化工学报 ›› 2022, Vol. 73 ›› Issue (11): 4826-4837.DOI: 10.11949/0438-1157.20221029
收稿日期:2022-07-26
修回日期:2022-09-21
出版日期:2022-11-05
发布日期:2022-12-06
通讯作者:
陈光进
作者简介:朱晨阳(1992—),男,博士,讲师,zcyzhu@cup.edu.cn
基金资助:
Chenyang ZHU1( ), Xiangyang LIU2, Maogang HE2, Guangjin CHEN3(
), Xiangyang LIU2, Maogang HE2, Guangjin CHEN3( )
)
Received:2022-07-26
Revised:2022-09-21
Online:2022-11-05
Published:2022-12-06
Contact:
Guangjin CHEN
摘要:
为了建立起一个统一的、可同时应用于纯质与混合物高压黏度计算的绝对速率理论模型,基于此前已有的纯质黏度模型,提出了相应的混合法则,将其扩展至对混合流体的计算当中。为验证模型精度,首先选取了27种纯质流体,通过拟合其黏度数据得到模型参数。随后,对27种二元混合物以及3种三元混合物的黏度进行了计算。其中,对于二元混合物,仍需要引入额外的二元交互参数,可通过拟合混合体系黏度数据得到。结果表明,该黏度模型对所选纯质、二元混合物和三元混合物的黏度均有着较高的计算精度,其相对偏差的平均绝对值分别为1.54%、2.35%和3.86%,最大偏差为8.62%、12.9%和19.7%。最后,将本文模型与自由体积模型进行了比较,其对高黏度和互缔合流体的计算精度均高于后者。
中图分类号:
朱晨阳, 刘向阳, 何茂刚, 陈光进. 基于Eyring绝对速率理论的流体混合物黏度推算[J]. 化工学报, 2022, 73(11): 4826-4837.
Chenyang ZHU, Xiangyang LIU, Maogang HE, Guangjin CHEN. Viscosity estimation of fluid mixtures based on Eyring's absolute rate theory[J]. CIESC Journal, 2022, 73(11): 4826-4837.
| 作者 | 流动活化能① | 温度/K | 压力/MPa | 物质 | 文献 |
|---|---|---|---|---|---|
| Kincaid等 | ΔG*=ΔUvap/2.45 | 273~353 | 饱和压力 | 纯质 | [ |
| Lei等 | ΔG*=αΔUvap | 77~653 | 饱和压力 | 纯质 | [ |
| Macías-Salinas等 | (ΔG*/RT)=α(ΔUvap/RT) β | 20~623 | 0.1~100 | 纯质 | [ |
| ΔG*E=αGE | 275~350 | 0.1~65 | 二元混合物 | [ | |
| Martins等 | ΔG*-ΔG0*=A-A0 | 65~460 | 0.1~253 | 纯质 | [ |
| ΔG*E=GE | 283~328 | 0.1 | 二元混合物 | [ | |
| Liu等 | ΔG*-ΔG0*=A-A0 | 65~460 | 0.1~253 | 纯质 | [ |
| Han等 | ΔG*=0.175NAε | 283~353 | 饱和压力 | 纯质 | [ |
| Tochigi等 | ΔG*E=αGE | 298~370 | 0.1~100 | 二元混合物 | [ |
| Wang等 | ΔG*E=GE | 298~343 | 0.1 | 二元混合物 | [ |
| He等 | ΔG*E=GE | 298~343 | 0.1 | 二元混合物 | [ |
| Atashrouz等 | ΔG*E=GE | 278~353 | 0.1 | 二元混合物 | [ |
| Xu等 | ΔG*E=GE | 298~343 | 0.1 | 二、三元混合物 | [ |
表1 绝对速率理论模型计算流体黏度研究汇总
Table 1 Summary of absolute rate theory models for the viscosities of fluids
| 作者 | 流动活化能① | 温度/K | 压力/MPa | 物质 | 文献 |
|---|---|---|---|---|---|
| Kincaid等 | ΔG*=ΔUvap/2.45 | 273~353 | 饱和压力 | 纯质 | [ |
| Lei等 | ΔG*=αΔUvap | 77~653 | 饱和压力 | 纯质 | [ |
| Macías-Salinas等 | (ΔG*/RT)=α(ΔUvap/RT) β | 20~623 | 0.1~100 | 纯质 | [ |
| ΔG*E=αGE | 275~350 | 0.1~65 | 二元混合物 | [ | |
| Martins等 | ΔG*-ΔG0*=A-A0 | 65~460 | 0.1~253 | 纯质 | [ |
| ΔG*E=GE | 283~328 | 0.1 | 二元混合物 | [ | |
| Liu等 | ΔG*-ΔG0*=A-A0 | 65~460 | 0.1~253 | 纯质 | [ |
| Han等 | ΔG*=0.175NAε | 283~353 | 饱和压力 | 纯质 | [ |
| Tochigi等 | ΔG*E=αGE | 298~370 | 0.1~100 | 二元混合物 | [ |
| Wang等 | ΔG*E=GE | 298~343 | 0.1 | 二元混合物 | [ |
| He等 | ΔG*E=GE | 298~343 | 0.1 | 二元混合物 | [ |
| Atashrouz等 | ΔG*E=GE | 278~353 | 0.1 | 二元混合物 | [ |
| Xu等 | ΔG*E=GE | 298~343 | 0.1 | 二、三元混合物 | [ |
| 物质 | T/K | p/MPa | 参数 | AAD/% | MD/% | 数据点数 | 文献 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a1 | 102a2 | b | c | d | |||||||
| 正戊烷 | 298~373 | 0.1~25 | 0.2455 | -5.1312 | 9.3775 | 5.1283 | -1.9272 | 1.07 | 2.99 | 31 | [ |
| 正己烷 | 273~373 | 0.1~205 | 0.1420 | -1.0610 | 2.1592 | 2.7046 | 0.1682 | 2.28 | 5.10 | 38 | [ |
| 正庚烷 | 298~474 | 0.1~100 | 0.1497 | -1.0627 | -0.5700 | 7.8378 | 0.6419 | 1.30 | 3.23 | 33 | [ |
| 正辛烷 | 273~467 | 0.1~203 | 0.1163 | -0.4310 | 1.1151 | 4.1198 | 0.2715 | 2.61 | 7.26 | 104 | [ |
| 正壬烷 | 300~420 | 0.1~50 | 0.1431 | -0.8425 | 2.6574 | 1.9450 | -0.0004 | 0.44 | 3.04 | 220 | [ |
| 正癸烷 | 293~373 | 0.1~100 | 0.1278 | -0.7410 | 3.4988 | 1.1772 | -0.1271 | 1.93 | 5.19 | 66 | [ |
| 正十二烷 | 293~373 | 0.1~200 | 0.0774 | 0.1410 | 3.7887 | 1.0225 | -0.1582 | 1.97 | 8.62 | 112 | [ |
| 正十三烷 | 293~353 | 0.1~100 | 0.0728 | 0.1678 | 2.8687 | 1.8857 | -0.0281 | 2.12 | 6.76 | 42 | [ |
| 正十四烷 | 313~393 | 0.7~60 | 0.1041 | -0.7250 | 2.7749 | 1.7020 | -0.0872 | 1.84 | 4.44 | 40 | [ |
| 正十六烷 | 298~413 | 0.1~202 | 0.0534 | 0.2920 | 3.6739 | 1.1963 | -0.1388 | 2.30 | 7.94 | 117 | [ |
| 环己烷 | 298~413 | 0.1~100 | 0.2955 | -2.2960 | 2.2599 | 2.1360 | -0.0745 | 2.60 | 7.01 | 120 | [ |
