化工学报 ›› 2023, Vol. 74 ›› Issue (1): 145-156.DOI: 10.11949/0438-1157.20221072
收稿日期:2022-08-01
修回日期:2022-12-15
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
刘昌俊
作者简介:沈辰阳(1995—),男,博士研究生,shenchenyang@tju.edu.cn
基金资助:
Chenyang SHEN1( ), Kaihang SUN1, Yueping ZHANG2, Changjun LIU1(
), Kaihang SUN1, Yueping ZHANG2, Changjun LIU1( )
)
Received:2022-08-01
Revised:2022-12-15
Online:2023-01-05
Published:2023-03-20
Contact:
Changjun LIU
摘要:
二氧化碳转化利用技术在“双碳”目标达成方面将发挥重要作用。在各种转化利用反应途径中,因为甲醇可以作为有机合成中间体和液态燃料,二氧化碳加氢合成甲醇受到广泛关注。开发高活性、高选择性催化剂是二氧化碳加氢合成甲醇技术发展的关键。近年来,氧化铟及其负载金属催化剂因其高活性、高甲醇选择性而备受关注。氧化铟与一些金属,如金、银、铂、钯、钌、铑、铱、镍、铼等有强相互作用,不仅可以稳定氧化铟、避免氧化铟过还原,还导致催化剂电子结构、反应途径等发生变化,也使得一些本身不具CO2加氢合成甲醇活性的金属催化剂变为高活性催化剂。CO2加氢合成甲醇氧化铟系催化剂是通过理论研究预测后经实验证实发现的。CO2加氢合成甲醇氧化铟及其负载金属催化剂涵盖单原子催化、团簇及纳米颗粒催化剂,是理论可预测的、难得的模型催化剂体系。相关反应途径可用于解释“催化剂”定义。CO2加氢合成甲醇氧化铟及其负载金属催化剂研究在基础研究和应用两方面都具有重要意义。
中图分类号:
沈辰阳, 孙楷航, 张月萍, 刘昌俊. 二氧化碳加氢合成甲醇氧化铟及其负载金属催化剂研究进展[J]. 化工学报, 2023, 74(1): 145-156.
Chenyang SHEN, Kaihang SUN, Yueping ZHANG, Changjun LIU. Research progresses on In2O3 and In2O3 supported metal catalysts for CO2 hydrogenation to methanol[J]. CIESC Journal, 2023, 74(1): 145-156.
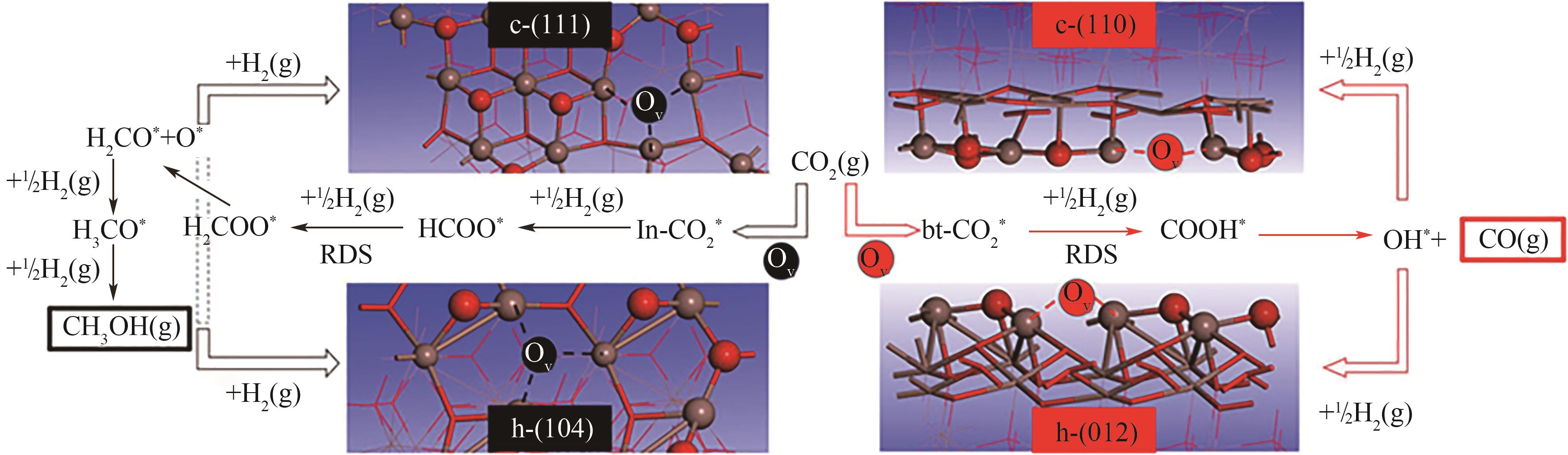
图3 c-In2O3和h-In2O3表面CO2加氢最优反应途径图示[16]
Fig.3 Schematic illustration of the most favorable CO2 hydrogenation pathways on c-In2O3 and h-In2O3 surfaces[16]
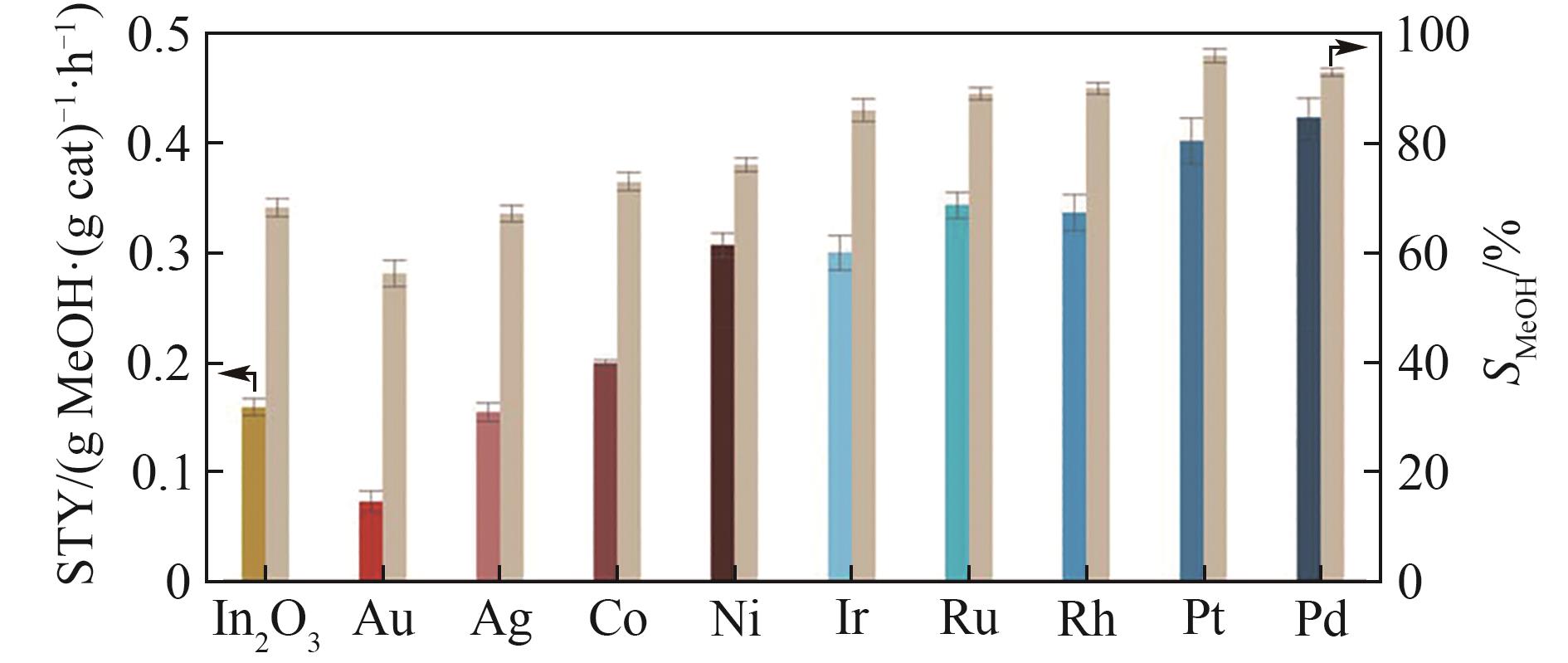
图6 火焰喷雾合成氧化铟负载金属催化剂(载量均为0.5%,质量分数)与氧化铟催化剂CO2加氢活性对比[34](图中甲醇时空收率以彩色柱状表示、甲醇选择性以米色表示,反应条件:5 MPa, 280℃, H2/CO2 = 4, GHSV = 24000 cm3·(g cat)-1·h-1)
Fig.6 Methanol space-time yield (STY, colored bars) and selectivity (SMeOH, beige bars) during CO2 hydrogenation over undoped In2O3 and M-In2O3 catalysts (0.5%(mass) of metal) prepared by FSP[34] (the methanol STY is assessed at GHSV = 24000 cm3·(g cat)-1·h-1,while SMeOH at constant CO2 conversion (≈3%) and variable WHSV, averaged values measured over 24 h on stream are presented with their corresponding error bars, reaction conditions: T = 280℃, P = 5 MPa, and H2/CO2 = 4)
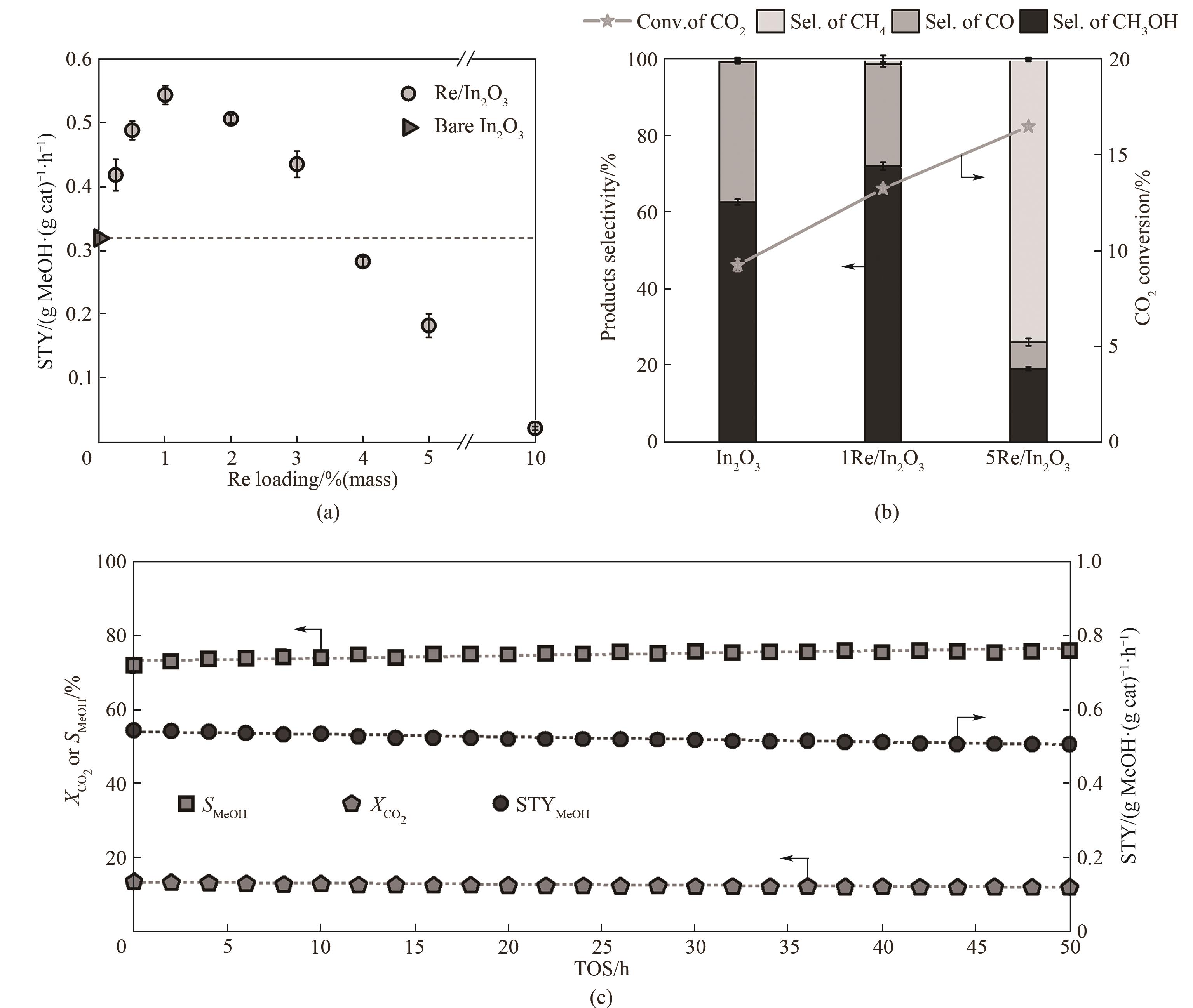
图7 Re/In2O3催化剂CO2加氢活性测试结果[36](a) 铼的负载量对甲醇时空收率的影响; (b) In2O3, 1Re/In2O3 (铼负载量为1%(质量)催化剂)以及5Re/In2O3(铼负载量为5%(质量)催化剂)在CO2加氢过程中CO2转化率以及产物选择性的对比;(c) 1Re/In2O3催化剂的稳定性测试结果(测试条件:5 MPa, 300℃, H2/CO2 = 4, GHSV = 21000 cm3·(g cat-1)·h-1)
