化工学报 ›› 2022, Vol. 73 ›› Issue (8): 3501-3510.DOI: 10.11949/0438-1157.20220477
收稿日期:2022-04-06
修回日期:2022-05-24
出版日期:2022-08-05
发布日期:2022-09-06
通讯作者:
苏海萍
作者简介:黄凯(1995—),男,博士研究生,y12203017@mail.ecust.edu.cn
基金资助:
Kai HUANG( ), Sijie WANG, Haiping SU(
), Sijie WANG, Haiping SU( ), Cheng LIAN, Honglai LIU
), Cheng LIAN, Honglai LIU
Received:2022-04-06
Revised:2022-05-24
Online:2022-08-05
Published:2022-09-06
Contact:
Haiping SU
摘要:
抑制锂枝晶生长是锂金属电池中亟需解决的关键问题之一。电极表面涂覆石墨烯可以有效抑制锂枝晶生长。然而,目前对石墨烯层间距影响锂枝晶生长的机制尚不明晰。采用第一性原理计算方法从吸附和扩散两个角度考察了石墨烯层间距对锂枝晶生长的影响。结果表明,石墨烯的层间距为0.45 ~ 0.55 nm时,电极表面对锂原子的吸附较弱,锂原子扩散性能最好,有利于抑制锂枝晶的生长。若小于该层间距,锂原子在层间的扩散较难。反之,锂原子则会在石墨烯层上吸附聚集,导致锂枝晶的快速生长。此外,在最佳层间距下,B掺杂和N掺杂改性的石墨烯,能促进锂原子在石墨烯层间的扩散,避免锂的不均匀沉积,从而抑制锂枝晶的形成。
中图分类号:
黄凯, 王思洁, 苏海萍, 练成, 刘洪来. 石墨烯层间距调控抑制锂枝晶生长的第一性原理研究[J]. 化工学报, 2022, 73(8): 3501-3510.
Kai HUANG, Sijie WANG, Haiping SU, Cheng LIAN, Honglai LIU. First principle study on inhibition of lithium dendrites growth by regulating graphene layer spacings[J]. CIESC Journal, 2022, 73(8): 3501-3510.

图1 计算构型示意图(图中灰色、粉色、蓝色、紫色和绿色的球分别代表C、B、N、P和Cl原子)
Fig. 1 The schematic diagram of calculation configurations (the gray, pink, blue, purple and green ball represent C, B, N, P and Cl atom, respectively)
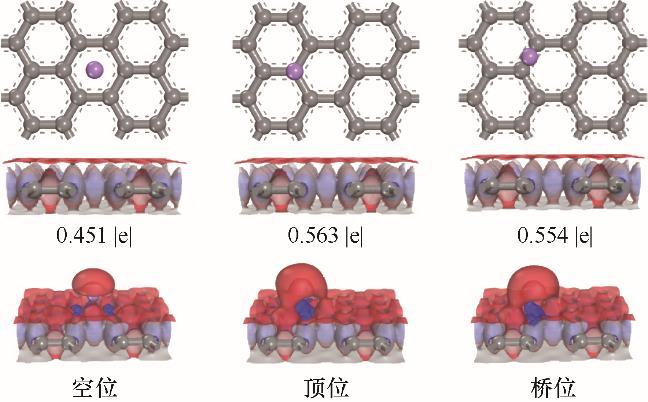
图2 锂原子在层间距为0.75 nm的石墨烯不同吸附位点的结合能、差分电荷密度和Hirshfeld电荷(红色和蓝色的区域分别代表电子云密度的减少和增加,等值面的值为± 0.02 e/Å3)
Fig. 2 The configuration, difference charge density and Hirshfeld charge of lithium atom adsorbed on different sites of graphene with d = 0.75 nm (red and blue isosurface denote the decrease and increase of electron density, respectively, and the value is ± 0.02 e/Å3)
| 层间距/nm | 顶位结合能/eV | 桥位结合能/eV | 空位结合能/eV |
|---|---|---|---|
| 0.35 | -0.90 | -1.10 | -1.90 |
| 0.45 | -1.63 | -1.64 | -1.68 |
| 0.55 | -1.15 | -1.17 | -1.32 |
| 0.65 | -0.92 | -0.97 | -1.24 |
| 0.75 | -0.86 | -0.86 | -1.19 |
表1 锂原子在不同层间距石墨烯上不同吸附位点的结合能
Table 1 The binding energy of Li atom at different sites on graphene with different layer spacings
| 层间距/nm | 顶位结合能/eV | 桥位结合能/eV | 空位结合能/eV |
|---|---|---|---|
| 0.35 | -0.90 | -1.10 | -1.90 |
| 0.45 | -1.63 | -1.64 | -1.68 |
| 0.55 | -1.15 | -1.17 | -1.32 |
| 0.65 | -0.92 | -0.97 | -1.24 |
| 0.75 | -0.86 | -0.86 | -1.19 |
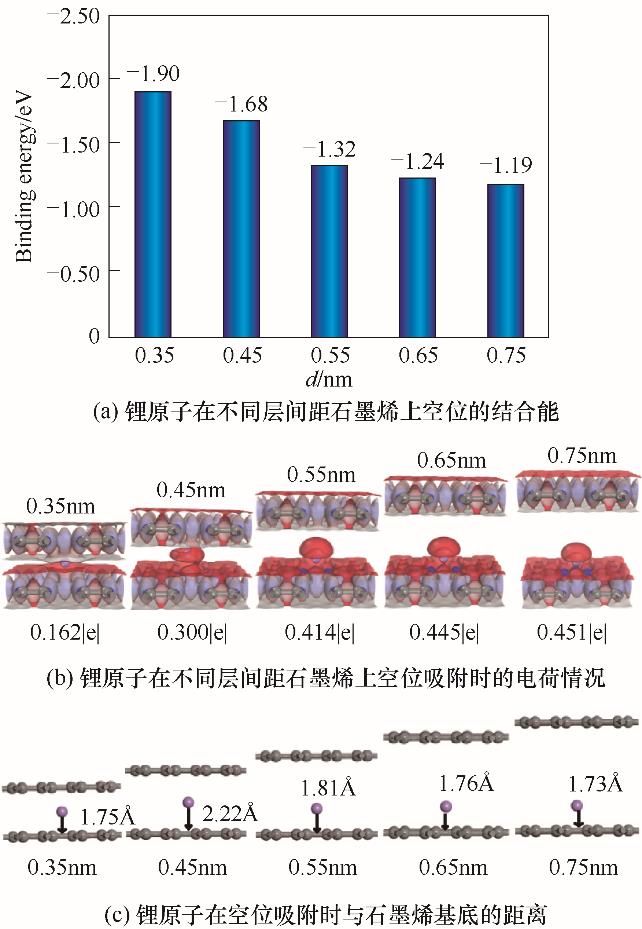
图3 锂原子在不同层间距石墨烯上的空位结合能、电荷密度和吸附状态(红色和蓝色的区域分别代表电子云密度的减少和增加,等值面的值为± 0.02 e/Å3)
Fig. 3 The binding energy, charge density and adsorption state of lithium atom on graphene hollow site with different layer spacings (red and blue isosurface denote the decrease and increase of electron density, respectively, and the value is ± 0.02 e/Å3)
| 掺杂原子 | 扩散活化能/eV | |||||
|---|---|---|---|---|---|---|
| 5.3% | 2.9% | 1.1% | ||||
| 路径1 | 路径3 | 路径1 | 路径3 | 路径1 | 路径3 | |
| B | 0.126 | -0.040 | 0.078 | -0.073 | 0.090 | -0.054 |
