化工学报 ›› 2023, Vol. 74 ›› Issue (8): 3366-3374.DOI: 10.11949/0438-1157.20230442
收稿日期:2023-05-05
修回日期:2023-08-18
出版日期:2023-08-25
发布日期:2023-10-18
通讯作者:
周维,倪中海
作者简介:杨菲菲(1991—),女,博士,副教授,feiyang@cumt.edu.cn
基金资助:
Feifei YANG1( ), Shixi ZHAO2, Wei ZHOU1(
), Shixi ZHAO2, Wei ZHOU1( ), Zhonghai NI1(
), Zhonghai NI1( )
)
Received:2023-05-05
Revised:2023-08-18
Online:2023-08-25
Published:2023-10-18
Contact:
Wei ZHOU, Zhonghai NI
摘要:
选择性加氢制甲醇是CO2资源化利用最主要的方式之一,因此十分需要开发高效的合成甲醇催化剂。本研究采用共沉淀法在In2O3中掺杂Sn来调控In2O3的还原能力和表面氧空位浓度,以提升In2O3催化剂在CO2加氢制甲醇中的催化性能。通过XRD、TEM、H2-TPR、H2-D2-TPSR、Raman、XPS、CO2-TPD等表征手段,研究了Sn助剂对催化剂结构和表面化学性质的影响;并通过高压固定床装置测试了催化剂的催化性能。结果表明,Sn在In2O3中高度分散并倾向于在表面富集,与In2O3形成Sn—O—In结构,促进了表面氧空位的生成,且能抑制In2O3的过度还原,从而使得Sn-In2O3催化剂表现出更高的甲醇选择性和产率。在300℃、3 MPa、空速为15000 ml·g-1·h-1的反应条件下,Sn/In摩尔比为0.5%的Sn-In2O3催化剂具有最优的催化性能,此时CO2转化率为6.2%,甲醇选择性为70.4%,当Sn含量进一步增加时,CO2转化率会略有下降,而甲醇选择性提升至80.8%。
中图分类号:
杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374.
Feifei YANG, Shixi ZHAO, Wei ZHOU, Zhonghai NI. Sn doped In2O3 catalyst for selective hydrogenation of CO2 to methanol[J]. CIESC Journal, 2023, 74(8): 3366-3374.
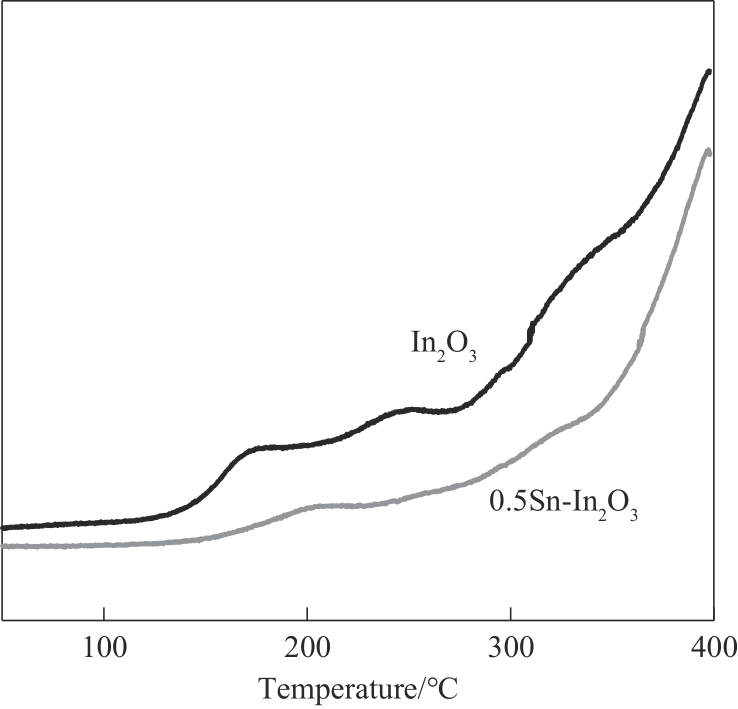
图5 In2O3和0.5Sn-In2O3催化剂H2-D2-TPSR测试中HD生成曲线(m/z=3)
Fig.5 HD formation profile during H2-D2-TPSR experiment over In2O3 and 0.5Sn-In2O3 catalysts (m/z=3)
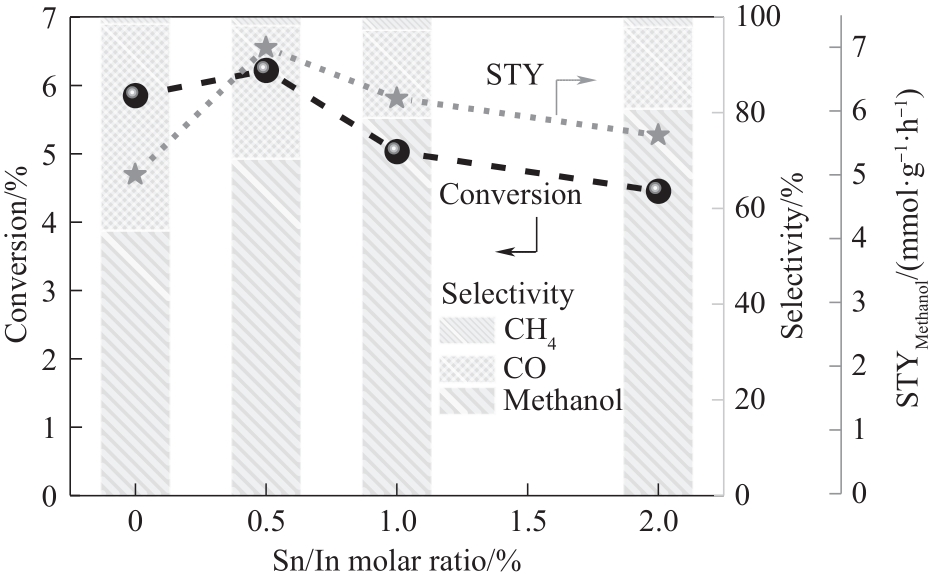
图9 Sn含量对CO2转化率、产物选择性以及甲醇的时空收率的影响
Fig.9 The effect of Sn content on CO2 conversion, product selectivity and the space time yield (STY) of methanol (reaction conditions: T=300℃, P=3 MPa, GHSV=15000 ml·g-1·h-1, TOS=1 h)
| Catalyst | Reaction condition | GHSV/ (ml·g-1·h-1) | CO2 conversion/% | SMethanol/% | STY/ (mmol·g-1·h-1) | Ref. | |
|---|---|---|---|---|---|---|---|
| T/℃ | P /MPa | ||||||
| In2O3 | 300 | 3 | 15000 | 5.9 | 55.4 | 5.0 | this work |
| 0.5Sn-In2O3 | 300 | 3 | 15000 | 6.2 | 70.4 | 6.9 | this work |
| 2Sn-In2O3 | 300 | 3 | 15000 | 4.5 | 80.8 | 5.6 | this work |
| In2O3 | 300 | 4 | 24000 | ~5 | ~60 | 7.4 | [ |
| Pt-In2O3 | 300 | 4 | 24000 | ~9 | ~67 | 14.8 | [ |