| 2,2,4-三甲基戊烷 | 293~353 | 0.1~140 | 0.1673 | -1.2950 | 3.1482 | 1.4400 | -0.0796 | 2.10 | 6.11 | 98 | [ |
| 七甲基壬烷 | 293~353 | 0.1~100 | 0.0786 | 0.8681 | 4.3655 | 0.8666 | -0.2015 | 0.96 | 2.89 | 42 | [ |
| 甲基环己烷 | 293~353 | 0.1~100 | 0.2426 | -2.0455 | -0.0096 | 5.9618 | 0.3825 | 1.17 | 3.20 | 42 | [ |
| 苯 | 298~393 | 0.1~178 | 0.1615 | 0.8100 | 3.2339 | 1.3008 | -0.1247 | 1.58 | 5.78 | 70 | [ |
| 甲苯 | 293~373 | 0.1~203 | 0.1697 | -1.1530 | 3.2276 | 1.3479 | -0.0745 | 1.33 | 6.28 | 201 | [ |
| 壬苯 | 313~353 | 0.1~40 | 0.1169 | -0.4350 | 0.3116 | 4.3219 | 0.1644 | 1.13 | 2.72 | 15 | [ |
| 1,2,4-三甲基苯 | 293~353 | 0.1~140 | 0.0699 | 0.9220 | 1.9018 | 2.8515 | 0.1272 | 0.57 | 2.40 | 56 | [ |
| 甲基萘 | 293~353 | 0.1~100 | 0.0859 | 0.8097 | 4.8653 | 0.5503 | -0.2135 | 2.11 | 6.14 | 42 | [ |
| 顺式十氢化萘 | 293~353 | 0.1~100 | 0.1482 | 0.3316 | 3.8357 | 1.1811 | -0.1438 | 1.25 | 3.12 | 42 | [ |
| 辛酸乙酯 | 288~362 | 0.1~30 | 0.1466 | -1.1153 | 3.8311 | 0.9913 | -0.1722 | 0.75 | 2.26 | 87 | [ |
| 十二酸甲酯 | 283~353 | 0.1~200 | 0.0483 | 0.5054 | 4.3916 | 0.7843 | -0.1991 | 1.35 | 8.57 | 105 | [ |
| 十二酸乙酯 | 283~354 | 0.1~200 | 0.0407 | 0.5081 | 4.2869 | 0.8756 | -0.1826 | 1.65 | 8.29 | 109 | [ |
| 乙醇 | 293~353 | 0.1~100 | 0.2585 | -2.7712 | 0.5546 | 4.3166 | 0.0860 | 1.13 | 5.25 | 59 | [ |
| 丁醇 | 293~353 | 0.1~140 | 0.2197 | -1.5720 | 1.3790 | 2.8759 | -0.0894 | 1.45 | 6.91 | 98 | [ |
| 异丙醇 | 298~343 | 0.1~118 | 0.2785 | -2.5450 | 2.5620 | 1.5637 | -0.2813 | 1.46 | 3.43 | 44 | [ |
| 二丙酮醇 | 303~343 | 0.1~100 | 0.1243 | 1.3320 | 3.7976 | 1.0327 | -0.2458 | 0.78 | 2.13 | 18 | [ |
| 总体 | 1.54 | 8.62 | |||||||||
表2 纯质流体黏度计算结果
Table 2 Calculated viscosity of pure fluids
| 物质 | T/K | p/MPa | 参数 | AAD/% | MD/% | 数据点数 | 文献 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a1 | 102a2 | b | c | d | |||||||
| 正戊烷 | 298~373 | 0.1~25 | 0.2455 | -5.1312 | 9.3775 | 5.1283 | -1.9272 | 1.07 | 2.99 | 31 | [ |
| 正己烷 | 273~373 | 0.1~205 | 0.1420 | -1.0610 | 2.1592 | 2.7046 | 0.1682 | 2.28 | 5.10 | 38 | [ |
| 正庚烷 | 298~474 | 0.1~100 | 0.1497 | -1.0627 | -0.5700 | 7.8378 | 0.6419 | 1.30 | 3.23 | 33 | [ |
| 正辛烷 | 273~467 | 0.1~203 | 0.1163 | -0.4310 | 1.1151 | 4.1198 | 0.2715 | 2.61 | 7.26 | 104 | [ |
| 正壬烷 | 300~420 | 0.1~50 | 0.1431 | -0.8425 | 2.6574 | 1.9450 | -0.0004 | 0.44 | 3.04 | 220 | [ |
| 正癸烷 | 293~373 | 0.1~100 | 0.1278 | -0.7410 | 3.4988 | 1.1772 | -0.1271 | 1.93 | 5.19 | 66 | [ |
| 正十二烷 | 293~373 | 0.1~200 | 0.0774 | 0.1410 | 3.7887 | 1.0225 | -0.1582 | 1.97 | 8.62 | 112 | [ |
| 正十三烷 | 293~353 | 0.1~100 | 0.0728 | 0.1678 | 2.8687 | 1.8857 | -0.0281 | 2.12 | 6.76 | 42 | [ |
| 正十四烷 | 313~393 | 0.7~60 | 0.1041 | -0.7250 | 2.7749 | 1.7020 | -0.0872 | 1.84 | 4.44 | 40 | [ |
| 正十六烷 | 298~413 | 0.1~202 | 0.0534 | 0.2920 | 3.6739 | 1.1963 | -0.1388 | 2.30 | 7.94 | 117 | [ |
| 环己烷 | 298~413 | 0.1~100 | 0.2955 | -2.2960 | 2.2599 | 2.1360 | -0.0745 | 2.60 | 7.01 | 120 | [ |
| 2,2,4-三甲基戊烷 | 293~353 | 0.1~140 | 0.1673 | -1.2950 | 3.1482 | 1.4400 | -0.0796 | 2.10 | 6.11 | 98 | [ |
| 七甲基壬烷 | 293~353 | 0.1~100 | 0.0786 | 0.8681 | 4.3655 | 0.8666 | -0.2015 | 0.96 | 2.89 | 42 | [ |
| 甲基环己烷 | 293~353 | 0.1~100 | 0.2426 | -2.0455 | -0.0096 | 5.9618 | 0.3825 | 1.17 | 3.20 | 42 | [ |
| 苯 | 298~393 | 0.1~178 | 0.1615 | 0.8100 | 3.2339 | 1.3008 | -0.1247 | 1.58 | 5.78 | 70 | [ |
| 甲苯 | 293~373 | 0.1~203 | 0.1697 | -1.1530 | 3.2276 | 1.3479 | -0.0745 | 1.33 | 6.28 | 201 | [ |
| 壬苯 | 313~353 | 0.1~40 | 0.1169 | -0.4350 | 0.3116 | 4.3219 | 0.1644 | 1.13 | 2.72 | 15 | [ |
| 1,2,4-三甲基苯 | 293~353 | 0.1~140 | 0.0699 | 0.9220 | 1.9018 | 2.8515 | 0.1272 | 0.57 | 2.40 | 56 | [ |
| 甲基萘 | 293~353 | 0.1~100 | 0.0859 | 0.8097 | 4.8653 | 0.5503 | -0.2135 | 2.11 | 6.14 | 42 | [ |
| 顺式十氢化萘 | 293~353 | 0.1~100 | 0.1482 | 0.3316 | 3.8357 | 1.1811 | -0.1438 | 1.25 | 3.12 | 42 | [ |
