化工学报 ›› 2024, Vol. 75 ›› Issue (1): 171-189.DOI: 10.11949/0438-1157.20230661
齐元帅1( ), 彭文朝1, 李阳1, 张凤宝1, 范晓彬1,2(
), 彭文朝1, 李阳1, 张凤宝1, 范晓彬1,2( )
)
收稿日期:2023-06-30
修回日期:2023-08-14
出版日期:2024-01-25
发布日期:2024-03-11
通讯作者:
范晓彬
作者简介:齐元帅(1998—),男,硕士研究生,qiyuanshuai@tju.edu.cn
基金资助:
Yuanshuai QI1( ), Wenchao PENG1, Yang LI1, Fengbao ZHANG1, Xiaobin FAN1,2(
), Wenchao PENG1, Yang LI1, Fengbao ZHANG1, Xiaobin FAN1,2( )
)
Received:2023-06-30
Revised:2023-08-14
Online:2024-01-25
Published:2024-03-11
Contact:
Xiaobin FAN
摘要:
电化学脱盐技术通过可逆的电化学过程实现离子固定化,是一种很有前途的节能水处理技术。有关脱盐机理的研究有助于深入了解离子传输和去除特性,进而为材料和电池的设计提供理论支持。根据电化学基本原理,可以将电化学脱盐机理分为电吸附机理与电荷转移机理两大类,后者包括氧化还原活性导电聚合物、离子插入(或插层)反应、转化反应以及氧化还原活性电解质。先进表征技术(包括原位X射线技术、原位波谱技术以及其他技术)和计算机建模与仿真(包括分子动力学模拟、密度泛函理论、有限元分析)在机理分析中起到关键作用。
中图分类号:
齐元帅, 彭文朝, 李阳, 张凤宝, 范晓彬. 电化学脱盐机理及相关研究进展[J]. 化工学报, 2024, 75(1): 171-189.
Yuanshuai QI, Wenchao PENG, Yang LI, Fengbao ZHANG, Xiaobin FAN. Research progress on electrochemical desalination mechanisms and related studies[J]. CIESC Journal, 2024, 75(1): 171-189.
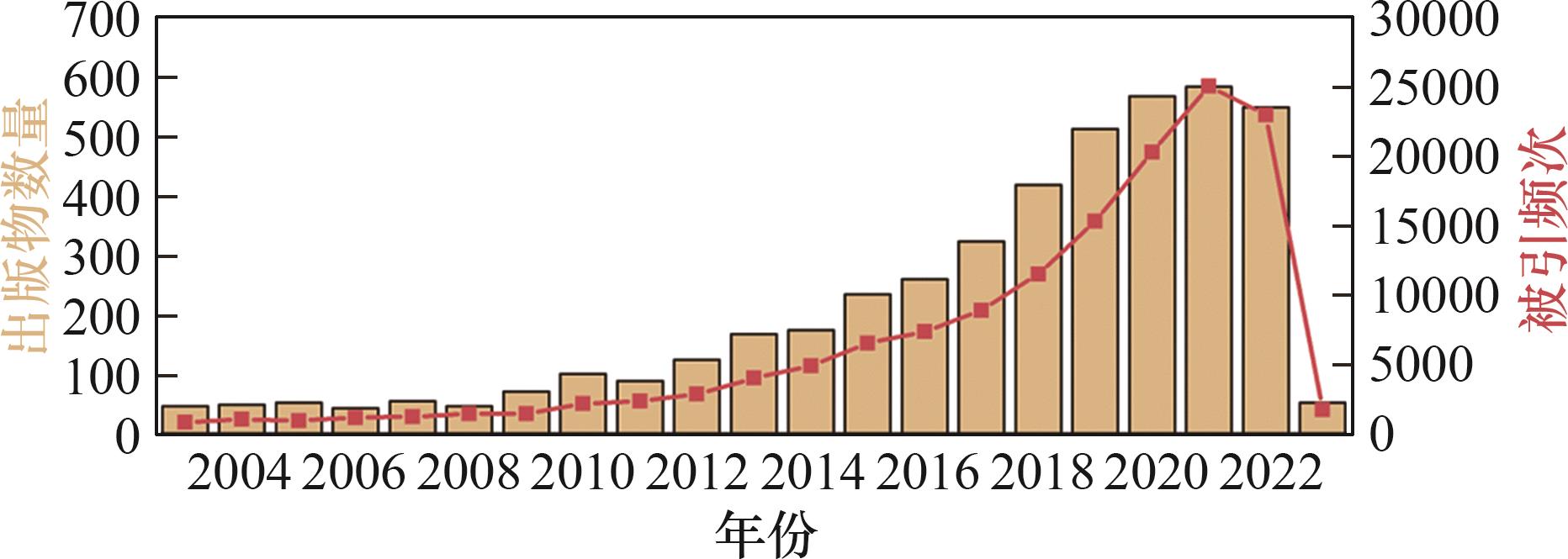
图1 过去20年Web of Science使用关键词“电容去离子”或“电吸附”的出版物和被引频次记录[16]
Fig.1 Record of publications and citations over the past 20 years using the keywords ‘capacitive deionization’ or ‘electrosorption’ from the Web of Science[16]
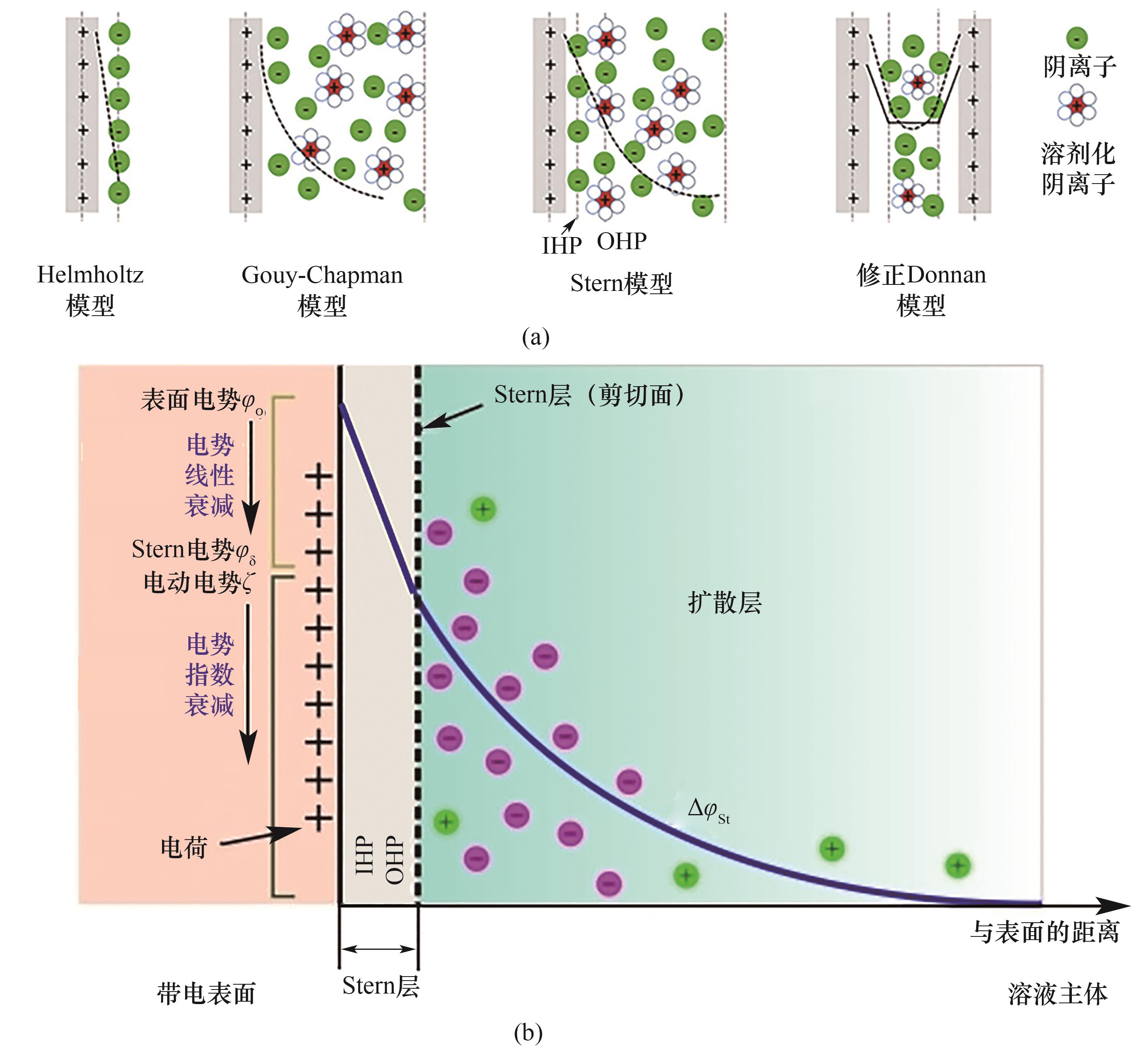
图3 (a) 描述带正电荷表面双电层结构的模型[20]; (b) 固-液界面处的双电层模型(以Stern模型为例)[28]
Fig.3 (a) Models for describing the structure of an electrical double layer at a positively charged surface[20]; (b) The model of electric double layer at the solid-liquid interface (taking the Stern model as an example)[28]
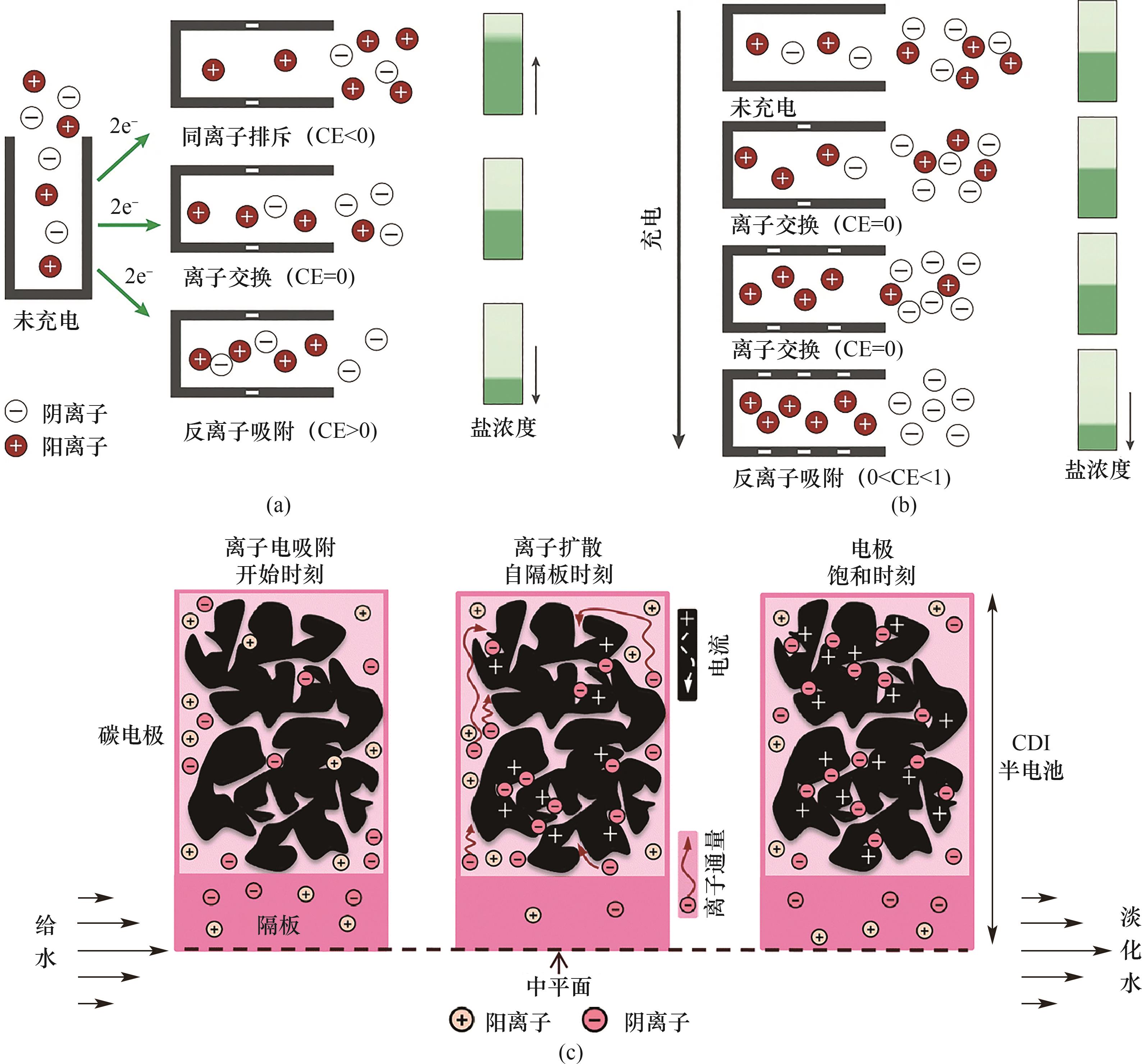
图5 (a) 离子电吸附过程的基本电荷补偿机理:未充电状态下的同离子排斥、离子交换和反离子吸附[26];(b) 随着电极电荷增加,电荷补偿发生演变:由未充电状态经两个离子交换过程到反离子吸附[26];(c) 随时间变化的二维多孔电极模型示意图[38]
Fig.5 (a) The fundamental mechanism of charge compensation during the ion electrosorption process: co-ion expulsion, ion exchange, and counterion adsorption in the uncharged state[26]; (b) The evolution of electric charge compensation upon increasing electrode charge, where two subsequent ion swapping events are followed by counterion adsorption[26]; (c) Schematic diagram of a two-dimensional porous electrode model that varies with time[38]
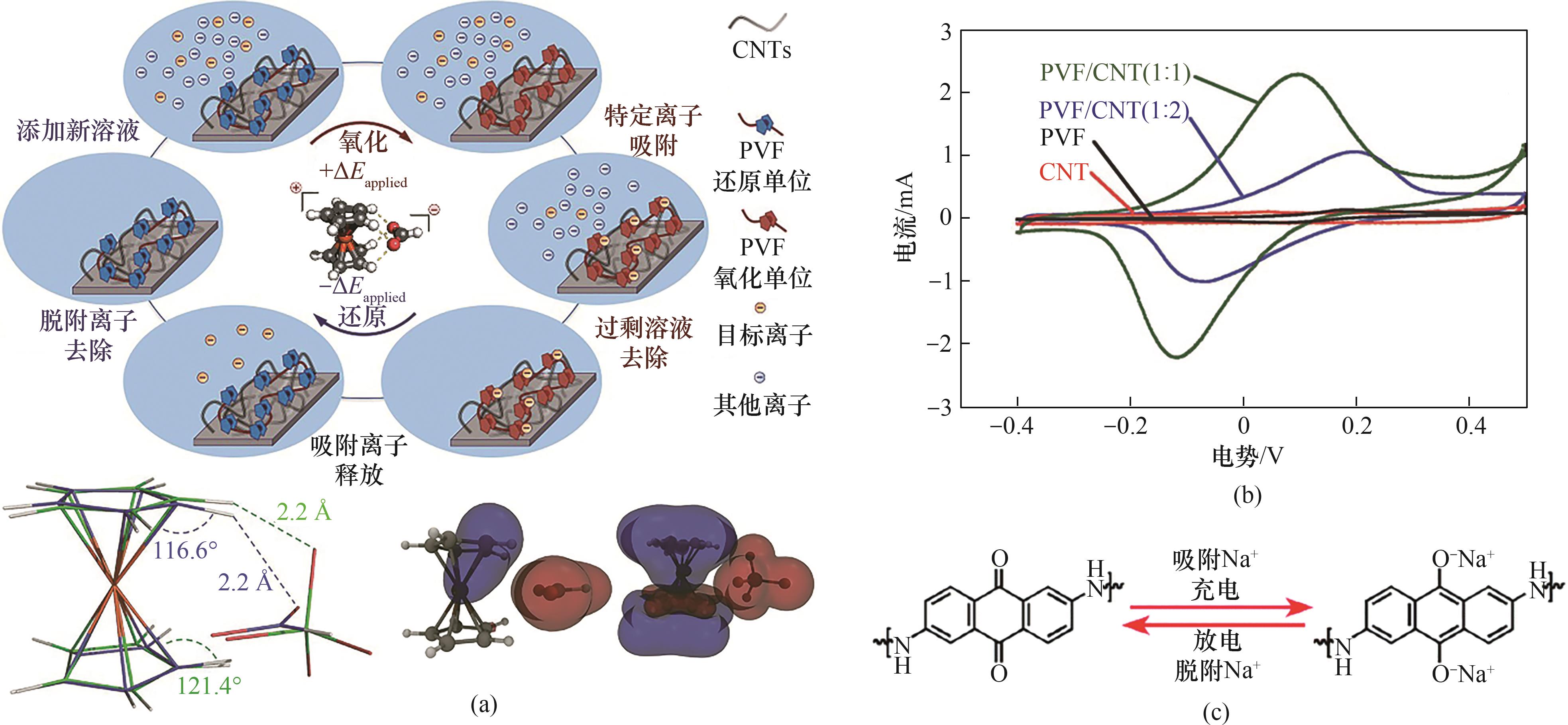
图6 (a) 通过定制表面基团的可逆电化学反应选择性去除离子[73];(b) 阴离子选择性氧化还原电极的电化学特征[73];(c) 引入氧化还原活性DAAQ单元和用于阳离子去除的电化学可逆醌/对苯二酚工艺[76] (1 Å=1×10-10 m)
Fig.6 (a) Selective ion removal through the reversible electrochemical reactions of tailored surface groups[73]; (b) Electrochemical characteristics of the anion-selective redox electrode[73]; (c) Introduction of redox-active DAAQ units and the electrochemically reversible quinone/hydroquinone process for cation removal[76]
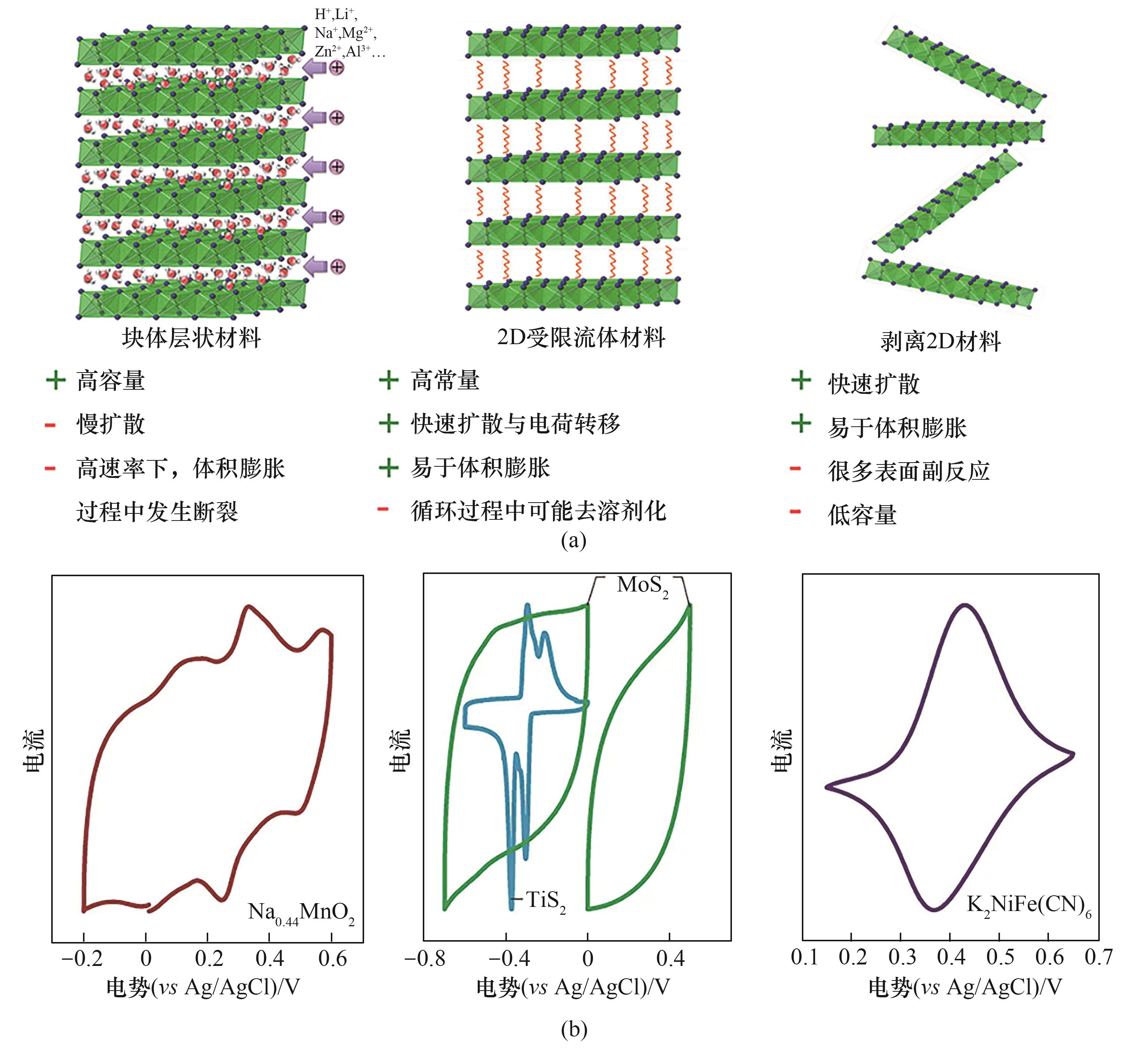
图7 离子插入型电极材料(块体层状材料、2D受限流体材料以及剥离2D材料)的优缺点(a)[78]及其电化学特征(b)[78, 83-84]
Fig.7 The advantages and disadvantages of ion-insertion-type electrode materials (a)[78] and their electrochemical characteristics (b)[78, 83-84]
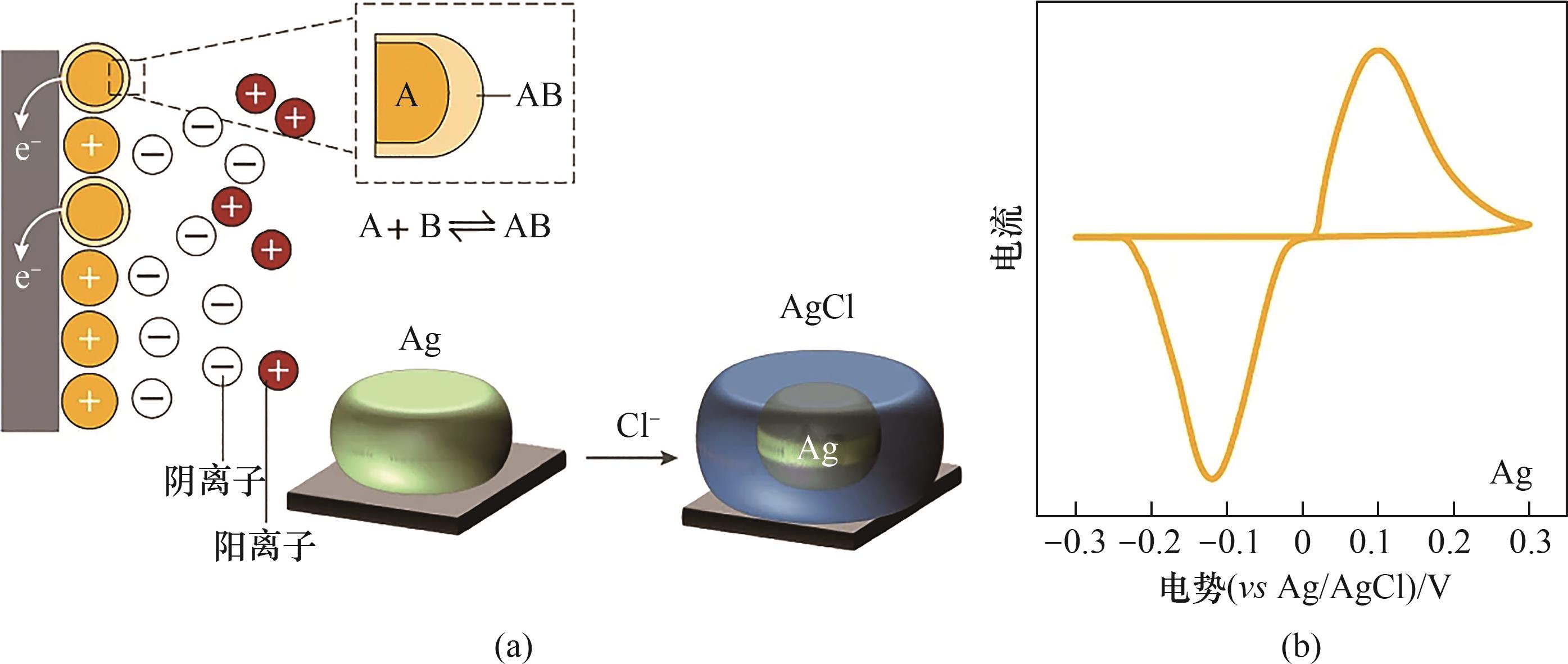
图8 以Ag/AgCl反应为例的离子转化型电极材料(a)及其电化学特征(b)[103]
Fig.8 Ion-conversion-type electrode materials (a) and the electrochemical characteristics (b), as exemplified for the Ag/AgCl reaction[103]
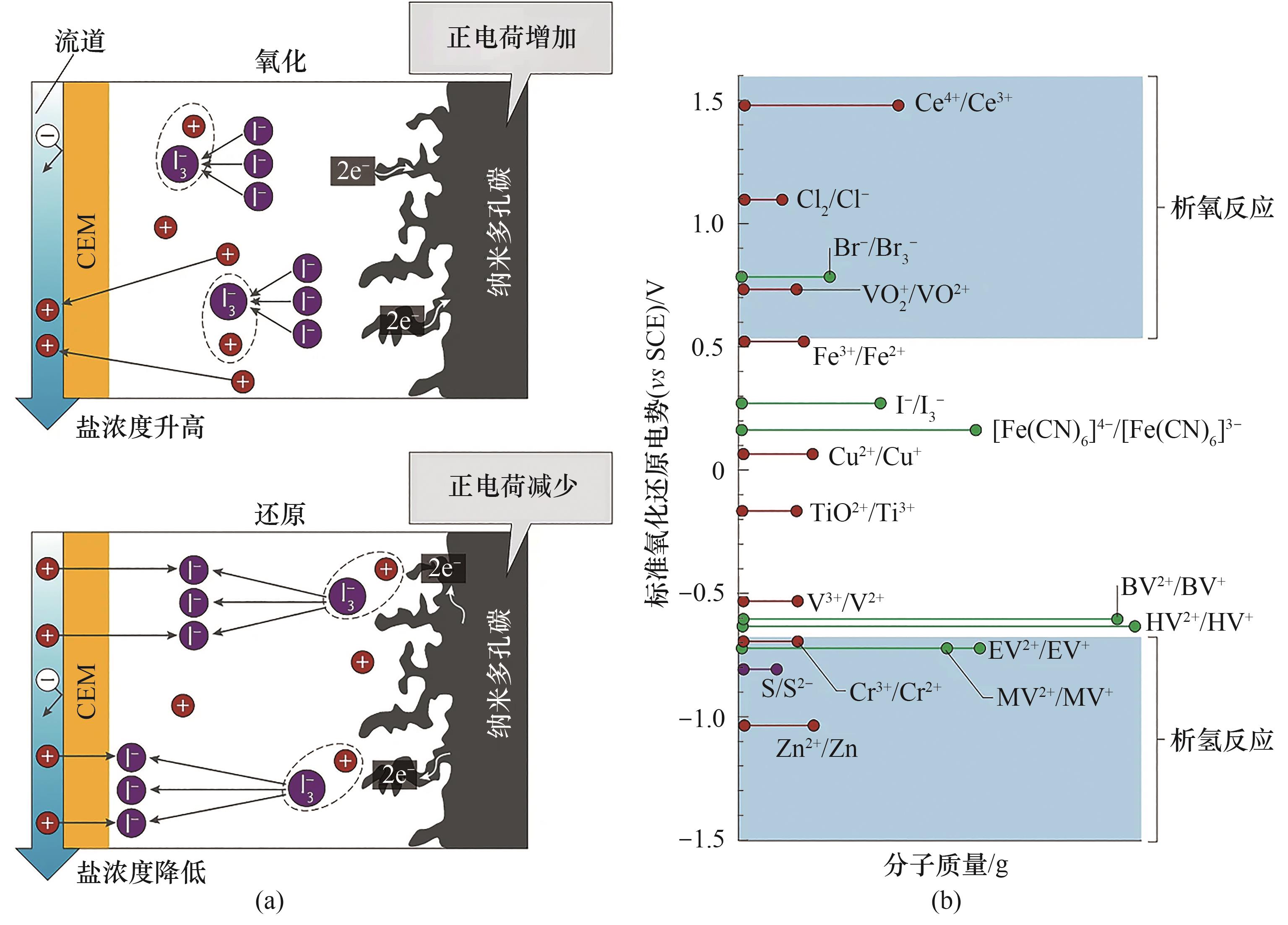
图9 (a) 氧化还原活性电解质的电化学脱盐机理[111];(b) 各种阴极电解质和阳极电解质的氧化还原电对的标准氧化还原电势[112]
Fig.9 (a) Electrochemical desalination mechanisms with redox-active electrolytes[111]; (b) Standard redox potentials of various catholyte and anolyte redox couples[112]

图10 原位XRD技术和原位SAXS技术在电化学脱盐机理研究中的应用[114, 116, 118]
Fig.10 Application of in situ XRD and in situ SAXS in the study of electrochemical desalination mechanisms[114, 116, 118]
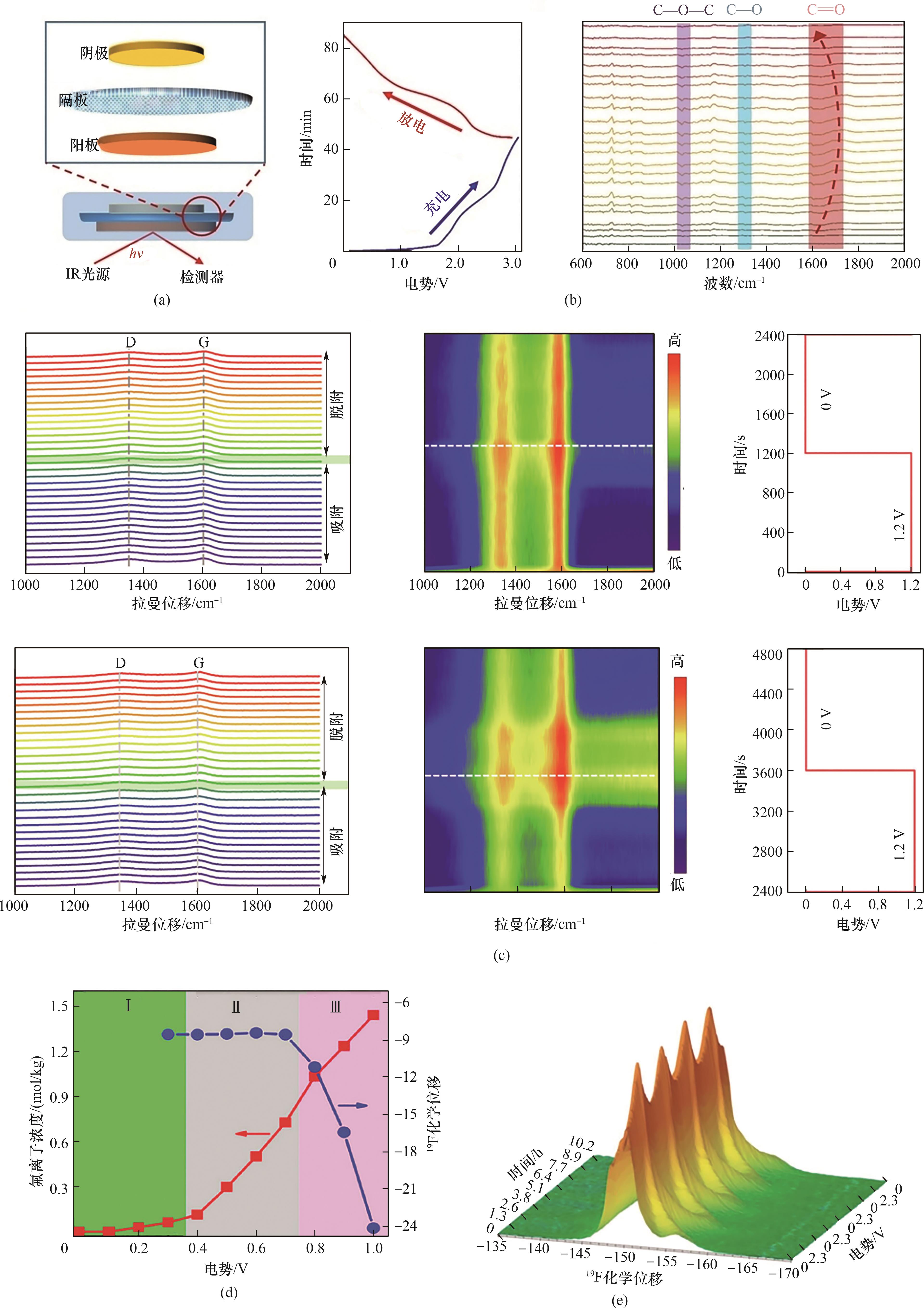
图11 原位FTIR技术、原位Raman技术和原位NMR技术在电化学脱盐机理研究中的应用[120, 123, 128-129]
Fig.11 Application of in situ FTIR, in situ Raman and in situ NMR in the study of electrochemical desalination mechanisms[120, 123, 128-129]
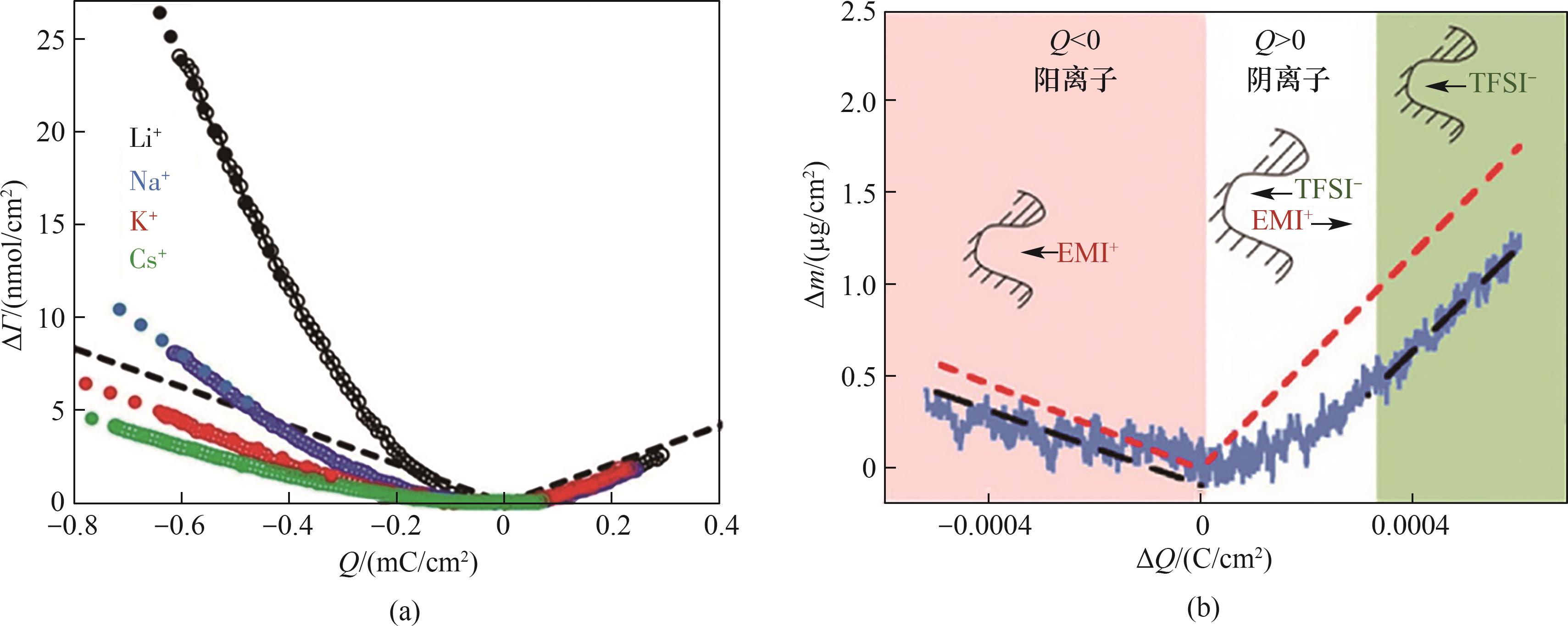
图12 原位电化学石英晶体微天平在电化学脱盐机理研究中的应用[131, 134]
Fig.12 Application of in situ electrochemical quartz crystal microbalance in the study of electrochemical desalination mechanisms[131, 134]