Fig.7 Catalytic performance of Re/In2O3 during CO2 hydrogenation[36](a) effect of Re loadings on the methanol STY; (b) comparison of selectivity and CO2 conversion in the CO2 hydrogenation over In2O3, 1Re/In2O3 and 5Re/In2O3 catalysts; (c) stability test of 1Re/In2O3 for 50 h on stream (reaction conditions: 5 MPa, 300℃, H2/CO2 = 4, GHSV = 21000 cm3·(g cat)-1·h-1)
| 12 | Shen C Y, Bao Q Q, Xue W J, et al. Synergistic effect of the metal-support interaction and interfacial oxygen vacancy for CO2 hydrogenation to methanol over Ni/In2O3 catalyst: a theoretical study[J]. Journal of Energy Chemistry, 2022, 65: 623-629. |
| 13 | Ye J Y, Liu C J, Ge Q F. DFT study of CO2 adsorption and hydrogenation on the In2O3 surface[J]. Journal of Physical Chemistry C, 2012, 116(14): 7817-7825. |
| 14 | Frei M S, Capdevila-Cortada M, Garcia-Muelas R, et al. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide[J]. Journal of Catalysis, 2018, 361: 313-321. |
| 15 | Tsoukalou A, Abdala P M, Stoian D, et al. Structural evolution and dynamics of an In2O3 catalyst for CO2 hydrogenation to methanol: an operando XAS-XRD and in situ TEM study[J]. Journal of the American Chemical Society, 2019, 141(34): 13497-13505. |
| 16 | Dang S S, Qin B, Yang Y, et al. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity[J]. Science Advances, 2020, 6(25): eaaz2060. |
| 17 | Cao A, Wang Z B, Li H, et al. Relations between surface oxygen vacancies and activity of methanol formation from CO2 hydrogenation over In2O3 surfaces[J]. ACS Catalysis, 2021, 11(3): 1780-1786. |
| 18 | Jia X Y, Sun K H, Wang J, et al. Selective hydrogenation of CO2 to methanol over Ni/In2O3 catalyst[J]. Journal of Energy Chemistry, 2020, 50: 409-415. |
| 19 | Zhang Z T, Shen C Y, Sun K H, et al. Advances in studies of the structural effects of supported Ni catalysts for CO2 hydrogenation: from nanoparticle to single atom catalyst[J]. Journal of Materials Chemistry A, 2022, 10(11): 5771-5791. |
| 20 | Lin D F, Zhang Z, Chen Y Y, et al. The Co-In2O3 interaction concerning the effect of amorphous Co metal on CO2 hydrogenation to methanol[J]. Journal of CO2 Utilization, 2022, 65: 102209. |
| 21 | Fang T F, Liu B, Lian Y, et al. Selective methanol synthesis from CO2 hydrogenation over an In2O3/Co/C-N catalyst[J]. Industrial & Engineering Chemical Research, 2020, 59(43): 19162-19167. |
