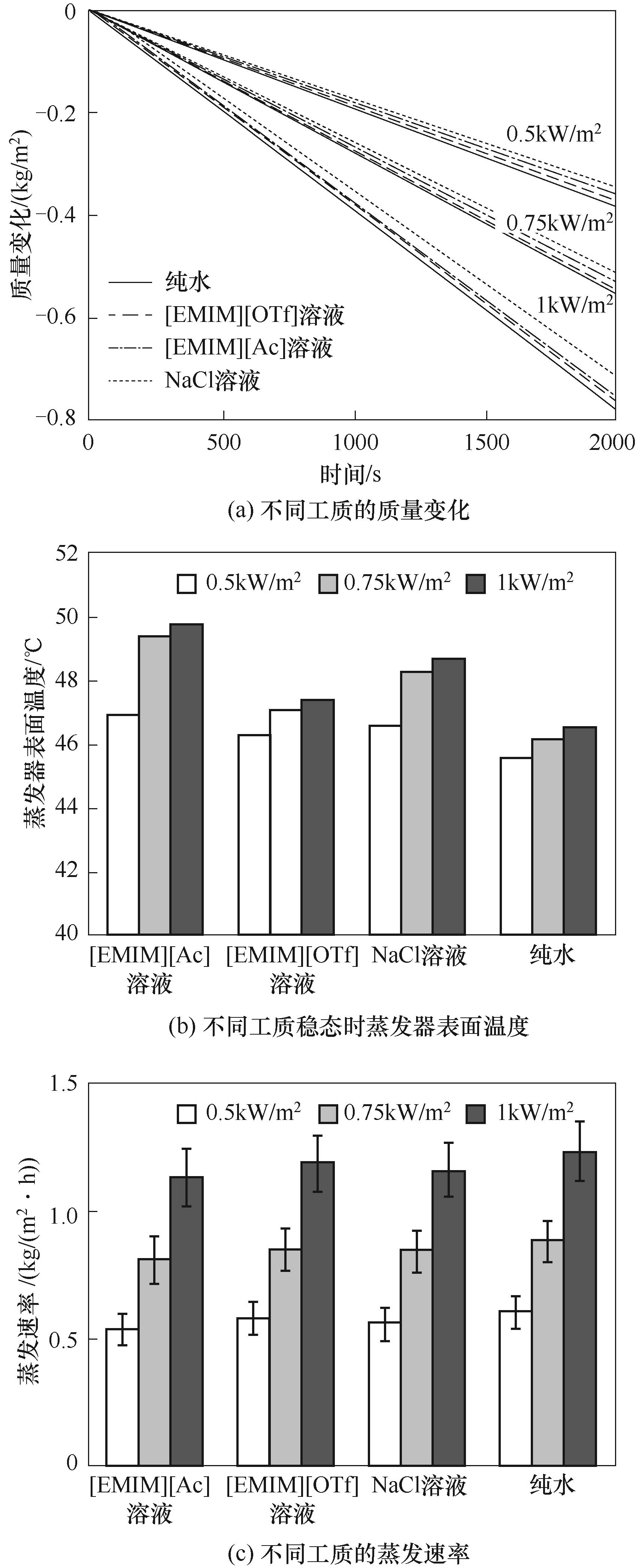
图7 不同光照强度下质量分数为5%的不同工质的质量变化、蒸发器表面温度和蒸发速率
Fig.7 The mass change, temperature of the evaporator surface and evaporation rate of different working fluids with a mass fraction of 5% under different sunlight intensities
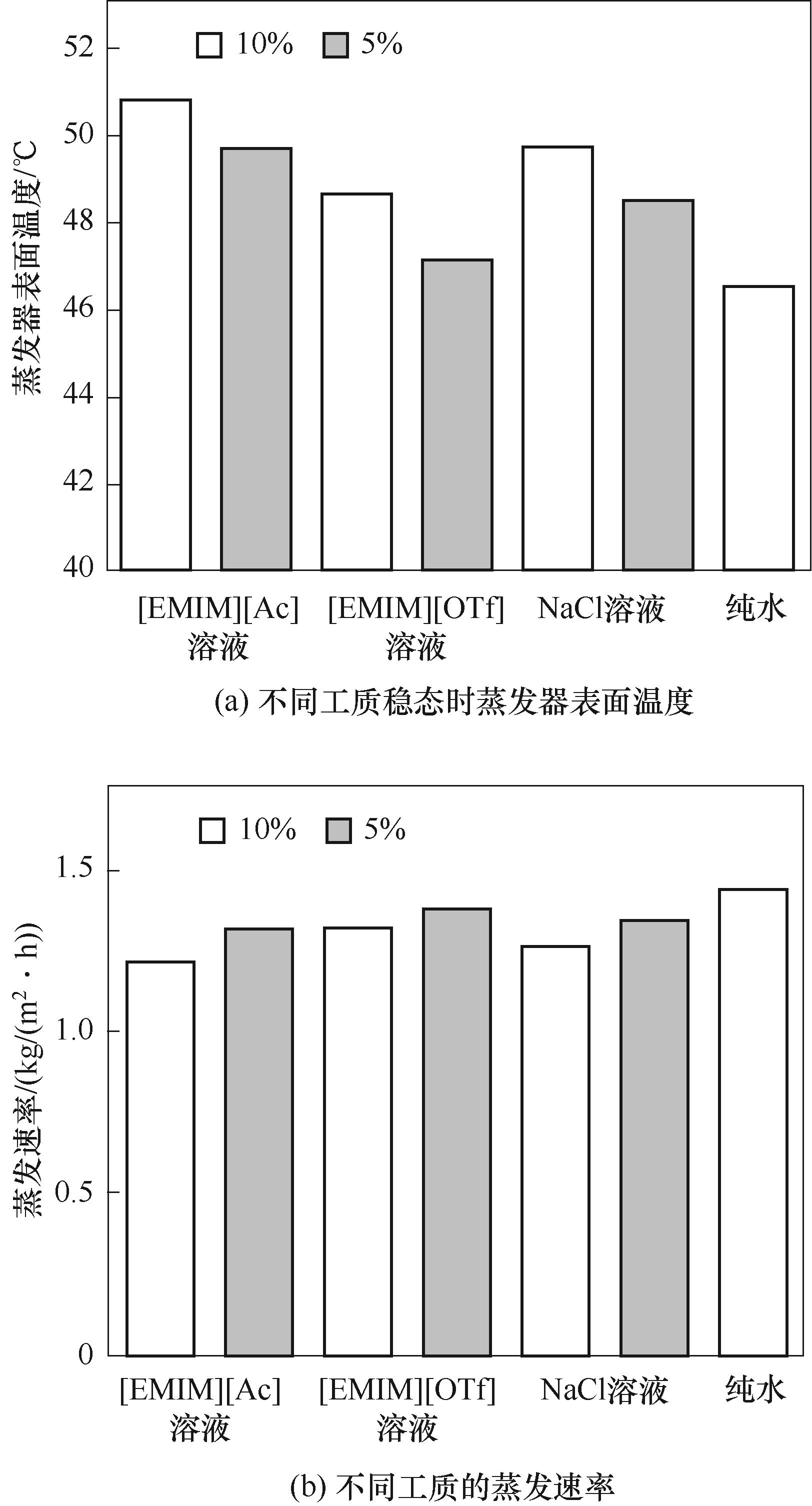
图8 1个光照强度下质量分数为5%和10%的不同工质的蒸发器表面温度与蒸发速率
Fig.8 The temperature of the evaporator surface and evaporation rate of different working fluids with a mass fraction of 5% and 10% under one sunlight intensity

图9 质量分数为3.5%的NaCl溶液和[EMIM][OTf]溶液长时间蒸发的质量变化、蒸发器表面温度变化、蒸发速率变化和3.5 h后蒸发器表面图
Fig.9 The mass change, the change of evaporator surface temperature, the change of evaporation rate, of 3.5% NaCl solution and [EMIM][OTf] solution for long-term evaporation, and the surface map of the evaporator after 3.5 h
| 1 | Wang Z H, Liu Y M, Tao P, et al. Bio-inspired evaporation through plasmonic film of nanoparticles at the air-water interface[J]. Small, 2014, 10(16): 3234-3239. |
| 2 | Ghasemi H, Ni G, Marconnet A M, et al. Solar steam generation by heat localization[J]. Nature Communications, 2014, 5: 4449. |
| 3 | Chang C, Tao P, Fu B W, et al. Three-dimensional porous solar-driven interfacial evaporator for high-efficiency steam generation under low solar flux[J]. ACS Omega, 2019, 4(2): 3546-3555. |
| 4 | Chang C, Tao P, Xu J, et al. High-efficiency superheated steam generation for portable sterilization under ambient pressure and low solar flux[J]. ACS Applied Materials & Interfaces, 2019, 11(20): 18466-18474. |
| 5 | Liu Z P, Yang Z J, Huang X C, et al. High-absorption recyclable photothermal membranes used in a bionic system for high-efficiency solar desalination via enhanced localized heating[J]. Journal of Materials Chemistry A, 2017, 5(37): 20044-20052. |
| 6 | Wang Z, Horseman T, Straub A P, et al. Pathways and challenges for efficient solar-thermal desalination[J]. Science Advances, 2019, 5(7): eaax0763. |