| N | -0.091 | 0.197 | -0.069 | 0.053 | -0.012 | 0.224 |
| P | -0.118 | 0.639 | -0.192 | 0.529 | -0.077 | 0.712 |
| Cl | -0.113 | 0.553 | -0.040 | 0.698 | -0.034 | 0.691 |
表2 锂原子在不同掺杂量的掺杂石墨烯上路径1和路径3的扩散活化能
Table 2 Diffusion activation energy of lithium atom on doped-graphene (path1 and path3) with different doping amount
| 掺杂原子 | 扩散活化能/eV | |||||
|---|---|---|---|---|---|---|
| 5.3% | 2.9% | 1.1% | ||||
| 路径1 | 路径3 | 路径1 | 路径3 | 路径1 | 路径3 | |
| B | 0.126 | -0.040 | 0.078 | -0.073 | 0.090 | -0.054 |
| N | -0.091 | 0.197 | -0.069 | 0.053 | -0.012 | 0.224 |
| P | -0.118 | 0.639 | -0.192 | 0.529 | -0.077 | 0.712 |
| Cl | -0.113 | 0.553 | -0.040 | 0.698 | -0.034 | 0.691 |
| 1 | Xu W, Wang J L, Ding F, et al. Lithium metal anodes for rechargeable batteries[J]. Energy & Environmental Science, 2014, 7(2): 513-537. |
| 2 | Fan X L, Chen L, Borodin O, et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries[J]. Nature Nanotechnology, 2018, 13(8): 715-722. |
| 3 | 张睿, 沈馨, 王金福, 等. 锂离子在三维骨架复合锂金属负极中的沉积规律[J]. 化工学报, 2020, 71(6): 2688-2695. |
| Zhang R, Shen X, Wang J F, et al. Plating of Li ions in 3D structured lithium metal anodes[J]. CIESC Journal, 2020, 71(6): 2688-2695. | |
| 4 | Tan S J, Yue J P, Hu X C, et al. Nitriding-interface-regulated lithium plating enables flame-retardant electrolytes for high-voltage lithium metal batteries[J]. Angewandte Chemie International Edition, 2019, 58(23): 7802-7807. |
| 5 | Cheng X B, Yan C, Huang J Q, et al. The gap between long lifespan Li-S coin and pouch cells: the importance of lithium metal anode protection[J]. Energy Storage Materials, 2017, 6: 18-25. |
| 6 | Wu F, Yuan Y X, Cheng X B, et al. Perspectives for restraining harsh lithium dendrite growth: towards robust lithium metal anodes[J]. Energy Storage Materials, 2018, 15: 148-170. |
| 7 | 丰闪闪, 刘晓斌, 郭石麟, 等. 锂枝晶的成核、生长与抑制[J]. 化工学报, 2022, 73(1): 97-109. |
| Feng S S, Liu X B, Guo S L, et al. Nucleation, growth and inhibition of lithium dendrites[J]. CIESC Journal, 2022, 73(1): 97-109. | |
| 8 | Kang H K, Woo S G, Kim J H, et al. Few-layer graphene island seeding for dendrite-free Li metal electrodes[J]. ACS Applied Materials & Interfaces, 2016, 8(40): 26895-26901. |
| 9 | An Y L, Tian Y, Wei C L, et al. Scalable and physical synthesis of 2D silicon from bulk layered alloy for lithium-ion batteries and lithium metal batteries[J]. ACS Nano, 2019, 13(12): 13690-13701. |
| 10 | Chen X, Shang M W, Niu J J. Inter-layer-calated thin Li metal electrode with improved battery capacity retention and dendrite suppression[J]. Nano Letters, 2020, 20(4): 2639-2646. |
| 11 | Cha E, Patel M D, Park J, et al. 2D MoS2 as an efficient protective layer for lithium metal anodes in high-performance Li-S batteries[J]. Nature Nanotechnology, 2018, 13(4): 337-344. |