| 辛酸乙酯 | 288~362 | 0.1~30 | 0.1466 | -1.1153 | 3.8311 | 0.9913 | -0.1722 | 0.75 | 2.26 | 87 | [ |
| 十二酸甲酯 | 283~353 | 0.1~200 | 0.0483 | 0.5054 | 4.3916 | 0.7843 | -0.1991 | 1.35 | 8.57 | 105 | [ |
| 十二酸乙酯 | 283~354 | 0.1~200 | 0.0407 | 0.5081 | 4.2869 | 0.8756 | -0.1826 | 1.65 | 8.29 | 109 | [ |
| 乙醇 | 293~353 | 0.1~100 | 0.2585 | -2.7712 | 0.5546 | 4.3166 | 0.0860 | 1.13 | 5.25 | 59 | [ |
| 丁醇 | 293~353 | 0.1~140 | 0.2197 | -1.5720 | 1.3790 | 2.8759 | -0.0894 | 1.45 | 6.91 | 98 | [ |
| 异丙醇 | 298~343 | 0.1~118 | 0.2785 | -2.5450 | 2.5620 | 1.5637 | -0.2813 | 1.46 | 3.43 | 44 | [ |
| 二丙酮醇 | 303~343 | 0.1~100 | 0.1243 | 1.3320 | 3.7976 | 1.0327 | -0.2458 | 0.78 | 2.13 | 18 | [ |
| 总体 | 1.54 | 8.62 | |||||||||
| 物质 | T/K | p/MPa | κ12 | AAD/% | MD/% | 数据点数 | 文献 |
|---|---|---|---|---|---|---|---|
| 正戊烷+正辛烷 | 298~373 | 0.1~25 | 0.2225 | 2.95 | 9.13 | 295 | [ |
| 正戊烷+正癸烷 | 298~373 | 0.1~25 | 0.3476 | 2.45 | 8.51 | 312 | [ |
| 正庚烷+正己烷 | 303~323 | 0.1~72 | 0.0735 | 1.14 | 3.15 | 53 | [ |
| 正庚烷+正壬烷 | 303~323 | 0.1~72 | 0.0434 | 1.14 | 3.03 | 57 | [ |
| 正庚烷+正辛烷 | 298~473 | 0.1~10 | -0.0601 | 1.02 | 4.01 | 122 | [ |
| 正辛烷+正癸烷 | 298~373 | 0.1~25 | 0.0872 | 1.90 | 8.78 | 324 | [ |
| 正癸烷+正十六烷 | 313~353 | 0.1~100 | 0.1812 | 1.80 | 4.57 | 54 | [ |
| 正庚烷+甲基萘 | 303~343 | 0.1~100 | -0.4344 | 3.37 | 11.3 | 126 | [ |
| 正己烷+正十二烷 | 298~373 | 0.1~204 | 0.3014 | 3.21 | 5.67 | 15 | [ |
| 正辛烷+正十二烷 | 298~373 | 0.1~203 | 0.0916 | 3.13 | 6.99 | 21 | [ |
| 正十三烷+七甲基壬烷 | 293~353 | 0.1~100 | -0.0641 | 1.96 | 6.65 | 294 | [ |
| 正庚烷+壬苯 | 313~353 | 0.1~40 | 0.3640 | 3.93 | 9.53 | 105 | [ |
| 环己烷+苯 | 313~393 | 0.7~60 | -0.1587 | 3.02 | 9.08 | 160 | [ |
| 甲基环己烷+正庚烷 | 303~343 | 0.1~100 | -0.1338 | 1.38 | 5.53 | 126 | [ |
| 甲基环己烷+顺式十氢化萘 | 293~353 | 0.1~100 | -0.0347 | 1.91 | 6.55 | 294 | [ |
| 甲基环己烷+七甲基壬烷 | 293~353 | 0.1~100 | 0.2681 | 2.34 | 7.20 | 294 | [ |
| 苯+正十四烷 | 313~393 | 0.7~60 | 0.3191 | 3.38 | 10.5 | 160 | [ |
| 甲苯+正己烷 | 298~373 | 0.1~203 | 0.0283 | 4.12 | 12.9 | 84 | [ |
| 甲基萘+甲基环己烷 | 303~343 | 0.1~100 | -0.3382 | 3.62 | 10.0 | 126 | [ |
| 甲基萘+七甲基壬烷 | 293~353 | 0.1~100 | -0.2426 | 2.00 | 6.78 | 294 | [ |
| 顺式十氢化萘+七甲基壬烷 | 293~353 | 0.1~100 | -0.0150 | 1.21 | 3.72 | 294 | [ |
| 辛酸乙酯+正十六烷 | 293~362 | 0.1~30 | 0.0050 | 1.99 | 4.77 | 168 | [ |
| 十二酸甲酯+辛酸乙酯 | 303~323 | 0.1~15 | 0.0243 | 1.48 | 3.79 | 162 | [ |
| 丁醇+2,2,4-三甲基戊烷 | 293~353 | 0.1~140 | -0.5304 | 3.42 | 9.94 | 168 | [ |
| 丁醇+1,2,4-三甲基苯 | 293~353 | 0.1~140 | -0.4963 | 2.38 | 8.95 | 168 | [ |
| 十二酸乙酯+乙醇 | 303~323 | 0.1~15 | 0.6092 | 3.58 | 9.97 | 216 | [ |
| 异丙醇+二丙酮醇 | 303~343 | 0.1~100 | -0.0659 | 1.91 | 5.34 | 162 | [ |
| 总体 | 2.35 | 12.9 |
表3 二元混合流体黏度计算结果
Table 3 Calculated viscosity of binary mixtures
| 物质 | T/K | p/MPa | κ12 | AAD/% | MD/% | 数据点数 | 文献 |
|---|---|---|---|---|---|---|---|
| 正戊烷+正辛烷 | 298~373 | 0.1~25 | 0.2225 | 2.95 | 9.13 | 295 | [ |
| 正戊烷+正癸烷 | 298~373 | 0.1~25 | 0.3476 | 2.45 | 8.51 | 312 | [ |
| 正庚烷+正己烷 | 303~323 | 0.1~72 | 0.0735 | 1.14 | 3.15 | 53 | [ |
| 正庚烷+正壬烷 | 303~323 | 0.1~72 | 0.0434 | 1.14 | 3.03 | 57 | [ |
| 正庚烷+正辛烷 | 298~473 | 0.1~10 | -0.0601 | 1.02 | 4.01 | 122 | [ |
| 正辛烷+正癸烷 | 298~373 | 0.1~25 | 0.0872 | 1.90 | 8.78 | 324 | [ |
| 正癸烷+正十六烷 | 313~353 | 0.1~100 | 0.1812 | 1.80 | 4.57 | 54 | [ |
| 正庚烷+甲基萘 | 303~343 | 0.1~100 | -0.4344 | 3.37 | 11.3 | 126 | [ |
| 正己烷+正十二烷 | 298~373 | 0.1~204 | 0.3014 | 3.21 | 5.67 | 15 | [ |
| 正辛烷+正十二烷 | 298~373 | 0.1~203 | 0.0916 | 3.13 | 6.99 | 21 | [ |
| 正十三烷+七甲基壬烷 | 293~353 | 0.1~100 | -0.0641 | 1.96 | 6.65 | 294 | [ |
| 正庚烷+壬苯 | 313~353 | 0.1~40 | 0.3640 | 3.93 | 9.53 | 105 | [ |
| 环己烷+苯 | 313~393 | 0.7~60 | -0.1587 | 3.02 | 9.08 | 160 | [ |
| 甲基环己烷+正庚烷 | 303~343 | 0.1~100 | -0.1338 | 1.38 | 5.53 | 126 | [ |
| 甲基环己烷+顺式十氢化萘 | 293~353 | 0.1~100 | -0.0347 | 1.91 | 6.55 | 294 | [ |