| 22 | Rui N, Sun K H, Shen C Y, et al. Density functional theoretical study of Au4/In2O3 catalyst for CO2 hydrogenation to methanol: the strong metal-support interaction and its effect[J]. Journal of CO2 Utilization, 2020, 42: 101313. |
| 23 | Rui N, Zhang F, Sun K H, et al. Hydrogenation of CO2 to methanol on a Au δ +-In2O3- x catalyst[J]. ACS Catalysis, 2020, 10(19): 11307-11317. |
| 24 | Sun K H, Zhang Z T, Shen C Y, et al. The feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol[J]. Green Energy & Environment, 2022, 7(4): 807-817. |
| 25 | Sun K H, Rui N, Shen C Y, et al. Theoretical study of selective hydrogenation of CO2 to methanol over Pt4/In2O3 model catalyst[J]. The Journal of Physical Chemistry C, 2021, 125(20): 10926-10936. |
| 26 | Sun K H, Rui N, Zhang Z T, et al. A highly active Pt/In2O3 catalyst for CO2 hydrogenation to methanol with enhanced stability[J]. Green Chemistry, 2020, 22(15): 5059-5066. |
| 27 | Han Z, Tang C Z, Wang J J, et al. Atomically dispersed Pt n + species as highly active sites in Pt/In2O3 catalysts for methanol synthesis from CO2 hydrogenation[J]. Journal of Catalysis, 2021, 394: 236-244. |
| 28 | Frei M S, Mondelli C, Garcia-Muelas R, et al. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation[J]. Nature Communications, 2019, 10: 1-11. |
| 29 | Rui N, Wang Z Y, Sun K H, et al. CO2 hydrogenation to methanol over Pd/In2O3: effects of Pd and oxygen vacancy[J]. Applied Catalysis B-Environmental, 2017, 218: 488-497. |
| 30 | Snider J L, Streibel V, Hubert M A, et al. Revealing the synergy between oxide and alloy phases on the performance of bimetallic In-Pd catalysts for CO2 hydrogenation to methanol[J]. ACS Catalysis, 2019, 9(4): 3399-3412. |
| 31 | Ye J Y, Liu C J, Mei D H, et al. Methanol synthesis from CO2 hydrogenation over a Pd4/In2O3 model catalyst: a combined DFT and kinetic study[J]. Journal of Catalysis, 2014, 317: 44-53. |
| 32 | Wu Q L, Shen C Y, Rui N, et al. Experimental and theoretical studies of CO2 hydrogenation to methanol on Ru/In2O3 [J]. Journal of CO2 Utilization, 2021, 53: 101720. |
| 33 | Wang J, Sun K H, Jia X Y, et al. CO2 hydrogenation to methanol over Rh/In2O3 catalyst[J]. Catalysis Today, 2020: 341-347. |