| 12 | Nie X, Zhang A Y, Liu Y H, et al. Synthesis of interconnected graphene framework with two-dimensional protective layers for stable lithium metal anodes[J]. Energy Storage Materials, 2019, 17: 341-348. |
| 13 | Kim J S, Kim D W, Jung H T, et al. Controlled lithium dendrite growth by a synergistic effect of multilayered graphene coating and an electrolyte additive[J]. Chemistry of Materials, 2015, 27(8): 2780-2787. |
| 14 | Huang G, Han J H, Zhang F, et al. Lithiophilic 3D nanoporous nitrogen-doped graphene for dendrite-free and ultrahigh-rate lithium-metal anodes[J]. Advanced Materials, 2019, 31(2): e1805334. |
| 15 | Li Z H, Li X L, Zhou L, et al. A collaborative strategy for stable lithium metal anodes by using three-dimensional nitrogen-doped graphene foams[J]. Nanoscale, 2018, 10(10): 4675-4679. |
| 16 | Lin D C, Liu Y Y, Liang Z, et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes[J]. Nature Nanotechnology, 2016, 11(7): 626-632. |
| 17 | Zhang R, Chen X R, Chen X, et al. Lithiophilic sites in doped graphene guide uniform lithium nucleation for dendrite-free lithium metal anodes[J]. Angewandte Chemie International Edition, 2017, 56(27): 7764-7768. |
| 18 | Tang Y H, Wang X, Chen J J, et al. PVP-assisted synthesis of g-C3N4-derived N-doped graphene with tunable interplanar spacing as high-performance lithium/sodium ions battery anodes[J]. Carbon, 2021, 174: 98-109. |
| 19 | Shi H D, Qin J Q, Huang K, et al. A two-dimensional mesoporous polypyrrole-graphene oxide heterostructure as a dual-functional ion redistributor for dendrite-free lithium metal anodes[J]. Angewandte Chemie, 2020, 132(29): 12245-12251. |
| 20 | Leggesse E G, Chen C L, Jiang J C. Lithium diffusion in graphene and graphite: effect of edge morphology[J]. Carbon, 2016, 103: 209-216. |
| 21 | Wasalathilake K C, Ayoko G A, Yan C. Effects of heteroatom doping on the performance of graphene in sodium-ion batteries: a density functional theory investigation[J]. Carbon, 2018, 140: 276-285. |
| 22 | Chen S Q, Zheng F F, Feng J, et al. Theoretical study on single-side fluorinated graphene for lithium storage[J]. Applied Surface Science, 2021, 560: 150033. |
| 23 | Wen Y, He K, Zhu Y J, et al. Expanded graphite as superior anode for sodium-ion batteries[J]. Nature Communications, 2014, 5: 4033. |
| 24 | Chen L, Shi G S, Shen J, et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing[J]. Nature, 2017, 550(7676): 380-383. |
| 25 | Shen J, Liu G P, Han Y, et al. Artificial channels for confined mass transport at the sub-nanometre scale[J]. Nature Reviews Materials, 2021, 6(4): 294-312. |
| 26 | Vishnugopi B S, Hao F, Verma A, et al. Double-edged effect of temperature on lithium dendrites[J]. ACS Applied Materials & Interfaces, 2020, 12(21): 23931-23938. |