| In2O3 | 300 | 5 | 21000 | 8 | 71 | 10 | [ |
| Ir-In2O3 | 300 | 5 | 21000 | 17.7 | 70 | 24.1 | [ |
| Pd-In2O3 | 300 | 5 | 21000 | 21 | 71 | 27.8 | [ |
| Au-In2O3 | 300 | 5 | 21000 | 11.7 | 67.8 | 14.7 | [ |
| In2O3 | 300 | 5 | 21000 | 9 | 61 | 10.3 | [ |
| Pt/In2O3 | 300 | 5 | 21000 | 17.5 | 53 | 16.6 | [ |
表1 Sn-In2O3催化剂与文献报道的In2O3基催化剂用于CO2加氢制甲醇的比较
Table 1 Comparison of Sn-In2O3 with the reported In2O3 based catalysts for CO2 hydrogenation to methanol
| Catalyst | Reaction condition | GHSV/ (ml·g-1·h-1) | CO2 conversion/% | SMethanol/% | STY/ (mmol·g-1·h-1) | Ref. | |
|---|---|---|---|---|---|---|---|
| T/℃ | P /MPa | ||||||
| In2O3 | 300 | 3 | 15000 | 5.9 | 55.4 | 5.0 | this work |
| 0.5Sn-In2O3 | 300 | 3 | 15000 | 6.2 | 70.4 | 6.9 | this work |
| 2Sn-In2O3 | 300 | 3 | 15000 | 4.5 | 80.8 | 5.6 | this work |
| In2O3 | 300 | 4 | 24000 | ~5 | ~60 | 7.4 | [ |
| Pt-In2O3 | 300 | 4 | 24000 | ~9 | ~67 | 14.8 | [ |
| In2O3 | 300 | 5 | 21000 | 8 | 71 | 10 | [ |
| Ir-In2O3 | 300 | 5 | 21000 | 17.7 | 70 | 24.1 | [ |
| Pd-In2O3 | 300 | 5 | 21000 | 21 | 71 | 27.8 | [ |
| Au-In2O3 | 300 | 5 | 21000 | 11.7 | 67.8 | 14.7 | [ |
| In2O3 | 300 | 5 | 21000 | 9 | 61 | 10.3 | [ |
| Pt/In2O3 | 300 | 5 | 21000 | 17.5 | 53 | 16.6 | [ |
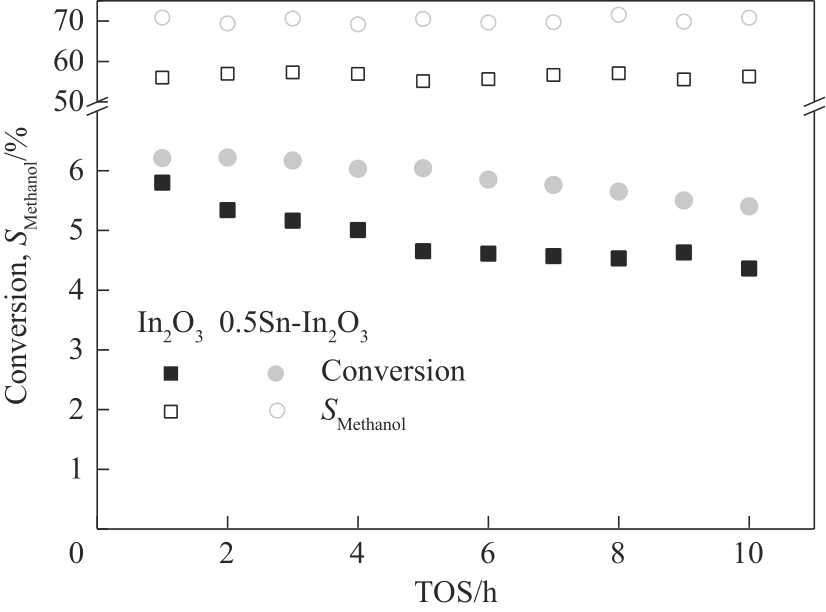
图10 不同催化剂中CO2的转化率随运行时间的变化
Fig.10 CO2 conversion as a function of TOS over different catalysts (reaction conditions: T=300℃, P=3 MPa, GHSV=15000 ml·g-1·h-1)
| 1 | Holdren J P. Science and technology for sustainable well-being[J]. Science, 2008, 319(5862): 424-434. |
| 2 | Wang W, Wang S P, Ma X B, et al. Recent advances in catalytic hydrogenation of carbon dioxide[J]. Chemical Society Reviews, 2011, 40(7): 3703-3727. |
| 3 | 巩金龙. CO2化学转化研究进展概述[J]. 化工学报, 2017, 68(4): 1282-1285. |
| Gong J L. A brief overview on recent progress on chemical conversion of CO2 [J]. CIESC Journal, 2017, 68(4): 1282-1285. | |
| 4 | Olah G A. Beyond oil and gas: the methanol economy[J]. Angewandte Chemie International Edition, 2005, 44(18): 2636-2639. |
