化工学报 ›› 2025, Vol. 76 ›› Issue (2): 667-685.DOI: 10.11949/0438-1157.20240947
• 催化、动力学与反应器 • 上一篇
党法璐1( ), 孙志国2, 高照1, 王刚1(
), 孙志国2, 高照1, 王刚1( ), 陈政宇1, 张霖宙1, 连竞存1, 刘美佳1, 张忠东2(
), 陈政宇1, 张霖宙1, 连竞存1, 刘美佳1, 张忠东2( ), 刘超伟2
), 刘超伟2
收稿日期:2024-08-22
修回日期:2024-09-05
出版日期:2025-03-25
发布日期:2025-03-10
通讯作者:
王刚,张忠东
作者简介:党法璐(1995—),男,博士研究生,dangfalu@163.com
基金资助:
Falu DANG1( ), Zhiguo SUN2, Zhao GAO1, Gang WANG1(
), Zhiguo SUN2, Zhao GAO1, Gang WANG1( ), Zhengyu CHEN1, Linzhou ZHANG1, Jingcun LIAN1, Meijia LIU1, Zhongdong ZHANG2(
), Zhengyu CHEN1, Linzhou ZHANG1, Jingcun LIAN1, Meijia LIU1, Zhongdong ZHANG2( ), Chaowei LIU2
), Chaowei LIU2
Received:2024-08-22
Revised:2024-09-05
Online:2025-03-25
Published:2025-03-10
Contact:
Gang WANG, Zhongdong ZHANG
摘要:
首先通过中试实验获得大庆原油制低碳烯烃的反应规律及优选反应域;然后基于智慧炼化技术进行了分子动力学建模,模型包括原料组成、反应机理制定、反应网络生成、动力学及反应器型式。通过全局优化算法训练实验数据得到动力学参数,模型在馏分产率、气体产率、液体产品烃族组成等方面的计算值和实验值基本一致。利用模型剖析了优选实验条件下原油制低碳烯烃的分子转化路径,定量对比了理论上关键裂解主反应及副反应对低碳烯烃产率的影响,探索了消除副反应后理想情况下的产物分布情况。模型计算发现影响低碳烯烃生成的三个最关键裂解主反应路径为:烯烃中间裂解、烯烃裂解生成丙烯、丁烯及烷烃中间裂解反应;三种影响低碳烯烃产率的主要副反应为:氢转移、烯烃环化及烯烃叠合反应。利用模型对最优化实验点进行计算,模拟优化在消除所有副反应后,乙烯、丙烯及丁烯产率分别提升3.91、10.88及10.51个百分点,总低碳烯烃产率相对提高约45.5%(质量分数)。
中图分类号:
党法璐, 孙志国, 高照, 王刚, 陈政宇, 张霖宙, 连竞存, 刘美佳, 张忠东, 刘超伟. 原油一步法催化裂解制低碳烯烃:实验和反应路径研究[J]. 化工学报, 2025, 76(2): 667-685.
Falu DANG, Zhiguo SUN, Zhao GAO, Gang WANG, Zhengyu CHEN, Linzhou ZHANG, Jingcun LIAN, Meijia LIU, Zhongdong ZHANG, Chaowei LIU. One-step catalytic cracking of crude oil to light olefins: experimental and reaction pathway studies[J]. CIESC Journal, 2025, 76(2): 667-685.