| 1 | Vörösmarty C J, Green P, Salisbury J, et al. Global water resources: vulnerability from climate change and population growth[J]. Science, 2000, 289(5477): 284-288. |
| 2 | Ercin A E, Hoekstra A Y. Water footprint scenarios for 2050: a global analysis[J]. Environment International, 2014, 64: 71-82. |
| 3 | Gleick P H, Gilbert F W. Water in Crisis: A Guide to the World’s Fresh Water Resources[M]. New York: Oxford University Press, 1993. |
| 4 | Oren Y. Capacitive deionization (CDI) for desalination and water treatment—past, present and future (a review)[J]. Desalination, 2008, 228(1/2/3): 10-29. |
| 5 | Porada S, Zhao R, van der Wal A, et al. Review on the science and technology of water desalination by capacitive deionization[J]. Progress in Materials Science, 2013, 58(8): 1388-1442. |
| 6 | Blair J W, Murphy G W. Electrochemical demineralization of water with porous electrodes of large surface area[M]//Advances in Chemistry. Washington, D. C.: American Chemical Society, 1960: 206-223. |
| 7 | Murphy G W, Caudle D D. Mathematical theory of electrochemical demineralization in flowing systems[J]. Electrochimica Acta, 1967, 12(12): 1655-1664. |
| 8 | Evans S, Hamilton W S. The mechanism of demineralization at carbon electrodes[J]. Journal of The Electrochemical Society, 1966, 113(12): 1314-1319. |
| 9 | Evans S, Accomazzo M A, Accomazzo J E. Electrochemically controlled ion exchange (Ⅰ): Mechanism[J]. Journal of the Electrochemical Society, 1969, 116(2): 307. |
| 10 | Accomazzo M A, Evans S. Electrochemically controlled ion exchange (Ⅱ): Transport processes[J]. Journal of the Electrochemical Society, 1969, 116(2): 309. |
| 11 | Johnson A M, Newman J. Desalting by means of porous carbon electrodes[J]. Journal of the Electrochemical Society, 1971, 118(3): 510-517. |
| 12 | Soffer A, Folman M. The electrical double layer of high surface porous carbon electrode[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1972, 38(1): 25-43. |
| 13 | Richardson J H, Farmer J C, Fix D V, et al. Desalting in wastewater reclamation using capacitive deionization with carbon aerogel electrodes[C]// American Desalting Association 1996 Biennial Conference and Exposition. Washington, DC (United States), 1996. |
| 14 | Farmer J C, Fix D V, Mack G V, et al. Capacitive deionization of NaCl and NaNO3 solutions with carbon aerogel electrodes[J]. Journal of the Electrochemical Society, 1996, 143(1): 159-169. |
| 15 | Farmer J C, Fix D V, Mack G V, et al. Capacitive deionization of NH4ClO4 solutions with carbon aerogel electrodes[J]. Journal of Applied Electrochemistry, 1996, 26(10): 1007-1018. |
| 16 | Xu X T, Eguchi M, Asakura Y, et al. Meta-organic framework derivatives for promoted capacitive deionization of oxygenated saline water[J]. Energy & Environmental Science, 2023, 16(5): 1815-1820. |
| 17 | Srimuk P, Su X, Yoon J, et al. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements[J]. Nature Reviews Materials, 2020, 5(7): 517-538. |
| 18 | 武婷婷. 碳材料的表面改性及其电容去离子性能研究[D]. 大连: 大连理工大学, 2017. |
| Wu T T. Surface modification of carbon materials for capacitive deionization[D]. Dalian: Dalian University of Technology, 2017. | |
| 19 | 王世轩, 蔡延萌, 徐世昌, 等. 聚间苯二胺/碳纳米管复合材料制备及其电容法脱盐研究[J]. 化学工业与工程, 2022, 39(2): 90-99. |
| Wang S X, Cai Y M, Xu S C, et al. Preparation and performance test of poly-m-phenylene diamine and CNT composite material in capacitive deionization process[J]. Chemical Industry and Engineering, 2022, 39(2): 90-99. | |
| 20 | Sun K G, Tebyetekerwa M, Wang C, et al. Electrocapacitive deionization: mechanisms, electrodes, and cell designs[J]. Advanced Functional Materials, 2023, 33(18): 2213578. |
| 21 | Liu E Y, Lee L Y, Ong S L, et al. Treatment of industrial brine using capacitive deionization (CDI) towards zero liquid discharge—challenges and optimization[J]. Water Research, 2020, 183: 116059. |
| 22 | Bone S E, Steinrück H G, Toney M F. Advanced characterization in clean water technologies[J]. Joule, 2020, 4(8): 1637-1659. |
| 23 | Sharma N, Peterson V K. In situ neutron powder diffraction studies of lithium-ion batteries[J]. Journal of Solid State Electrochemistry, 2012, 16(5): 1849-1856. |