| 5 | Jiang X, Nie X W, Guo X W, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chemical Reviews, 2020, 120(15): 7984-8034. |
| 6 | Zhong J W, Yang X F, Wu Z L, et al. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol[J]. Chemical Society Reviews, 2020, 49(5): 1385-1413. |
| 7 | 王莉, 姜枫, 胥月兵, 等. Ga助剂对Cu@ZnO催化CO2加氢制甲醇性能的影响[J]. 化工时刊, 2021, 35(5): 1-4. |
| Wang L, Jiang F, Xu Y B, et al. Effect of Ga promoter on performance of Cu@ZnO catalyst for CO2 hydrogenation to methanol[J]. Chemical Industry Times, 2021, 35(5): 1-4. | |
| 8 | Ye J Y, Liu C J, Mei D H, et al. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): a DFT study[J]. ACS Catalysis, 2013, 3(6): 1296-1306. |
| 9 | Martin O, Martín A J, Mondelli C, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2016, 55(21): 6261-6265. |
| 10 | 沈辰阳, 孙楷航, 张月萍, 等. 二氧化碳加氢合成甲醇氧化铟及其负载金属催化剂研究进展[J]. 化工学报, 2023, 74(1): 145-156. |
| Shen C Y, Sun K H, Zhang Y P, et al. Research progresses on In2O3 and In2O3 supported metal catalysts for CO2 hydrogenation to methanol[J]. CIESC Journal, 2023, 74(1): 145-156. | |
| 11 | 焦春学, 慕红梅, 高鹏, 等. In2O3基催化剂在热催化二氧化碳加氢反应中的研究进展[J]. 燃料化学学报, DOI: 10.19906/j.cnki.JFCT.2022086 . |
| Jiao C X, Mu H M, Gao P, et al. Progress of In2O3-based catalysts in thermal catalytic CO2 hydrogenation reaction[J]. Journal of Fuel Chemistry and Technology, DOI: 10.19906/j.cnki.JFCT.2022086 . | |
| 12 | Pinheiro Araújo T, Mondelli C, Agrachev M, et al. Flame-made ternary Pd-In2O3-ZrO2 catalyst with enhanced oxygen vacancy generation for CO2 hydrogenation to methanol[J]. Nature Communications, 2022, 13(1): 1-12. |
| 13 | Jamalpoor N, Ghasemi M, Soleimanian V. Investigation of the role of deposition rate on optical, microstructure and ethanol sensing characteristics of nanostructured Sn doped In2O3 films[J]. Materials Research Bulletin, 2018, 106: 49-56. |
| 14 | Ri J S, Li X W, Shao C L, et al. Sn-doping induced oxygen vacancies on the surface of the In2O3 nanofibers and their promoting effect on sensitive NO2 detection at low temperature[J]. Sensors and Actuators B: Chemical, 2020, 317: 128194. |