| 7 | Lv B, Gao C, Xu Y L, et al. A self-floating, salt-resistant 3D Janus radish-based evaporator for highly efficient solar desalination[J]. Desalination, 2021, 510: 115093. |
| 8 | Cheng G, Wang X Z, Liu X, et al. Enhanced interfacial solar steam generation with composite reduced graphene oxide membrane[J]. Solar Energy, 2019, 194: 415-430. |
| 9 | Ni G, Li G, Boriskina S V, et al. Steam generation under one sun enabled by a floating structure with thermal concentration[J]. Nature Energy, 2016, 1: 16126. |
| 10 | Ito Y, Tanabe Y, Han J H, et al. Multifunctional porous graphene for high-efficiency steam generation by heat localization[J]. Advanced Materials, 2015, 27(29): 4302-4307. |
| 11 | Chen C L, Zhou L, Yu J Y, et al. Dual functional asymmetric plasmonic structures for solar water purification and pollution detection[J]. Nano Energy, 2018, 51: 451-456. |
| 12 | Feng R, Qiao Y M, Song C Y. A perspective on bio-inspired interfacial systems for solar clean-water generation[J]. MRS Communications, 2019, 9(1): 3-13. |
| 13 | Li X Q, Li J L, Lu J Y, et al. Enhancement of interfacial solar vapor generation by environmental energy[J]. Joule, 2018, 2(7): 1331-1338. |
| 14 | Tao P, Ni G, Song C Y, et al. Solar-driven interfacial evaporation[J]. Nature Energy, 2018, 3(12): 1031-1041. |
| 15 | Bai H Y, Zhao T H, Cao M Y. Interfacial solar evaporation for water production: from structure design to reliable performance[J]. Molecular Systems Design & Engineering, 2020, 5(2): 419-432. |
| 16 | Li H R, Yan Z, Li Y, et al. Latest development in salt removal from solar-driven interfacial saline water evaporators: advanced strategies and challenges[J]. Water Research, 2020, 177: 115770. |
| 17 | Zhang Y X, Xiong T, Nandakumar D K, et al. Structure architecting for salt-rejecting solar interfacial desalination to achieve high-performance evaporation with in situ energy generation[J]. Advanced Science, 2020, 7(9): 1903478. |