| 24 | Dixit M B, Park J S, Kenesei P, et al. Status and prospect of in situ and operando characterization of solid-state batteries[J]. Energy & Environmental Science, 2021, 14(9): 4672-4711. |
| 25 | Atkins D, Ayerbe E, Benayad A, et al. Understanding battery interfaces by combined characterization and simulation approaches: challenges and perspectives[J]. Advanced Energy Materials, 2022, 12(17): 2102687. |
| 26 | Suss M E, Porada S, Sun X, et al. Water desalination via capacitive deionization: what is it and what can we expect from it?[J]. Energy & Environmental Science, 2015, 8(8): 2296-2319. |
| 27 | Béguin F, Frąckowiak E. 超级电容器:材料、系统及应用[M]. 北京: 机械工业出版社, 2014. |
| Béguin F, Frąckowiak E. Supercapacitors: Materials, Systems, and Applications[M]. Beijing: China Machine Press, 2014. | |
| 28 | Kumar S, Aldaqqa N M, Alhseinat E, et al. Electrode materials for desalination of water via capacitive deionization[J]. Angewandte Chemie International Edition, 2023: e202302180. |
| 29 | Helmholtz H. Studien über electrische grenzschichten[J]. Annalen Der Physik, 1879, 243(7): 337-382. |
| 30 | Gouy M. Sur la constitution de la charge électrique à la surface d’un électrolyte[J]. Journal De Physique Théorique et Appliquée, 1910, 9(1): 457-468. |
| 31 | Chapman D L. A contribution to the theory of electrocapillarity[J]. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 1913, 25(148): 475-481. |
| 32 | Stern O. Zur theorie der elektrolytischen doppelschicht[J]. Zeitschrift Für Elektrochemie Und Angewandte Physikalische Chemie, 1924, 30(21/22): 508-516. |
| 33 | Biesheuvel P M, Fu Y, Bazant M Z. Electrochemistry and capacitive charging of porous electrodes in asymmetric multicomponent electrolytes[J]. Russian Journal of Electrochemistry, 2012, 48(6): 580-592. |
| 34 | Zhao R, Biesheuvel P M, van der Wal A. Energy consumption and constant current operation in membrane capacitive deionization[J]. Energy & Environmental Science, 2012, 5(11): 9520-9527. |
| 35 | Biesheuvel P M, Fu Y Q, Bazant M Z. Diffuse charge and Faradaic reactions in porous electrodes[J]. Physical Review E, 2011, 83(6): 061507. |
| 36 | Biesheuvel P M, Porada S, Levi M, et al. Attractive forces in microporous carbon electrodes for capacitive deionization[J]. Journal of Solid State Electrochemistry, 2014, 18(5): 1365-1376. |
| 37 | Biesheuvel P M, Zhao R, Porada S, et al. Theory of membrane capacitive deionization including the effect of the electrode pore space[J]. Journal of Colloid and Interface Science, 2011, 360(1): 239-248. |
| 38 | Porada S, Borchardt L, Oschatz M, et al. Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization[J]. Energy & Environmental Science, 2013, 6(12): 3700-3712. |
| 39 | Porada S, Bryjak M, van der Wal A, et al. Effect of electrode thickness variation on operation of capacitive deionization[J]. Electrochimica Acta, 2012, 75: 148-156. |
| 40 | Biesheuvel P M, Bazant M Z. Nonlinear dynamics of capacitive charging and desalination by porous electrodes[J]. Physical Review E, 2010, 81(3): 031502. |
| 41 | Kim T, Dykstra J E, Porada S, et al. Enhanced charge efficiency and reduced energy use in capacitive deionization by increasing the discharge voltage[J]. Journal of Colloid and Interface Science, 2015, 446: 317-326. |
| 42 | 刘红, 王刚, 王六平, 等. 电容去离子脱盐技术: 离子交换膜复合活性炭电极的性能[J]. 化工学报, 2012, 63(5): 1512-1516. |
| Liu H, Wang G, Wang L P, et al. Capacitive deionization (CDI) technology for desalination of sea water: properties of carbon electrode materials made of activated carbon and ion-exchange membranes[J]. CIESC Journal, 2012, 63(5): 1512-1516. | |
| 43 | 王刚, 车小平, 汪仕勇, 等. 水溶性带电聚合物黏结剂修饰炭电极用于增强电容去离子性能[J]. 化工学报, 2022, 73(4): 1763-1771. |
| Wang G, Che X P, Wang S Y, et al. Carbon electrodes modified with water-soluble charged polymer binder for enhanced capacitive deionization performance[J]. CIESC Journal, 2022, 73(4): 1763-1771. | |
| 44 | Wang G, Pan C, Wang L P, et al. Activated carbon nanofiber webs made by electrospinning for capacitive deionization[J]. Electrochimica Acta, 2012, 69: 65-70. |