| 34 | Pinheiro A T, Morales-Vidal J, Zou T S, et al. Flame spray pyrolysis as a synthesis platform to assess metal promotion in In2O3-catalyzed CO2 hydrogenation[J]. Advanced Energy Materials, 2022, 12(14): 2103707. |
| 35 | Zhu J, Cannizzaro F, Liu L, et al. Ni-In synergy in CO2 hydrogenation to methanol[J]. ACS Catalysis, 2021, 11(18), 11371-11384. |
| 36 | Shen C Y, Sun K H, Zou R, et al. CO2 hydrogenation to methanol on indium oxide-supported rhenium catalysts: the effects of size[J]. ACS Catalysis, 2022, 12(20): 12658-12669. |
| 37 | Bai J L, Luo, Y B, Chen C, et al. Functionalization of 1D In2O3 nanotubes with abundant oxygen vacancies by rare earth dopant for ultra-high sensitive ethanol detection[J]. Sensors and Actuators B: Chemical, 2020, 324: 128755. |
| 38 | Xu P C, Cheng Z X, Pan Q Y, et al. High aspect ratio In2O3 nanowires: synthesis, mechanism and NO2 gas-sensing properties[J]. Sensors and Actuators B: Chemical, 2008, 130: 802-808. |
| 39 | Schühle P, Schmidt M, Schill L, et al. Influence of gas impurities on the hydrogenation of CO2 to methanol using indium-based catalysts[J]. Catalysis Science Technology, 2020, 10: 7309-7322. |
| 40 | Jiang X, Nie X W, Guo X W, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chemical Reviews, 2020, 120(15): 7984-8034. |
| 41 | Fayisa B A, Yang Y W, Zhen Z H, et al. Engineered chemical utilization of CO2 to methanol via direct and indirect hydrogenation pathways: a review[J]. Industrial & Engineering Chemistry Research, 2022, 61(29): 10319-10335. |
| 42 | Xu D, Wang Y Q, Ding M Y, et al. Advances in higher alcohol synthesis from CO2 hydrogenation[J]. Chem, 2021, 7(4): 849-881. |
| 43 | Estevez R, Aguado-Deblas L, Bautista F M, et al. A review on green hydrogen valorization by heterogeneous catalytic hydrogenation of captured CO2 into value-added products[J]. Catalysts, 2022, 12(12):1555. |
| 44 | Iwasa N, Suzuki H, Terashita M, al et, Methanol synthesis from CO 2 under atmospheric pressure over supported Pd catalysts[J]. Catalysis Letters, 2004, 96(1/2): 75-78. |
| 45 | Fujitani T, Saito M, Kanai Y, et al. Development of an active Ga2O3 supported palladium catalyst for the synthesis of methanol from carbon dioxide and hydrogen[J]. Applied Catalysis A: General, 1995, 125(2): 199-202. |