| 18 | Shan X, Lin Y, Zhao A, et al. Porous reduced graphene oxide/nickel foam for highly efficient solar steam generation[J]. Nanotechnology, 2019, 30(42): 425403. |
| 19 | Shi Y, Li R Y, Jin Y, et al. A 3D photothermal structure toward improved energy efficiency in solar steam generation[J]. Joule, 2018, 2(6): 1171-1186. |
| 20 | Ni G, Zandavi S H, Javid S M, et al. A salt-rejecting floating solar still for low-cost desalination[J]. Energy & Environmental Science, 2018, 11(6): 1510-1519. |
| 21 | Xia Y, Hou Q F, Jubaer H, et al. Spatially isolating salt crystallisation from water evaporation for continuous solar steam generation and salt harvesting[J]. Energy & Environmental Science, 2019, 12(6): 1840-1847. |
| 22 | Wu L, Dong Z C, Cai Z R, et al. Highly efficient three-dimensional solar evaporator for high salinity desalination by localized crystallization[J]. Nature Communications, 2020, 11: 521. |
| 23 | Cooper T A, Zandavi S H, Ni G W, et al. Contactless steam generation and superheating under one sun illumination[J]. Nature Communications, 2018, 9: 5086. |
| 24 | Hou Q, Zhou H Y, Zhang W, et al. Boosting adsorption of heavy metal ions in wastewater through solar-driven interfacial evaporation of chemically-treated carbonized wood[J]. Science of the Total Environment, 2021, 759: 144317. |
| 25 | Bülow M, Greive M, Zaitsau D H, et al. Extremely low vapor-pressure data as access to PC-SAFT parameter estimation for ionic liquids and modeling of precursor solubility in ionic liquids[J]. ChemistryOpen, 2021, 10(2): 216-226. |
| 26 | 张润楠, 范晓晨, 贺明睿, 等. 煤气化废水深度处理与回用研究进展[J]. 化工学报, 2015, 66(9): 3341-3349. |
| Zhang R N, Fan X C, He M R, et al. Research progress on deep treatment and reclamation of coal gasification wastewater[J]. CIESC Journal, 2015, 66(9): 3341-3349. | |
| 27 | 张兰河, 万洒, 陈子成, 等. 高分子絮凝剂处理高浓度化妆品原料生产废水研究[J]. 化工学报, 2020, 71(8): 3730-3740. |
| Zhang L H, Wan S, Chen Z C, et al. Treatment of high-strength wastewater generated in cosmetics raw materials production using polymer flocculants[J]. CIESC Journal, 2020, 71(8): 3730-3740. | |