| 45 | Fleischmann S, Mitchell J B, Wang R C, et al. Pseudocapacitance: from fundamental understanding to high power energy storage materials[J]. Chemical Reviews, 2020, 120(14): 6738-6782. |
| 46 | Shao H, Wu Y C, Lin Z F, et al. Nanoporous carbon for electrochemical capacitive energy storage[J]. Chemical Society Reviews, 2020, 49(10): 3005-3039. |
| 47 | Conway B E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications[M]. New York: Plenum Press, 1999. |
| 48 | Huang J S, Sumpter B G, Meunier V. Theoretical model for nanoporous carbon supercapacitors[J]. Angewandte Chemie International Edition, 2008, 47(3): 520-524. |
| 49 | Huang J S, Sumpter B G, Meunier V. A universal model for nanoporous carbon supercapacitors applicable to diverse pore regimes, carbon materials, and electrolytes[J]. Chemistry-A European Journal, 2008, 14(22): 6614-6626. |
| 50 | Largeot C, Portet C, Chmiola J, et al. Relation between the ion size and pore size for an electric double-layer capacitor[J]. Journal of the American Chemical Society, 2008, 130(9): 2730-2731. |
| 51 | Ania C O, Pernak J, Stefaniak F, et al. Polarization-induced distortion of ions in the pores of carbon electrodes for electrochemical capacitors[J]. Carbon, 2009, 47(14): 3158-3166. |
| 52 | de Levie R. On porous electrodes in electrolyte solutions (Ⅰ): Capacitance effects[J]. Electrochimica Acta, 1963, 8(10): 751-780. |
| 53 | Newman J S, Balsara N P. Electrochemical Systems[M]. John Wiley & Sons, 2021. |
| 54 | Porada S, Weinstein L, Dash R, et al. Water desalination using capacitive deionization with microporous carbon electrodes[J]. ACS Applied Materials & Interfaces, 2012, 4(3): 1194-1199. |
| 55 | Schipper F, Nayak P K, Erickson E M, et al. Study of cathode materials for lithium-ion batteries: recent progress and new challenges[J]. Inorganics, 2017, 5(2): 32. |
| 56 | Li H, Zou L, Pan L, et al. Novel graphene-like electrodes for capacitive deionization[J]. Environmental Science & Technology, 2010, 44(22): 8692-8697. |
| 57 | Chang L M, Li J R, Duan X Y, et al. Porous carbon derived from metal-organic framework (MOF) for capacitive deionization electrode[J]. Electrochimica Acta, 2015, 176: 956-964. |
| 58 | Wang Z M, Xu X T, Kim J, et al. Nanoarchitectured metal-organic framework/polypyrrole hybrids for brackish water desalination using capacitive deionization[J]. Materials Horizons, 2019, 6(7): 1433-1437. |
| 59 | Yan C J, Zou L D, Short R. Single-walled carbon nanotubes and polyaniline composites for capacitive deionization[J]. Desalination, 2012, 290: 125-129. |
| 60 | Zhang Y, Prehal C, Jiang H L, et al. Ionophobicity of carbon sub-nanometer pores enables efficient desalination at high salinity[J]. Cell Reports Physical Science, 2022, 3(1): 100689. |
| 61 | Kim C, Lee J H, Srimuk P, et al. Concentration-gradient multichannel flow-stream membrane capacitive deionization cell for high desalination capacity of carbon electrodes[J]. ChemSusChem, 2017, 10(24): 4914-4920. |
| 62 | Kim C, Srimuk P, Lee J H, et al. Semi-continuous capacitive deionization using multi-channel flow stream and ion exchange membranes[J]. Desalination, 2018, 425: 104-110. |
| 63 | Kang J S, Kim S, Chung D Y, et al. Rapid inversion of surface charges in heteroatom-doped porous carbon: a route to robust electrochemical desalination[J]. Advanced Functional Materials, 2020, 30(9): 1909387. |
| 64 | Peng C, Zhang S W, Jewell D, et al. Carbon nanotube and conducting polymer composites for supercapacitors[J]. Progress in Natural Science, 2008, 18(7): 777-788. |
| 65 | Ahualli S, Iglesias G R, Fernández M M, et al. Use of soft electrodes in capacitive deionization of solutions[J]. Environmental Science & Technology, 2017, 51(9): 5326-5333. |
| 66 | Kong H, Yang M, Miao Y C, et al. Polypyrrole as a novel chloride-storage electrode for seawater desalination[J]. Energy Technology, 2019, 7(11): 1900835. |
| 67 | Park J H, Park O O, Shin K H, et al. An electrochemical capacitor based on a Ni(OH)2/activated carbon composite electrode[J]. Electrochemical and Solid-State Letters, 2002, 5(2): H7-H10. |
| 68 | Zhao Y, Lai Q Y, Hao Y J, et al. Study of electrochemical performance for AC/(Ni1/3Co1/3Mn1/3)(OH)2 [J]. Journal of Alloys and Compounds, 2009, 471(1/2): 466-469. |