| 甲基环己烷+七甲基壬烷 | 293~353 | 0.1~100 | 0.2681 | 2.34 | 7.20 | 294 | [ |
| 苯+正十四烷 | 313~393 | 0.7~60 | 0.3191 | 3.38 | 10.5 | 160 | [ |
| 甲苯+正己烷 | 298~373 | 0.1~203 | 0.0283 | 4.12 | 12.9 | 84 | [ |
| 甲基萘+甲基环己烷 | 303~343 | 0.1~100 | -0.3382 | 3.62 | 10.0 | 126 | [ |
| 甲基萘+七甲基壬烷 | 293~353 | 0.1~100 | -0.2426 | 2.00 | 6.78 | 294 | [ |
| 顺式十氢化萘+七甲基壬烷 | 293~353 | 0.1~100 | -0.0150 | 1.21 | 3.72 | 294 | [ |
| 辛酸乙酯+正十六烷 | 293~362 | 0.1~30 | 0.0050 | 1.99 | 4.77 | 168 | [ |
| 十二酸甲酯+辛酸乙酯 | 303~323 | 0.1~15 | 0.0243 | 1.48 | 3.79 | 162 | [ |
| 丁醇+2,2,4-三甲基戊烷 | 293~353 | 0.1~140 | -0.5304 | 3.42 | 9.94 | 168 | [ |
| 丁醇+1,2,4-三甲基苯 | 293~353 | 0.1~140 | -0.4963 | 2.38 | 8.95 | 168 | [ |
| 十二酸乙酯+乙醇 | 303~323 | 0.1~15 | 0.6092 | 3.58 | 9.97 | 216 | [ |
| 异丙醇+二丙酮醇 | 303~343 | 0.1~100 | -0.0659 | 1.91 | 5.34 | 162 | [ |
| 总体 | 2.35 | 12.9 |
| 物质 | T/K | p/MPa | AAD/% | MD/% | 数据点数 | 文献 |
|---|---|---|---|---|---|---|
| 正戊烷+正辛烷+正癸烷 | 298~373 | 0.1~25 | 5.81 | 19.7 | 530 | [ |
| 正庚烷+甲基环己烷+甲基萘 | 303~343 | 0.1~100 | 4.04 | 10.8 | 378 | [ |
| 甲基环己烷+顺式十氢化萘+七甲基壬烷 | 293~353 | 0.1~100 | 1.79 | 7.82 | 546 | [ |
| 总体 | 3.86 | 19.7 |
表4 三元混合流体黏度计算结果
Table 4 Calculated viscosity of ternary mixtures
| 物质 | T/K | p/MPa | AAD/% | MD/% | 数据点数 | 文献 |
|---|---|---|---|---|---|---|
| 正戊烷+正辛烷+正癸烷 | 298~373 | 0.1~25 | 5.81 | 19.7 | 530 | [ |
| 正庚烷+甲基环己烷+甲基萘 | 303~343 | 0.1~100 | 4.04 | 10.8 | 378 | [ |
| 甲基环己烷+顺式十氢化萘+七甲基壬烷 | 293~353 | 0.1~100 | 1.79 | 7.82 | 546 | [ |
| 总体 | 3.86 | 19.7 |
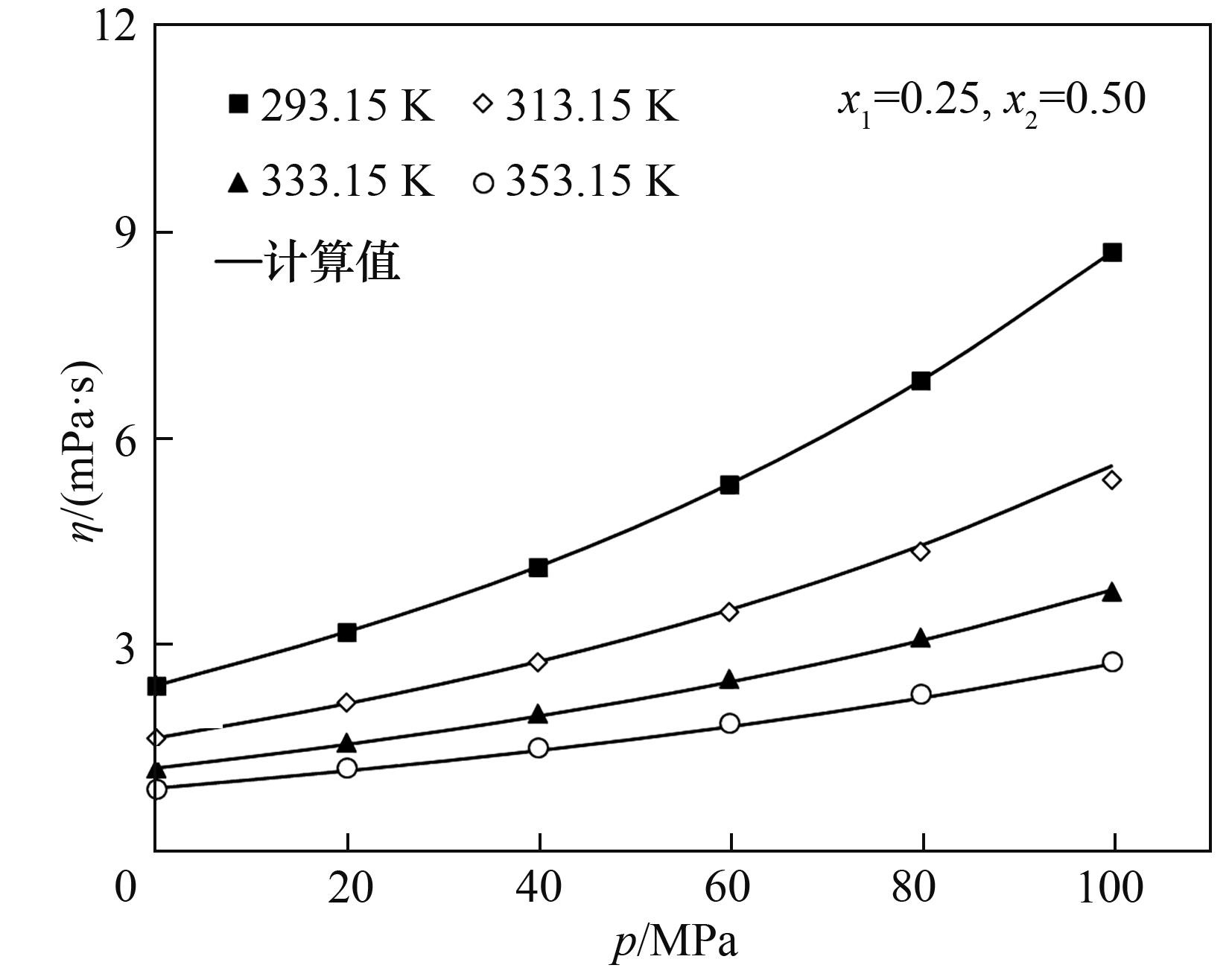
图8 绝对速率理论模型对甲基环己烷+顺式十氢化萘+七甲基壬烷黏度计算结果与实验结果的比较
Fig.8 Comparison of viscosity of methylcyclohexane + cis-decaline + heptamethylnonane between results using the absolute rate theory model and experiment

图10 本文模型(AR)与自由体积模型(FV)对异丙醇+二丙酮醇黏度计算结果
Fig.10 Calculated viscosity of 2-propanol + diacetone alcohol using the present model (AR) and the free-volume model (FV)
| 1 | Liu H T, Yang F F, Yang Z, et al. Modeling the viscosity of hydrofluorocarbons, hydrofluoroolefins and their binary mixtures using residual entropy scaling and cubic-plus-association equation of state[J]. Journal of Molecular Liquids, 2020, 308: 113027. |
| 2 | 韩光泽, 房增科, 陈明东. Eyring黏度公式的几率修正及基于液体准晶模型分子活化能的计算[J]. 中国科学: 物理学 力学 天文学, 2010, 40(9): 1092-1098. |
| Han G Z, Fang Z K, Chen M D. Probability correction of Eyring viscosity equation and the calculation of activation energy based on the liquid quasicrystal model[J]. Scientia Sinica (Physica, Mechanica & Astronomica), 2010, 40(9): 1092-1098. | |
| 3 | 房升. 基于Eyring反应速率理论的溶液黏度模型[J]. 化学进展, 2010, 22(Z1): 309-314. |
| Fang S. Viscosity model for mixtures based on Eyring's absolute reaction theory[J]. Progress in Chemistry, 2010, 22(Z1): 309-314. | |
| 4 | Quiñones-Cisneros S E, Zéberg-Mikkelsen C K, Stenby E H. The friction theory (f-theory) for viscosity modeling[J]. Fluid Phase Equilibria, 2000, 169(2): 249-276. |
| 5 | Allal A, Boned C, Baylaucq A. Free-volume viscosity model for fluids in the dense and gaseous states[J]. Physical Review E, 2001, 64(1): 011203. |
| 6 | Allal A, Moha-ouchane M, Boned C. A new free volume model for dynamic viscosity and density of dense fluids versus pressure and temperature[J]. Physics and Chemistry of Liquids, 2001, 39(1): 1-30. |
| 7 | Kincaid J F, Eyring H, Stearn A E. The theory of absolute reaction rates and its application to viscosity and diffusion in the liquid state[J]. Chemical Reviews, 1941, 28(2): 301-365. |
| 8 | Lei Q F, Hou Y C, Lin R S. Correlation of viscosities of pure liquids in a wide temperature range[J]. Fluid Phase Equilibria, 1997, 140(1/2): 221-231. |
| 9 | Macías-Salinas R, García-Sánchez F, Hernández-Garduza O. Viscosity model for pure liquids based on Eyring theory and cubic EOS[J]. AIChE Journal, 2003, 49(3): 799-804. |