| 46 | Richard A R, Fan M H. Low-pressure hydrogenation of CO2 to CH3OH using Ni-In-Al/SiO2 catalyst synthesized via a phyllosilicate precursor[J]. ACS Catalysis, 2017, 7(9): 5679-5692. |
| 47 | Meng C, Zhao G F, Shi X R, et al. Oxygen-deficient metal oxides supported nano-intermetallic InNi3C0.5 toward efficient CO2 hydrogenation to methanol[J]. Science Advances, 2021, 7(32): eabi6012. |
| 48 | Frei M S, Mondelli C, García-Muelas R, et al. Nanostructure of nickel-promoted indium oxide catalysts drives selectivity in CO2 hydrogenation[J]. Nature Communications, 2021, 12(1):1-9. |
| 49 | Cherevotan A, Raj J, Dheer L, et al. Operando generated ordered heterogeneous catalyst for the selective conversion of CO2 to methanol[J]. ACS Energy Letters 2021, 6(2): 509-516. |
| 50 | García-Trenco A, Regoutz A, White E R, et al. PdIn intermetallic nanoparticles for the hydrogenation of CO2 to methanol[J]. Applied Catalysis B: Environmental, 2018, 220: 9-18. |
| 51 | Cai Z J, Huang M, Dai J J, et al. Fabrication of Pd/In2O3 nanocatalysts derived from MIL-68(In) loaded with molecular metalloporphyrin (TCPP(Pd)) toward CO2 hydrogenation to methanol[J]. ACS Catalysis, 2022, 12(1): 709-723. |
| 52 | Ye J Y, Ge Q F, Liu C J. Effect of PdIn bimetallic particle formation on CO2 reduction over the Pd-In/SiO2 catalyst[J]. Chemical Engineering Science, 2015, 135: 193-201. |
| 53 | Geng F Y, Zhan X, Hicks J C. Promoting methanol synthesis and inhibiting CO2 methanation with bimetallic In-Ru catalysts[J]. ACS Sustainable Chemistry & Engineering 2021, 9(35): 11891-11902. |
| 54 | Li M M-J, Zou H B, Zheng J W, et al. Methanol synthesis at a wide range of H2/CO2 ratios over a Rh-In bimetallic catalyst[J]. Angewandte Chemie International Edition, 2020, 132: 16173-16180. |
| 55 | Bavykina A, Yarulina I, Al Abdulghani A J, et al. Turning a methanation Co catalyst into an In-Co methanol producer[J]. ACS Catalysis, 2019, 9(8): 6910-6918. |
| 56 | Zhang H, Mao D L, Zhang J X, et al. Regulating the crystal structure of layered double hydroxide-derived Co-In catalysts for highly selective CO2 hydrogenation to methanol[J]. Chemical Engineering Journal, 2023, 452: 139144. |