| 69 | Ren Y Y, Mao X W, Hatton T A. An asymmetric electrochemical system with complementary tunability in hydrophobicity for selective separations of organics[J]. ACS Central Science, 2019, 5(8): 1396-1406. |
| 70 | Raudsepp T, Marandi M, Tamm T, et al. Influence of ion-exchange on the electrochemical properties of polypyrrole films[J]. Electrochimica Acta, 2014, 122: 79-86. |
| 71 | Kim Y, Lin Z, Jeon I, et al. Polyaniline nanofiber electrodes for reversible capture and release of mercury(Ⅱ) from water[J]. Journal of the American Chemical Society, 2018, 140(43): 14413-14420. |
| 72 | Cui H, Li Q, Qian Y, et al. Defluoridation of water via electrically controlled anion exchange by polyaniline modified electrode reactor[J]. Water Research, 2011, 45(17): 5736-5744. |
| 73 | Su X, Kulik H J, Jamison T F, et al. Anion-selective redox electrodes: electrochemically mediated separation with heterogeneous organometallic interfaces[J]. Advanced Functional Materials, 2016, 26(20): 3394-3404. |
| 74 | Su X, Tan K J, Elbert J, et al. Asymmetric Faradaic systems for selective electrochemical separations[J]. Energy & Environmental Science, 2017, 10(5): 1272-1283. |
| 75 | Su X, Kushima A, Halliday C, et al. Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water[J]. Nature Communications, 2018, 9(1): 4701. |
| 76 | Li Y Q, Ding Z B, Zhang X L, et al. Novel hybrid capacitive deionization constructed by a redox-active covalent organic framework and its derived porous carbon for highly efficient desalination[J]. Journal of Materials Chemistry A, 2019, 7(44): 25305-25313. |
| 77 | Huggins R A. Advanced Batteries: Materials Science Aspects[M]. New York: Springer, 2008. |
| 78 | Augustyn V, Gogotsi Y. 2D materials with nanoconfined fluids for electrochemical energy storage[J]. Joule, 2017, 1(3): 443-452. |
| 79 | Guo L, Huang Y X, Ding M, et al. A high performance electrochemical deionization method to desalinate brackish water with an FePO4/RGO nanocomposite[J]. Journal of Materials Chemistry A, 2018, 6(19): 8901-8908. |
| 80 | Chayambuka K, Mulder G, Danilov D L, et al. Sodium-ion battery materials and electrochemical properties reviewed[J]. Advanced Energy Materials, 2018, 8(16): 1800079. |
| 81 | Meng J S, Guo H C, Niu C J, et al. Advances in structure and property optimizations of battery electrode materials[J]. Joule, 2017, 1(3): 522-547. |
| 82 | Sun Y, Zhao L, Pan H L, et al. Direct atomic-scale confirmation of three-phase storage mechanism in Li4Ti5O12 anodes for room-temperature sodium-ion batteries[J]. Nature Communications, 2013, 4: 1870. |
| 83 | Srimuk P, Lee J H, Fleischmann S, et al. Faradaic deionization of brackish and sea water via pseudocapacitive cation and anion intercalation into few-layered molybdenum disulfide[J]. Journal of Materials Chemistry A, 2017, 5(30): 15640-15649. |
| 84 | Srimuk P, Lee J H, Budak Ö, et al. In situ tracking of partial sodium desolvation of materials with capacitive, pseudocapacitive, and battery-like charge/discharge behavior in aqueous electrolytes[J]. Langmuir, 2018, 34(44): 13132-13143. |
| 85 | Mathis T S, Kurra N, Wang X H, et al. Energy storage data reporting in perspective—guidelines for interpreting the performance of electrochemical energy storage systems[J]. Advanced Energy Materials, 2019, 9(39): 1902007. |
| 86 | Ridley P, Andris R, Pomerantseva E A. HCDI performance of Na-2×3 and Na-2×4 nanowires for water desalination[C]//SPIE Nanoscience + Engineering. San Diego, California, USA, 2019, 11085: 142-149. |
| 87 | Nayak P K, Yang L T, Brehm W, et al. From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises[J]. Angewandte Chemie International Edition, 2018, 57(1): 102-120. |
| 88 | Lee J H, Srimuk P, Zwingelstein R, et al. Sodium ion removal by hydrated vanadyl phosphate for electrochemical water desalination[J]. Journal of Materials Chemistry A, 2019, 7(8): 4175-4184. |
| 89 | Zhu Y, Peng L L, Chen D H, et al. Intercalation pseudocapacitance in ultrathin VOPO4 nanosheets: toward high-rate alkali-ion-based electrochemical energy storage[J]. Nano Letters, 2016, 16(1): 742-747. |