| 15 | Ye X, Yang C Y, Pan X L, et al. Highly selective hydrogenation of CO2 to ethanol via designed bifunctional Ir1-In2O3 single-atom catalyst[J]. Journal of the American Chemical Society, 2020, 142(45): 19001-19005. |
| 16 | Finetti M, Ottavianelli E E, Pis Diez R, et al. A density functional study of the Ni5Sn and Ni6Sn clusters[J]. Journal of Molecular Structure: THEOCHEM, 2003, 634(1/2/3): 171-179. |
| 17 | Tian H, Li X Y, Chen S, et al. Role of Sn in Ni-Sn/CeO2 catalysts for ethanol steam reforming[J]. Chinese Journal of Chemistry, 2017, 35(5): 651-658. |
| 18 | Gan J Y, Lu X H, Wu J H, et al. Oxygen vacancies promoting photoelectrochemical performance of In2O3 nanocubes[J]. Scientific Reports, 2013, 3(1): 1-7. |
| 19 | Wang L, Ghoussoub M, Wang H, et al. Photocatalytic hydrogenation of carbon dioxide with high selectivity to methanol at atmospheric pressure[J]. Joule, 2018, 2(7): 1369-1381. |
| 20 | Shen C Y, Sun K H, Zhang Z T, et al. Highly active Ir/In2O3 catalysts for selective hydrogenation of CO2 to methanol: experimental and theoretical studies[J]. ACS Catalysis, 2021, 11(7): 4036-4046. |
| 21 | Frei M S, Mondelli C, García-Muelas R, et al. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation[J]. Nature Communications, 2019, 10(1): 1-11. |
| 22 | Rui N, Wang Z Y, Sun K H, et al. CO2 hydrogenation to methanol over Pd/In2O3: effects of Pd and oxygen vacancy[J]. Applied Catalysis B: Environmental, 2017, 218: 488-497. |
| 23 | 戴文华, 辛忠. Si掺杂对Cu/ZrO2催化CO2加氢制甲醇性能的影响[J]. 化工学报, 2022, 73(8): 3586-3596. |
| Dai W H, Xin Z. Effect of Si-doped Cu/ZrO2 on the performance of catalysts for CO2 hydrogenation to methanol[J]. CIESC Journal, 2022, 73(8): 3586-3596. | |
| 24 | Dang S S, Qin B, Yang Y, et al. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity[J]. Science Advances, 2020, 6(25): eaaz2060. |