| 57 | Li L T, Yang B, Gao B, et al. CO2 hydrogenation selectivity shift over In-Co binary oxides catalysts: catalytic mechanism and structure-property relationship[J]. Chinese Journal of Catalysis, 2022, 43(3): 862-876. |
| 58 | Shi Z S, Pan M, Wei X L, et al. Cu-In intermetallic compounds as highly active catalysts for CH3OH formation from CO2 hydrogenation[J]. International Journal of Energy Research, 2022, 46(2): 1285-1298. |
| 59 | Shi Z S, Tan Q Q, Tian C, et al. CO2 hydrogenation to methanol over Cu-In intermetallic catalysts: effect of reduction temperature[J]. Journal of Catalysis, 2019, 379: 78-89. |
| 60 | Ziemba M, Radtke M, Schumacher L, et al. Elucidating CO2 hydrogenation over In2O3 nanoparticles using operando UV/Vis and impedance spectroscopies[J]. Angewandte Chemie International Edition, 2022, 61(39): e202209388. |
| 1 | Liu C J. Do we have a rapid solution for CO2 utilization? A perspective from China[J]. Greenhouse Gases: Science and Technology, 2012, 2(2): 75-76. |
| 2 | 曹晨熙,陈天元,丁晓旭,等. 负载型铟基催化剂二氧化碳加氢动力学研究[J]. 化工学报, 2019, 70(10): 3985-3993. |
| Cao C X, Chen T Y, Ding X X, et al. Kinetics study on supported indium-based catalysts in carbon dioxide hydrogenation[J]. CIESC Journal, 2019, 70(10): 3985-3993. | |
| 3 | 戴文华,辛忠. Si掺杂对Cu/ZrO2催化CO2加氢制甲醇性能的影响[J]. 化工学报, 2022, 73(8): 3586-3596. |
| Dai W H, Xin Z. Effect of Si-doped Cu/ZrO2 on the performance of catalysts for CO2 hydrogenation to methanol[J]. CIESC Journal, 2022, 73(8): 3586-3596. | |
| 4 | Zhong J W, Yang X F, Wu Z L, et al. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol[J]. Chemical Society Reviews, 2020, 49(5): 1385-1413. |
| 5 | Wang J Y, Zhang G H, Zhu J, et al. CO2 hydrogenation to methanol over In2O3-based catalysts: from mechanism to catalyst development[J]. ACS Catalysis, 2021, 11(3): 1406-1423. |
| 6 | Kattel S, Liu P, Chen J G. Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface[J]. Journal of the American Chemical Society, 2017, 139(29): 9739-9754. |
| 7 | Wang W, Wang S P, Ma X B, et al. Recent advances in catalytic hydrogenation of carbon dioxide[J]. Chemical Society Reviews, 2011, 40(7): 3703-3727. |
| 8 | Ye J Y, Liu C J, Mei D H, et al. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): a DFT study[J]. ACS Catalysis, 2013, 3(6): 1296-1306. |