| 10 | Macías-Salinas R, Garcı́a-Sánchez F, Eliosa-Jiménez G. An equation-of-state-based viscosity model for non-ideal liquid mixtures[J]. Fluid Phase Equilibria, 2003, 210(2): 319-334. |
| 11 | Martins R J, de M Cardoso M J E, Barcia O E. A new model for calculating the viscosity of pure liquids at high pressures[J]. Industrial & Engineering Chemistry Research, 2003, 42(16): 3824-3830. |
| 12 | Martins R J, de M Cardoso M J E, Barcia O E. Excess Gibbs free energy model for calculating the viscosity of binary liquid mixtures[J]. Industrial & Engineering Chemistry Research, 2000, 39(3): 849-854. |
| 13 | Liu X Y, Zhu C Y, He M G, et al. Correlation for viscosities of pure liquids at high pressures[J]. Journal of Molecular Liquids, 2017, 231: 404-410. |
| 14 | Han G Z, Fang Z K, Chen M D. Modified Eyring viscosity equation and calculation of activation energy based on the liquid quasi-lattice model[J]. Science China Physics, Mechanics and Astronomy, 2010, 53(10): 1853-1860. |
| 15 | Tochigi K, Okamura T, Rattan V K. Prediction of high-pressure viscosities for binary liquid mixtures using the EOS-GE mixing rule with low-pressure viscosity data[J]. Fluid Phase Equilibria, 2007, 257(2): 228-232. |
| 16 | Wang Y G, Chen D X, Ouyang X K. Viscosity calculations for ionic liquid-cosolvent mixtures based on Eyring's absolute rate theory and activity coefficient models[J]. Journal of Chemical & Engineering Data, 2010, 55(11): 4878-4884. |
| 17 | He Y C, Xu X J, Yang L J, et al. Viscosity modeling for ionic liquid solutions by Eyring-Wilson equation[J]. Chemical Industry and Chemical Engineering Quarterly, 2012, 18(3): 441-447. |
| 18 | Atashrouz S, Zarghampour M, Abdolrahimi S, et al. Estimation of the viscosity of ionic liquids containing binary mixtures based on the Eyring's theory and a modified Gibbs energy model[J]. Journal of Chemical & Engineering Data, 2014, 59(11): 3691-3704. |
| 19 | Xu Y J, Tang X C, Li J H, et al. Viscosity estimation of ternary mixtures containing ionic liquid from their binary subsystems: a comparison of three viscosity equations[J]. Fluid Phase Equilibria, 2016, 427: 166-174. |
| 20 | 朱晨阳, 刘向阳, 何茂刚. 基于绝对速率理论的高压液体黏度模型[J]. 工程热物理学报, 2018, 39(7): 1407-1411. |
| Zhu C Y, Liu X Y, He M G. A viscosity model of liquid based on absolute rate theory at high pressures[J]. Journal of Engineering Thermophysics, 2018, 39(7): 1407-1411. | |
| 21 | He M G, Zhu C Y, Liu X Y. Estimating the viscosity of ionic liquid at high pressure using Eyring's absolute rate theory[J]. Fluid Phase Equilibria, 2018, 458: 170-176. |
| 22 | Zhu C Y, Yang F, Liu X Y, et al. Viscosity of oxygenated fuel: a model based on Eyring's absolute rate theory[J]. Fuel, 2019, 241: 218-226. |
| 23 | 刘国杰, 胡英. 液体的黏度与内压[J]. 化学学报, 1991, 49(7): 649-655. |
| Liu G J, Hu Y. Viscosity and internal pressure for liquids[J]. Acta Chimica Sinica, 1991, 49(7): 649-655. | |
| 24 | Poling B E, Prausnitz J M, O'connell J P. Properties of Gases and Liquids[M]. 5th ed. New York: McGraw-Hill Education, 2001: A.5-A.19. |
| 25 | Grunberg L, Nissan A H. Mixture law for viscosity[J]. Nature, 1949, 164(4175): 799-800. |
| 26 | Yaws C L, Narasimhan P K. Critical properties and acentric factor—organic compounds[M]//Thermophysical Properties of Chemicals and Hydrocarbons. New York: William Andrew Publishing, 2009: 1-95. |
| 27 | Design Institute for Physical Property Data. DIPPR 801 database[DB]. New York: AIChE, 1998. |
| 28 | Comuñas M J P, Baylaucq A, Plantier F, et al. Influence of the number of CH2-CH2-O groups on the viscosity of polyethylene glycol dimethyl ethers at high pressure[J]. Fluid Phase Equilibria, 2004, 222/223: 331-338. |
| 29 | Estrada-Baltazar A, Iglesias-Silva G A, Barrufet M A. Liquid viscosities of pentane and pentane + decane from 298.15 K to 373.15 K and up to 25 MPa[J]. Journal of Chemical & Engineering Data, 1998, 43(4): 601-604. |