| 90 | Anasori B, Lukatskaya M R, Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage[J]. Nature Reviews Materials, 2017, 2(2): 16098. |
| 91 | Naguib M, Mashtalir O, Carle J, et al. Two-dimensional transition metal carbides[J]. ACS Nano, 2012, 6(2): 1322-1331. |
| 92 | Tan C L, Cao X H, Wu X J, et al. Recent advances in ultrathin two-dimensional nanomaterials[J]. Chemical Reviews, 2017, 117(9): 6225-6331. |
| 93 | Chen Z Q, Xu X T, Liu Y, et al. Ultra-durable and highly-efficient hybrid capacitive deionization by MXene confined MoS2 heterostructure[J]. Desalination, 2022, 528: 115616. |
| 94 | Srimuk P, Halim J, Lee J H, et al. Two-dimensional molybdenum carbide (MXene) with divacancy ordering for brackish and seawater desalination via cation and anion intercalation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(3): 3739-3747. |
| 95 | Huang S H, Mochalin V N. Hydrolysis of 2D transition-metal carbides (MXenes) in colloidal solutions[J]. Inorganic Chemistry, 2019, 58(3): 1958-1966. |
| 96 | He H, Lu P F, Wu L Y, et al. Structural properties and phase transition of Na adsorption on monolayer MoS2 [J]. Nanoscale Research Letters, 2016, 11(1): 330. |
| 97 | Wang Q H, Kalantar-Zadeh K, Kis A, et al. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides[J]. Nature Nanotechnology, 2012, 7(11): 699-712. |
| 98 | Wang X Z, Yao Z P, Hwang S, et al. In situ electron microscopy investigation of sodiation of titanium disulfide nanoflakes[J]. ACS Nano, 2019, 13(8): 9421-9430. |
| 99 | Paulitsch B, Yun J, Bandarenka A S. Electrodeposited Na2VO x [Fe(CN)6] films as a cathode material for aqueous Na-ion batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(9): 8107-8112. |
| 100 | Masquelier C, Croguennec L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries[J]. Chemical Reviews, 2013, 113(8): 6552-6591. |
| 101 | Scrosati B, Hassoun J, Sun Y K. Lithium-ion batteries. A look into the future[J]. Energy & Environmental Science, 2011, 4(9): 3287-3295. |
| 102 | Armand M, Tarascon J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. |
| 103 | Srimuk P, Husmann S, Presser V. Low voltage operation of a silver/silver chloride battery with high desalination capacity in seawater[J]. RSC Advances, 2019, 9(26): 14849-14858. |
| 104 | Hao Z W, Sun X Q, Chen J B, et al. Recent progress and challenges in Faradic capacitive desalination: from mechanism to performance[J]. Small, 2023, 19(33): e2300253. |
| 105 | Grygolowicz-Pawlak E, Sohail M, Pawlak M, et al. Coulometric sodium chloride removal system with nafion membrane for seawater sample treatment[J]. Analytical Chemistry, 2012, 84(14): 6158-6165. |
| 106 | Nam D H, Choi K S. Bismuth as a new chloride-storage electrode enabling the construction of a practical high capacity desalination battery[J]. Journal of the American Chemical Society, 2017, 139(32): 11055-11063. |
| 107 | Lee J H, Srimuk P, Fleischmann S, et al. Redox-electrolytes for non-flow electrochemical energy storage: a critical review and best practice[J]. Progress in Materials Science, 2019, 101: 46-89. |
| 108 | Narayanan R, Bandaru P R. High rate capacity through redox electrolytes confined in macroporous electrodes[J]. Journal of the Electrochemical Society, 2014, 162(1): A86-A91. |
| 109 | Chen L B, Bai H, Huang Z F, et al. Mechanism investigation and suppression of self-discharge in active electrolyte enhanced supercapacitors[J]. Energy & Environmental Science, 2014, 7(5): 1750-1759. |
| 110 | Lee J H, Krüner B, Tolosa A, et al. Tin/vanadium redox electrolyte for battery-like energy storage capacity combined with supercapacitor-like power handling[J]. Energy & Environmental Science, 2016, 9(11): 3392-3398. |
| 111 | Lee J H, Srimuk P, Zornitta R L, et al. High electrochemical seawater desalination performance enabled by an iodide redox electrolyte paired with a sodium superionic conductor[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(11): 10132-10142. |
| 112 | Chun S E, Evanko B, Wang X F, et al. Design of aqueous redox-enhanced electrochemical capacitors with high specific energies and slow self-discharge[J]. Nature Communications, 2015, 6(1): 7818. |