| 9 | Sun K H, Fan Z G, Ye J Y, et al. Hydrogenation of CO2 to methanol over In2O3 catalyst[J]. Journal of CO2 Utilization, 2015, 12: 1-6. |
| 10 | Martin O, Martin A J, Mondelli C, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2016, 55(21): 6261-6265. |
| 11 | Shen C Y, Sun K H, Zhang Z T, et al. Highly active Ir/In2O3 catalysts for selective hydrogenation of CO2 to methanol: experimental and theoretical studies[J]. ACS Catalysis, 2021, 11(7): 4036-4046. |
| 61 | Jia X Y, Zhang X S, Rui N, et al. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity[J]. Applied Catalysis B: Environmental, 2019, 244: 159-169. |
| 62 | Zhang Z T, Shen C Y, Sun K H, et al. Improvement in the activity of Ni/In2O3 with the addition of ZrO2 for CO2 hydrogenation to methanol[J]. Catalysis Communications, 2022, 162: 106386. |
| 63 | Deng S W, Qiu C L, Yao Z H, et al. Multiscale simulation on thermal stability of supported metal nanocatalysts[J]. WIREs Computational Molecular Science, 2019, 9(4): e1405. |
| 64 | Qiu C L, Zhao C X, Sun X, et al. Multiscale simulation of morphology evolution of supported Pt nanoparticles via interfacial control[J]. Langmuir, 2019, 35(19): 6393-6402. |
| 65 | Hu J T, Yu L, Deng J, et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol[J]. Nature Catalysis, 2021, 4: 242-250. |
| 66 | Yang Z M, Zhang D Z, Chen H N. MOF-derived indium oxide hollow microtubes/MoS2 nanoparticles for NO2 gas sensing[J]. Sensors and Actuators B: Chemical, 2019, 300: 127037. |
| 67 | Liu Z, Lv H, Xie Y, et al. A 2D/2D/2D Ti3C2Tx@TiO2@MoS2 heterostructure as an ultrafast and high-sensitivity NO2 gas sensor at room-temperature[J]. Journal of Materials Chemistry A, 2022, 10: 11980-11989. |
| 68 | Li H, Gong H, Jin Z. In2O3-modified three-dimensional nanoflower MoS x form S-scheme heterojunction for efficient hydrogen production[J]. Acta Physico-Chimica Sinica, 2022, 38(12): 2201037. |
| 69 | Hülsey M J, Fung V, Hou X D, et al. Hydrogen spillover and its relation to hydrogenation: observations on structurally defined single-atom sites[J]. Angewandte Chemie International Edition, 2022, 61(40): e202208237. |
| 70 | Lu Z, Wang J, Sun K H, et al. CO2 hydrogenation to methanol over Rh/In2O3-ZrO2 catalyst with improved activity[J]. Green Chemical Engineering, 2022, 3(2): 165-170. |