| 30 | Dymond J H, Young K J, Isdale J D. Transport properties of nonelectrolyte liquid mixtures (Ⅱ): Viscosity coefficients for the n-hexane + n-hexadecane system at temperatures from 25 to 100℃ at pressures up to the freezing pressure or 500 MPa[J]. International Journal of Thermophysics, 1980, 1(4): 345-373. |
| 31 | Kumagai A, Tomida D, Yokoyama C. Measurements of the liquid viscosities of mixtures of n-butane, n-hexane, and n-octane with squalane to 30 MPa[J]. International Journal of Thermophysics, 2006, 27(2): 376-393. |
| 32 | Baylaucq A, Boned C, Dauge P, et al. Measurements of the viscosity and density of three hydrocarbons and the three associated binary mixtures versus pressure and temperature[J]. International Journal of Thermophysics, 1997, 18(1): 3-23. |
| 33 | Abdulagatov I M, Azizov N D. (p, ρ, T, x) and viscosity measurements of {x1 n-heptane + (1-x1)n-octane} mixtures at high temperatures and high pressures[J]. The Journal of Chemical Thermodynamics, 2006, 38(11): 1402-1415. |
| 34 | Barrufet M A, Hall K R, Estrada-Baltazar A, et al. Liquid viscosity of octane and pentane + octane mixtures from 298.15 K to 373.15 K up to 25 MPa[J]. Journal of Chemical & Engineering Data, 1999, 44(6): 1310-1314. |
| 35 | Dymond J H, Robertson J, Isdale J D. Transport properties of nonelectrolyte liquid mixtures (Ⅲ): Viscosity coefficients for n-octane, n-dodecane, and equimolar mixtures of n-octane + n-dodecane and n-hexane + n-dodecane from 25 to 100℃ at pressures up to the freezing pressure or 500 MPa[J]. International Journal of Thermophysics, 1981, 2(2): 133-154. |
| 36 | Tanaka Y, Hosokawa H, Kubota H, et al. Viscosity and density of binary mixtures of cyclohexane with n-octane, n-dodecane, and n-hexadecane under high pressures[J]. International Journal of Thermophysics, 1991, 12(2): 245-264. |
| 37 | Stephan K. Numerical data on viscosity[M]//Viscosity of Dense Fluids. Boston: Springer, 1979: 188-190. |
| 38 | Estrada-Baltazar A, Alvarado J F J, Iglesias-Silva G A, et al. Experimental liquid viscosities of decane and octane + decane from 298.15 K to 373.15 K and up to 25 MPa[J]. Journal of Chemical & Engineering Data, 1998, 43(3): 441-446. |
| 39 | Ducoulombier D, Zhou H, Boned C, et al. Pressure (1—1000 bars) and temperature (20—100℃) dependence of the viscosity of liquid hydrocarbons[J]. The Journal of Physical Chemistry, 1986, 90(8): 1692-1700. |
| 40 | Zambrano J R, Sobrino M, Martín M C, et al. Contributing to accurate high pressure viscosity measurements: vibrating wire viscometer and falling body viscometer techniques[J]. The Journal of Chemical Thermodynamics, 2016, 96: 104-116. |
| 41 | Daugé P, Canet X, Baylaucq A, et al. Measurements of the density and viscosity of the tridecane + 2,2,4,4,6,8,8-heptamethylnonane mixtures in the temperature range 293.15—353.15 K at pressures up to 100 MPa[J]. High Temperatures-High Pressures, 2001, 33(2): 213-230. |
| 42 | Hernández-Galván M A, García-Sánchez F, Macías-Salinas R. Liquid viscosities of benzene, n-tetradecane, and benzene + n-tetradecane from 313 to 393 K and pressures up to 60 MPa: experiment and modeling[J]. Fluid Phase Equilibria, 2007, 262(1/2): 51-60. |
| 43 | Rajagopal K, Andrade L L P R, Paredes M L L. High-pressure viscosity measurements for the binary system cyclohexane + n-hexadecane in the temperature range of (318.15 to 413.15) K[J]. Journal of Chemical & Engineering Data, 2009, 54(10): 2967-2970. |
| 44 | Hernández-Galván M A, García-Sánchez F, García-Flores B E, et al. Liquid viscosities of cyclohexane, cyclohexane + tetradecane, and cyclohexane + benzene from (313 to 393) K and pressures up to 60 MPa[J]. Journal of Chemical & Engineering Data, 2009, 54(10): 2831-2838. |