| 27 | Mayers M Z, Kaminski J W, Miller T F III. Suppression of dendrite formation via pulse charging in rechargeable lithium metal batteries[J]. The Journal of Physical Chemistry C, 2012, 116(50): 26214-26221. |
| 28 | Liu W, Lin D C, Pei A, et al. Stabilizing lithium metal anodes by uniform Li-ion flux distribution in nanochannel confinement[J]. Journal of the American Chemical Society, 2016, 138(47): 15443-15450. |
| 29 | Huang K, Liu Y, Liu H L. Understanding and predicting lithium crystal growth on perfect and defective interfaces: a Kohn-Sham density functional study[J]. ACS Applied Materials & Interfaces, 2019, 11(40): 37239-37246. |
| 30 | Delley B. An all-electron numerical method for solving the local density functional for polyatomic molecules[J]. The Journal of Chemical Physics, 1990, 92(1): 508-517. |
| 31 | Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868. |
| 32 | Perdew J P, Chevary J A, Vosko S H, et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation[J]. Physical Review B: Condensed Matter, 1992, 46(11): 6671-6687. |
| 33 | Hammer B, Hansen L B, Nørskov J K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals[J]. Physical Review B, 1999, 59(11): 7413-7421. |
| 34 | Olsson E, Chai G L, Dove M, et al. Adsorption and migration of alkali metals (Li, Na, and K) on pristine and defective graphene surfaces[J]. Nanoscale, 2019, 11(12): 5274-5284. |
| 35 | Chen Y X, Dou X Y, Wang K, et al. Lithium dendrites inhibition via diffusion enhancement[J]. Advanced Energy Materials, 2019, 9(17): 1900019. |
| 36 | Li K, Hu Z Y, Ma J Z, et al. A 3D and stable lithium anode for high-performance lithium-iodine batteries[J]. Advanced Materials, 2019, 31(33): 1902399. |
| 37 | Zhou L J, Hou Z F, Gao B, et al. Doped graphenes as anodes with large capacity for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2016, 4(35): 13407-13413. |
| 38 | Liu W, Zhai P B, Qin S J, et al. Boron-doping induced lithophilic transition of graphene for dendrite-free lithium growth[J]. Journal of Energy Chemistry, 2021, 56: 463-469. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [7] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [8] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [9] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [10] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [11] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [12] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [13] | 顾浩, 张福建, 刘珍, 周文轩, 张鹏, 张忠强. 力电耦合作用下多孔石墨烯膜时间维度的脱盐性能及机理研究[J]. 化工学报, 2023, 74(5): 2067-2074. |
| [14] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [15] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号