| 25 | Han Z, Tang C Z, Wang J J, et al. Atomically dispersed Pt n + species as highly active sites in Pt/In2O3 catalysts for methanol synthesis from CO2 hydrogenation[J]. Journal of Catalysis, 2021, 394: 236-244. |
| 26 | Rui N, Zhang F, Sun K H, et al. Hydrogenation of CO2 to methanol on a Au δ +-In2O3– x catalyst[J]. ACS Catalysis, 2020, 10(19): 11307-11317. |
| 27 | Sun K H, Rui N, Zhang Z T, et al. A highly active Pt/In2O3 catalyst for CO2 hydrogenation to methanol with enhanced stability[J]. Green Chemistry, 2020, 22(15): 5059-5066. |
| 28 | Frei M S, Capdevila-Cortada M, García-Muelas R, et al. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide[J]. Journal of Catalysis, 2018, 361: 313-321. |
| 29 | Tsoukalou A, Abdala P M, Stoian D, et al. Structural evolution and dynamics of an In2O3 catalyst for CO2 hydrogenation to methanol: an operando XAS-XRD and in situ TEM study[J]. Journal of the American Chemical Society, 2019, 141(34): 13497-13505. |
| 30 | Cao A, Wang Z B, Li H, et al. Relations between surface oxygen vacancies and activity of methanol formation from CO2 hydrogenation over In2O3 surfaces[J]. ACS Catalysis, 2021, 11(3): 1780-1786. |
| 31 | Guharoy U, Le Saché E, Cai Q, et al. Understanding the role of Ni-Sn interaction to design highly effective CO2 conversion catalysts for dry reforming of methane[J]. Journal of CO2 Utilization, 2018, 27: 1-10. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 曹跃, 余冲, 李智, 杨明磊. 工业数据驱动的加氢裂化装置多工况切换过渡状态检测[J]. 化工学报, 2023, 74(9): 3841-3854. |
| [6] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [7] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [8] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [9] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [10] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [11] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [12] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [13] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [14] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [15] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号