| 28 | Yu K F, Shao P F, Meng P W, et al. Superhydrophilic and highly elastic monolithic sponge for efficient solar-driven radioactive wastewater treatment under one sun[J]. Journal of Hazardous Materials, 2020, 392: 122350. |
| 29 | 马文清. 基于热分解稳定性的咪唑类离子液体热力学性质研究[D]. 包头: 内蒙古科技大学, 2020. |
| Ma W Q. Thermodynamic properties of imidazole ionic liquids on the basis of thermal decomposition stability[D]. Baotou: Inner Mongolia University of Science & Technology, 2020. | |
| 30 | Huang S, Wang Z H, Liu S L, et al. Measurement and prediction of vapor pressure in binary systems containing the ionic liquid [EMIM][DCA[J]. Journal of Molecular Liquids, 2020, 309: 113126. |
| 31 | Kline S, McClintock F. Describing uncertainties in single-sample experiments[J]. Mechanical Engineering, 1953, 75: 3-8. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 常明慧, 王林, 苑佳佳, 曹艺飞. 盐溶液蓄能型热泵循环特性研究[J]. 化工学报, 2023, 74(S1): 329-337. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 张化福, 童莉葛, 张振涛, 杨俊玲, 王立, 张俊浩. 机械蒸汽压缩蒸发技术研究现状与发展趋势[J]. 化工学报, 2023, 74(S1): 8-24. |
| [5] | 吴馨, 龚建英, 靳龙, 王宇涛, 黄睿宁. 超声波激励下铝板表面液滴群输运特性的研究[J]. 化工学报, 2023, 74(S1): 104-112. |
| [6] | 叶展羽, 山訸, 徐震原. 用于太阳能蒸发的折纸式蒸发器性能仿真[J]. 化工学报, 2023, 74(S1): 132-140. |
| [7] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [8] | 陈爱强, 代艳奇, 刘悦, 刘斌, 吴翰铭. 基板温度对HFE7100液滴蒸发过程的影响研究[J]. 化工学报, 2023, 74(S1): 191-197. |
| [9] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [10] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| [11] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [12] | 程小松, 殷勇高, 车春文. 不同工质在溶液除湿真空再生系统中的性能对比[J]. 化工学报, 2023, 74(8): 3494-3501. |
| [13] | 汪尔奇, 彭书舟, 杨震, 段远源. 含HFO混合体系气液相平衡的理论模型评价[J]. 化工学报, 2023, 74(8): 3216-3225. |
| [14] | 张媛媛, 曲江源, 苏欣欣, 杨静, 张锴. 循环流化床燃煤机组SNCR脱硝过程气液传质和反应特性[J]. 化工学报, 2023, 74(6): 2404-2415. |
| [15] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号