| 45 | Barrouhou M, Zéberg-Mikkelsen C K, Baylaucq A, et al. High-pressure viscosity and density measurements for the asymmetric binary system cis-decalin + 2,2,4,4,6,8,8-heptamethylnonane[J]. International Journal of Thermophysics, 2003, 24(4): 937-952. |
| 46 | Zéberg-Mikkelsen C K, Barrouhou M, Baylaucq A, et al. Viscosity and density measurements of binary mixtures composed of methylcyclohexane + cis-decalin versus temperature and pressure[J]. International Journal of Thermophysics, 2003, 24(2): 361-374. |
| 47 | Vieira dos Santos F J, Nieto de Castro C A. Viscosity of toluene and benzene under high pressure[J]. International Journal of Thermophysics, 1997, 18(2): 367-378. |
| 48 | Dymond J H, Awan M A, Glen N F, et al. Transport properties of nonelectrolyte liquid mixtures (Ⅷ): Viscosity coefficients for toluene and for three mixtures of toluene + hexane from 25 to 100℃ at pressures up to 500 MPa[J]. International Journal of Thermophysics, 1991, 12(2): 275-287. |
| 49 | Zéberg-Mikkelsen C K, Baylaucq A, Watson G, et al. High-pressure viscosity measurements for the ethanol + toluene binary system[J]. International Journal of Thermophysics, 2005, 26(5): 1289-1302. |
| 50 | Kanti M, Lagourette B, Alliez J, et al. Viscosity of binary heptane-nonylbenzene as a function of pressure and temperature: application of Flory's theory[J]. Fluid Phase Equilibria, 1991, 65: 291-304. |
| 51 | Canet X, Daugé P, Baylaucq A, et al. Density and viscosity of the 1-methylnaphthalene + 2,2,4,4,6,8,8-heptamethylnonane system from 293.15 to 353.15 K at pressures up to 100 MPa[J]. International Journal of Thermophysics, 2001, 22(6): 1669-1689. |
| 52 | Sheu Y W, Tu C H. Densities, viscosities, refractive indices, and surface tensions for 12 flavor esters from T= 288.15 K to T= 358.15 K[J]. Journal of Chemical & Engineering Data, 2005, 50(5): 1706-1710. |
| 53 | Liu X Y, Lai T W, Guo X D, et al. Densities and viscosities of ethyl heptanoate and ethyl octanoate at temperatures from 303 to 353 K and at pressures up to 15 MPa[J]. Journal of Chemical & Engineering Data, 2017, 62(8): 2454-2460. |
| 54 | Wang X J, Du W B, Wang X P. Liquid viscosities of ethyl caprylate and ethyl caprate at elevated temperatures and pressures[J]. Journal of Molecular Liquids, 2020, 309: 113203. |
| 55 | Pratas M J, Freitas S, Oliveira M B, et al. Densities and viscosities of fatty acid methyl and ethyl esters[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3983-3990. |
| 56 | He M G, Lai T W, Liu X Y. Measurement and correlation of viscosities and densities of methyl dodecanoate and ethyl dodecanoate at elevated pressures[J]. Thermochimica Acta, 2018, 663: 85-92. |
| 57 | Habrioux M, Nasri D, Daridon J L. Measurement of speed of sound, density compressibility and viscosity in liquid methyl laurate and ethyl laurate up to 200 MPa by using acoustic wave sensors[J]. The Journal of Chemical Thermodynamics, 2018, 120: 1-12. |
| 58 | Assael M J, Polimatidou S K. Measurements of the viscosity of alcohols in the temperature range 290—340 K at pressures up to 30 MPa[J]. International Journal of Thermophysics, 1994, 15(1): 95-107. |
| 59 | Tanaka Y, Matsuda Y, Fujiwara H, et al. Viscosity of (water + alcohol) mixtures under high pressure[J]. International Journal of Thermophysics, 1987, 8(2): 147-163. |
| 60 | Zambrano J, Martín M C, Martín Á, et al. Viscosities of binary mixtures containing 1-butanol + 2,2,4-trimethylpentane or + 1,2,4-trimethylbenzene at high pressures for the thermophysical characterization of biofuels[J]. The Journal of Chemical Thermodynamics, 2016, 102: 140-146. |
| 61 | Moha-Ouchane M, Boned C, Allal A, et al. Viscosity and excess volume at high pressures in associative binaries[J]. International Journal of Thermophysics, 1998, 19(1): 161-189. |