| 参数 | 数值 | 参数 | 数值 |
|---|---|---|---|
| 密度(20℃)/(kg/m3) | 862.8 | 蒸馏数据 | |
| 黏度(50℃)/(mm2/s) | 27.2 | 初馏点 | 73.6℃ |
| 残炭/%(质量分数) | 3.07 | 10%(质量分数) | 209.2℃ |
| 蜡含量/%(质量分数) | 30.2 | 30%(质量分数) | 383.8℃ |
| 胶质含量/%(质量分数) | 6.6 | 50%(质量分数) | 448.2℃ |
| 沥青质含量/%(质量分数) | 0.08 | 70%(质量分数) | 602.2℃ |
| 镍/(mg/kg) | 3.28 | 90%(质量分数) | 707.2℃ |
| 铁/(mg/kg) | 4.13 | 95%(质量分数) | 741.2℃ |
| 钒/(mg/kg) | 0.57 | 馏分切割 | |
| C/%(质量分数) | 86.11 | 石脑油(C5~200℃) | 9.56%(质量分数) |
| H/%(质量分数) | 13.55 | 柴油(200~350℃) | 19.58%(质量分数) |
| S/%(质量分数) | 0.12 | 减压馏分油(350~500℃) | 26.56%(质量分数) |
| N/%(质量分数) | 0.11 | 减压渣油(>500℃) | 44.30%(质量分数) |
表1 大庆原油常规物理化学性质
Table 1 The general physical and chemical properties of Daqing crude oil
| 参数 | 数值 | 参数 | 数值 |
|---|---|---|---|
| 密度(20℃)/(kg/m3) | 862.8 | 蒸馏数据 | |
| 黏度(50℃)/(mm2/s) | 27.2 | 初馏点 | 73.6℃ |
| 残炭/%(质量分数) | 3.07 | 10%(质量分数) | 209.2℃ |
| 蜡含量/%(质量分数) | 30.2 | 30%(质量分数) | 383.8℃ |
| 胶质含量/%(质量分数) | 6.6 | 50%(质量分数) | 448.2℃ |
| 沥青质含量/%(质量分数) | 0.08 | 70%(质量分数) | 602.2℃ |
| 镍/(mg/kg) | 3.28 | 90%(质量分数) | 707.2℃ |
| 铁/(mg/kg) | 4.13 | 95%(质量分数) | 741.2℃ |
| 钒/(mg/kg) | 0.57 | 馏分切割 | |
| C/%(质量分数) | 86.11 | 石脑油(C5~200℃) | 9.56%(质量分数) |
| H/%(质量分数) | 13.55 | 柴油(200~350℃) | 19.58%(质量分数) |
| S/%(质量分数) | 0.12 | 减压馏分油(350~500℃) | 26.56%(质量分数) |
| N/%(质量分数) | 0.11 | 减压渣油(>500℃) | 44.30%(质量分数) |
| 参数 | 数值 | 参数 | 数值 |
|---|---|---|---|
| 孔体积/(cm3/g) | 0.17 | 粒度分布 | |
| 比表面积/(m2/g) | 210 | 20~40 μm | 5.64%(质量分数) |
| 微反活性 | 57 | 40~80 μm | 19.90%(质量分数) |
| 堆积密度/(g/cm3) | 0.79 | 80~110 μm | 48.01%(质量分数) |
| 平均孔径/nm | 6.82 | >110 μm | 17.58%(质量分数) |
表2 水热老化后催化剂的物化性质
Table 2 Physical and chemical properties of catalysts after hydrothermal aging