| 113 | 蓝闽波. 纳米材料测试技术[M]. 上海: 华东理工大学出版社, 2009. |
| Lan M B. Nano-Material Testing Technology[M]. Shanghai: East China University of Science and Technology Press, 2009. | |
| 114 | Shi W H, Liu X Y, Deng T Q, et al. Enabling superior sodium capture for efficient water desalination by a tubular polyaniline decorated with prussian blue nanocrystals[J]. Advanced Materials, 2020, 32(33):1907404. |
| 115 | Vafakhah S, Saeedikhani M, Huang S Z, et al. Tungsten disulfide-reduced GO/CNT aerogel: a tuned interlayer spacing anode for efficient water desalination[J]. Journal of Materials Chemistry A, 2021, 9(17): 10758-10768. |
| 116 | Vafakhah S, Saeedikhani M, Tanhaei M, et al. An energy efficient bi-functional electrode for continuous cation-selective capacitive deionization[J]. Nanoscale, 2020, 12(45): 22917-22927. |
| 117 | Chang W T, Chen P A, Chen W R, et al. Simultaneous capacitive deionisation and disinfection of saltwater by Ag@C/rGO electrodes[J]. Environmental Chemistry, 2022, 18(8): 352-359. |
| 118 | Prehal C, Weingarth D, Perre E, et al. Tracking the structural arrangement of ions in carbon supercapacitor nanopores using in situ small-angle X-ray scattering[J]. Energy & Environmental Science, 2015, 8(6): 1725-1735. |
| 119 | Jiang Q, Zhang L Q, Wang H L, et al. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3- based perovskite solar cells[J]. Nature Energy, 2017, 2: 16177. |
| 120 | Li J, Han C J, Ou X W, et al. Concentrated electrolyte for high-performance Ca-ion battery based on organic anode and graphite cathode[J]. Angewandte Chemie International Edition, 2022, 61(14): e202116668. |
| 121 | Richey F W, Tran C, Kalra V, et al. Ionic liquid dynamics in nanoporous carbon nanofibers in supercapacitors measured with in operando infrared spectroelectrochermistry[J]. The Journal of Physical Chemistry C, 2014, 118(38): 21846-21855. |
| 122 | Papaderakis A A, Ejigu A, Yang J, et al. Anion intercalation into graphite drives surface wetting[J]. Journal of the American Chemical Society, 2023, 145(14): 8007-8020. |
| 123 | Sun K G, Wang C, Tebyetekerwa M, et al. Electrocapacitive desalination with nitrogen-doped hierarchically structured carbon prepared using a sustainable salt-template method[J]. Chemical Engineering Journal, 2022, 446: 137211. |
| 124 | Mao M L, Yan T T, Chen G R, et al. Selective capacitive removal of Pb2+ from wastewater over redox-active electrodes[J]. Environmental Science & Technology, 2021, 55(1): 730-737. |
| 125 | Wang G Z, Yan T T, Shen J J, et al. Beneficial synergy of adsorption-intercalation-conversion mechanisms in Nb2O5@nitrogen-doped carbon frameworks for promoted removal of metal ions via hybrid capacitive deionization[J]. Environmental Science. Nano, 2021, 8(1): 122-130. |
| 126 | Zhou R J, Li J X, Wei W H, et al. Atomic substituents effect on boosting desalination performances of Zn-doped Na x CoO2 [J]. Desalination, 2020, 496: 114695. |
| 127 | Han J L, Yan T T, Shen J J, et al. Capacitive deionization of saline water by using MoS2-graphene hybrid electrodes with high volumetric adsorption capacity[J]. Environmental Science & Technology, 2019, 53(21): 12668-12676. |
| 128 | Wang H, Forse A C, Griffin J M, et al. In situ NMR spectroscopy of supercapacitors: insight into the charge storage mechanism[J]. Journal of the American Chemical Society, 2013, 135(50): 18968-18980. |
| 129 | Luo Z X, Xing Y Z, Liu S B, et al. Dehydration of ions in voltage-gated carbon nanopores observed by in situ NMR[J]. The Journal of Physical Chemistry Letters, 2015, 6(24): 5022-5026. |
| 130 | Levi M D, Salitra G, Levy N, et al. Application of a quartz-crystal microbalance to measure ionic fluxes in microporous carbons for energy storage[J]. Nature Materials, 2009, 8(11): 872-875. |
| 131 | Levi M D, Sigalov S, Aurbach D, et al. In situ electrochemical quartz crystal admittance methodology for tracking compositional and mechanical changes in porous carbon electrodes[J]. The Journal of Physical Chemistry C, 2013, 117(29): 14876-14889. |