| 62 | Assael M J, Charitidou E, Dymond J H, et al. Viscosity and thermal conductivity of binary n-heptane + n-alkane mixtures[J]. International Journal of Thermophysics, 1992, 13(2): 237-249. |
| 63 | Zéberg-Mikkelsen C K, Barrouhou M, Baylaucq A, et al. Measurements of the viscosity and density versus temperature and pressure for the binary system methylcyclohexane + 2,2,4,4,6,8,8-heptamethylnonane[J]. High Temperatures-High Pressures, 2002, 34(5): 591-601. |
| 64 | Wang X P, Wen K, Wang X J. High-pressure liquid viscosity of n-hexadecane/ethyl octanoate mixtures[J]. Journal of Chemical & Engineering Data, 2021, 66(2): 1185-1190. |
| 65 | Liu X Y, Yang F, Lai T W, et al. Densities and viscosities of mixtures of methyl dodecanoate + ethyl octanoate at pressures up to 15 MPa[J]. Journal of Chemical & Engineering Data, 2018, 63(11): 4085-4094. |
| 66 | Zhu C Y, Zhang Z W, Xue S, et al. Association effect on the density, viscosity and excess properties of fatty acid ester+alcohol mixtures: experiment and modeling[J]. Fuel, 2022, 316: 123425. |
| 67 | Soave G. Equilibrium constants from a modified Redlich-Kwong equation of state[J]. Chemical Engineering Science, 1972, 27(6): 1197-1203. |
| 68 | Iglesias-Silva G A, Estrada-Baltazar A, Hall K R, et al. Experimental liquid viscosity of pentane+octane+decane mixtures from 298.15 to 373.15 K up to 25 MPa[J]. Journal of Chemical & Engineering Data, 1999, 44(6): 1304-1309. |
| 69 | Baylaucq A, Daugé P, Boned C. Viscosity and density of the ternary mixture heptane + methylcyclohexane + 1-methylnaphthalene[J]. International Journal of Thermophysics, 1997, 18(5): 1089-1107. |
| 70 | Zéberg-Mikkelsen C K, Barrouhou M, Baylaucq A, et al. High-pressure viscosity and density measurements of the ternary system methylcyclohexane+cis-decalin+2,2,4,4,6,8,8-heptamethylnonane[J]. Journal of Chemical & Engineering Data, 2003, 48(6): 1387-1392. |
| 71 | Khemka Y, Sisco C J, Abutaqiya M I L, et al. One-parameter friction theory viscosity model for the cubic-plus-chain equation of state[J]. Fluid Phase Equilibria, 2021, 530: 112896. |
| 72 | Khosharay S, Karimi R, Khosharay K. Modeling the viscosity for (nC5+nC8), (nC5+nC10), (nC8+nC10) and (nC5+nC8+nC10) systems with Peng-Robinson viscosity equation of state[J]. Periodica Polytechnica Chemical Engineering, 2016, 60(4): 259-265. |
| 73 | Cain N, Roberts G, Kiserow D, et al. Modeling the thermodynamic and transport properties of decahydronaphthalene/propane mixtures: phase equilibria, density, and viscosity[J]. Fluid Phase Equilibria, 2011, 305(1): 25-33. |
| [1] | 宋嘉豪, 王文. 斯特林发动机与高温热管耦合运行特性研究[J]. 化工学报, 2023, 74(S1): 287-294. |
| [2] | 连梦雅, 谈莹莹, 王林, 陈枫, 曹艺飞. 地下水预热新风一体化热泵空调系统制热性能研究[J]. 化工学报, 2023, 74(S1): 311-319. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 王浩, 王振雷. 基于自适应谱方法的裂解炉烧焦模型化简策略[J]. 化工学报, 2023, 74(9): 3855-3864. |
| [5] | 李科, 文键, 忻碧平. 耦合蒸气冷却屏的真空多层绝热结构对液氢储罐自增压过程的影响机制研究[J]. 化工学报, 2023, 74(9): 3786-3796. |
| [6] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [7] | 汪尔奇, 彭书舟, 杨震, 段远源. 含HFO混合体系气液相平衡的理论模型评价[J]. 化工学报, 2023, 74(8): 3216-3225. |
| [8] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [9] | 诸程瑛, 王振雷. 基于改进深度强化学习的乙烯裂解炉操作优化[J]. 化工学报, 2023, 74(8): 3429-3437. |
| [10] | 闫琳琦, 王振雷. 基于STA-BiLSTM-LightGBM组合模型的多步预测软测量建模[J]. 化工学报, 2023, 74(8): 3407-3418. |
| [11] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [12] | 刘春雨, 周桓宇, 马跃, 岳长涛. CaO调质含油污泥干燥特性及数学模型[J]. 化工学报, 2023, 74(7): 3018-3027. |
| [13] | 郭雨莹, 敬加强, 黄婉妮, 张平, 孙杰, 朱宇, 冯君炫, 陆洪江. 稠油管道水润滑减阻及压降预测模型修正[J]. 化工学报, 2023, 74(7): 2898-2907. |
| [14] | 李艳辉, 丁邵明, 白周央, 张一楠, 于智红, 邢利梅, 高鹏飞, 王永贞. 非常规服役超临界锅炉的微纳尺度腐蚀动力学模型建立及应用[J]. 化工学报, 2023, 74(6): 2436-2446. |
| [15] | 刘起超, 周云龙, 陈聪. 起伏振动垂直上升管气液两相流截面含气率分析与计算[J]. 化工学报, 2023, 74(6): 2391-2403. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号