| 参数 | 数值 | 参数 | 数值 |
|---|---|---|---|
| 孔体积/(cm3/g) | 0.17 | 粒度分布 | |
| 比表面积/(m2/g) | 210 | 20~40 μm | 5.64%(质量分数) |
| 微反活性 | 57 | 40~80 μm | 19.90%(质量分数) |
| 堆积密度/(g/cm3) | 0.79 | 80~110 μm | 48.01%(质量分数) |
| 平均孔径/nm | 6.82 | >110 μm | 17.58%(质量分数) |
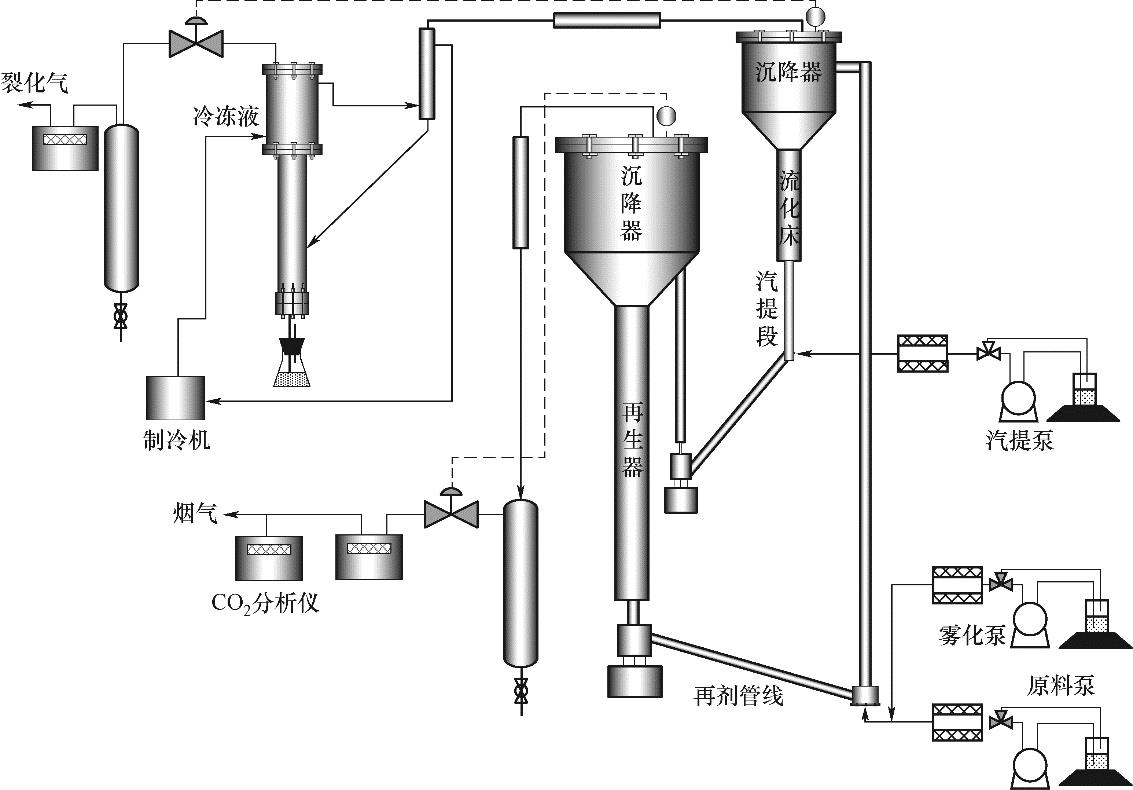
图1 连续反应-再生流化催化裂解提升管反应器实验装置示意图
Fig.1 Schematic diagram of the experimental setup of continuous reaction-regeneration fluidized catalytic cracking riser reactor
| 编号 | 提升管出口温度/℃ | 反应时间/s | 剂油比 |
|---|---|---|---|
| 1 | 600 | 2.48 | 15.70 |
| 2 | 600 | 3.80 | 21.60 |
| 3 | 600 | 2.52 | 18.20 |
| 4 | 600 | 2.55 | 17.35 |
| 5 | 600 | 3.72 | 17.81 |
| 6 | 600 | 1.70 | 24.10 |
| 7 | 600 | 3.70 | 28.00 |
| 8 | 605 | 3.75 | 30.80 |
| 9 | 610 | 3.78 | 32.60 |
| 10 | 620 | 2.55 | 19.60 |
| 11 | 620 | 3.80 | 29.00 |
| 12 | 620 | 3.80 | 33.50 |
| 13 | 620 | 3.80 | 30.00 |
| 14 | 620 | 3.80 | 32.16 |
| 15 | 620 | 3.60 | 35.80 |
| 16 | 620 | 3.30 | 36.00 |
| 17 | 620 | 3.00 | 35.70 |
| 18 | 620 | 2.85 | 35.20 |
| 19 | 620 | 1.70 | 35.90 |
| 20 | 650 | 2.30 | 18.20 |
| 21 | 650 | 4.00 | 36.00 |
| 22 | 650 | 3.50 | 42.20 |
表3 原油直接催化裂解主要工艺条件
Table 3 Primary process conditions for direct catalytic cracking of crude oil
| 编号 | 提升管出口温度/℃ | 反应时间/s | 剂油比 |
|---|---|---|---|
| 1 | 600 | 2.48 | 15.70 |
| 2 | 600 | 3.80 | 21.60 |
| 3 | 600 | 2.52 | 18.20 |
| 4 | 600 | 2.55 | 17.35 |
| 5 | 600 | 3.72 | 17.81 |
| 6 | 600 | 1.70 | 24.10 |
| 7 | 600 | 3.70 | 28.00 |
| 8 | 605 | 3.75 | 30.80 |
| 9 | 610 | 3.78 | 32.60 |
| 10 | 620 | 2.55 | 19.60 |
| 11 | 620 | 3.80 | 29.00 |
| 12 | 620 | 3.80 | 33.50 |
| 13 | 620 | 3.80 | 30.00 |
| 14 | 620 | 3.80 | 32.16 |
| 15 | 620 | 3.60 | 35.80 |