| 71 | Lu Z, Sun K H, Wang J, et al. A highly active Au/In2O3-ZrO2 catalyst for selective hydrogenation of CO2 to methanol[J]. Catalysts, 2020, 10(11): 1360. |
| 72 | Sun K H, Shen C Y, Zou R, et al. Highly active Pt/In2O3-ZrO2 catalyst for CO2 hydrogenation to methanol with enhnced CO tolerance: the effects of ZrO2 [J]. Applied Catalysis B: Environmental, 2023, 320: 122018. |
| 73 | Tsoukalou A, Serykh A, Willinger E, et al. Hydrogen dissociation sites on indium-based ZrO2-supported catalysts forhydrogenation of CO2 to methanol[J]. Catalysis Today, 2022, 387: 38-46. |
| 74 | Araújo T P, Mondelli C, Agrachev M, et al. Flame-made ternary Pd-In2O3-ZrO2 catalyst with enhanced oxygen vacancy generation for CO2 hydrogenation to methanol[J]. Nature Communications, 2022, 13: 5610. |
| 75 | Yao L B, Shen X C, Pan Y B, et al. Synergy between active sites of Cu-In-Zr-O catalyst in CO2 hydrogenation to methanol[J]. Journal of Catalysis, 2019, 372: 74-85. |
| 76 | Pan Y X, You Y, Xin S, et al. Photocatalytic CO2 reduction by carbon-coated indium-oxide nanobelts[J]. Journal of the American Chemical Society, 2017, 139(11): 4123-4129. |
| 77 | Zhang Z S, Mao C L, Meira D M, et al. New black indium oxide-tandem photothermal CO2-H2 methanol selective catalyst[J]. Nature Communications, 2022, 13: 1512. |
| 78 | Yang Y X, Pan Y X, Tu X, et al. Nitrogen doping of indium oxide for enhanced photocatalytic reduction of CO2 to methanol[J]. Nano Energy, 2022, 101: 107613. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [5] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [6] | 曹跃, 余冲, 李智, 杨明磊. 工业数据驱动的加氢裂化装置多工况切换过渡状态检测[J]. 化工学报, 2023, 74(9): 3841-3854. |
| [7] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [8] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [9] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [10] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [11] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [12] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [13] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [14] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [15] | 李贵贤, 曹阿波, 孟文亮, 王东亮, 杨勇, 周怀荣. 耦合固体氧化物电解槽的CO2制甲醇过程设计与评价研究[J]. 化工学报, 2023, 74(7): 2999-3009. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号