| 132 | Shpigel N, Levi M D, Sigalov S, et al. Novel in situ multiharmonic EQCM-D approach to characterize complex carbon pore architectures for capacitive deionization of brackish water[J]. Journal of Physics: Condensed Matter, 2016, 28(11): 114001. |
| 133 | Yu F, Yang Z Q, Zhang X C, et al. V2CT x -MXene partially derived hybrid VS2/V2CT x electrode for capacitive deionization with exceptional rate and capacity[J]. Journal of Materials Chemistry A, 2022, 10(44): 23531-23541. |
| 134 | Tsai W Y, Taberna P L, Simon P. Electrochemical quartz crystal microbalance (EQCM) study of ion dynamics in nanoporous carbons[J]. Journal of the American Chemical Society, 2014, 136(24): 8722-8728. |
| 135 | Breitsprecher K, Janssen M, Srimuk P, et al. How to speed up ion transport in nanopores[J]. Nature Communications, 2020, 11(1): 6085. |
| 136 | Jeanmairet G, Rotenberg B, Salanne M. Microscopic simulations of electrochemical double-layer capacitors[J]. Chemical Reviews, 2022, 122(12): 10860-10898. |
| 137 | Xu K, Shao H, Lin Z F, et al. Computational insights into charge storage mechanisms of supercapacitors[J]. Energy & Environmental Materials, 2020, 3(3): 235-246. |
| 138 | Spohr E, Sovyak E, Trokhymchuk A, et al. Electrostatic control of occupancy and valence selectivity in a charged nanometer-sized cylindrical pore[J]. Materialwissenschaft Und Werkstofftechnik-Materialwiss Werkstofftech, 2009, 40(4): 247-254. |
| 139 | Li X W, Xu S P, Ke P L, et al. Thickness dependence of properties and structure of ultrathin tetrahedral amorphous carbon films: a molecular dynamics simulation[J]. Surface & Coatings Technology, 2014, 258: 938-942. |
| 140 | Kiyohara K, Yamamoto Y, Kawai Y. Selective adsorption of monovalent cations in porous electrodes[J]. Physical Chemistry Chemical Physics, 2020, 22(43): 25184-25194. |
| 141 | Liang M X, Liu N N, Zhang X C, et al. A reverse-defect-engineering strategy toward high edge-nitrogen-doped nanotube-like carbon for high-capacity and stable sodium ion capture[J]. Advanced Functional Materials, 2022, 32(49): 2209741. |
| 142 | Huo S L, Zhang P, He M M, et al. Sustainable development of ultrathin porous carbon nanosheets with highly accessible defects from biomass waste for high-performance capacitive desalination[J]. Green Chemistry, 2021, 23(21): 8554-8565. |
| 143 | Huo S L, Song X, Zhao Y B, et al. Insight into the significant contribution of intrinsic carbon defects for the high-performance capacitive desalination of brackish water[J]. Journal of Materials Chemistry A, 2020, 8(38): 19927-19937. |
| 144 | Li L S, Xu S Z, Carter E A. First-principles modeling of sodium ion and water intercalation into titanium disulfide interlayers for water desalination[J]. Chemistry of Materials, 2020, 32(24): 10678-10687. |
| 145 | Qing L Y, Li Y, Tang W Q, et al. Dynamic adsorption of ions into like-charged nanospace: a dynamic density functional theory study[J]. Langmuir, 2019, 35(12): 4254-4262. |
| 146 | Liu M Q, Xu M, Xue Y F, et al. Efficient capacitive deionization using natural basswood-derived, freestanding, hierarchically porous carbon electrodes[J]. ACS Applied Materials & Interfaces, 2018, 10(37): 31260-31270. |
| [1] | 王尤佳, 赵亮, 高金森, 徐春明. 柴油烃类族组成分离技术研究进展[J]. 化工学报, 2024, 75(1): 20-32. |
| [2] | 闻文, 王慧艳, 周静红, 曹约强, 周兴贵. 石墨负极颗粒对锂离子电池容量衰减及SEI膜生长影响的模拟研究[J]. 化工学报, 2024, 75(1): 366-376. |
| [3] | 咸国义, 陈立芳, 漆志文. 基于DFT的环己酮肟液相贝克曼重排机理研究[J]. 化工学报, 2024, 75(1): 302-311. |
| [4] | 孟祥军, 花莹曦, 张长金, 张弛, 杨林睿, 杨若昔, 刘鉴漪, 许春建. 6N电子级氘气的制备与纯化技术研究[J]. 化工学报, 2024, 75(1): 377-390. |
| [5] | 闫可欣, 姜洪涛, 高维群, 郭晓晖, 孙伟振, 赵玲. 电子级多晶硅原料中痕量硼磷杂质的脱除研究进展[J]. 化工学报, 2024, 75(1): 83-94. |
| [6] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [7] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [8] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [9] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [10] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [11] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [12] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [13] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [14] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [15] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号