| 16 | 620 | 3.30 | 36.00 |
| 17 | 620 | 3.00 | 35.70 |
| 18 | 620 | 2.85 | 35.20 |
| 19 | 620 | 1.70 | 35.90 |
| 20 | 650 | 2.30 | 18.20 |
| 21 | 650 | 4.00 | 36.00 |
| 22 | 650 | 3.50 | 42.20 |
| 编号 | 实验值/ %(质量分数) | 计算值/ %(质量分数) | (计算-实验差值)/ %(质量分数) |
|---|---|---|---|
| 1 | 58.03 | 57.58 | -0.45 |
| 2 | 70.74 | 69.89 | -0.85 |
| 3 | 65.62 | 64.37 | -1.25 |
| 4 | 61.91 | 61.78 | -0.13 |
| 5 | 66.56 | 66.52 | -0.04 |
| 6 | 67.05 | 67.6 | 0.55 |
| 7 | 74.01 | 73.57 | -0.44 |
| 8 | 74.42 | 74.13 | -0.29 |
| 9 | 75.79 | 74.99 | -0.80 |
| 10 | 67.9 | 67.12 | -0.78 |
| 11 | 74.65 | 74.27 | -0.38 |
| 12 | 79.41 | 79.14 | -0.27 |
| 13 | 75.71 | 75.21 | -0.50 |
| 14 | 77.23 | 77.55 | 0.32 |
| 15 | 80.45 | 81.01 | 0.56 |
| 16 | 80.05 | 79.78 | -0.27 |
| 17 | 78.11 | 77.92 | -0.19 |
| 18 | 77.05 | 76.66 | -0.39 |
| 19 | 72.15 | 72.11 | -0.04 |
| 20 | 67.75 | 67.31 | -0.44 |
| 21 | 78.92 | 79.13 | 0.21 |
| 22 | 82.39 | 81.43 | -0.96 |
表4 不同实验点馏分转化率的实验值与计算值
Table 4 The experimental and calculated values of fraction conversion at different tests
| 编号 | 实验值/ %(质量分数) | 计算值/ %(质量分数) | (计算-实验差值)/ %(质量分数) |
|---|---|---|---|
| 1 | 58.03 | 57.58 | -0.45 |
| 2 | 70.74 | 69.89 | -0.85 |
| 3 | 65.62 | 64.37 | -1.25 |
| 4 | 61.91 | 61.78 | -0.13 |
| 5 | 66.56 | 66.52 | -0.04 |
| 6 | 67.05 | 67.6 | 0.55 |
| 7 | 74.01 | 73.57 | -0.44 |
| 8 | 74.42 | 74.13 | -0.29 |
| 9 | 75.79 | 74.99 | -0.80 |
| 10 | 67.9 | 67.12 | -0.78 |
| 11 | 74.65 | 74.27 | -0.38 |
| 12 | 79.41 | 79.14 | -0.27 |
| 13 | 75.71 | 75.21 | -0.50 |
| 14 | 77.23 | 77.55 | 0.32 |
| 15 | 80.45 | 81.01 | 0.56 |
| 16 | 80.05 | 79.78 | -0.27 |
| 17 | 78.11 | 77.92 | -0.19 |
| 18 | 77.05 | 76.66 | -0.39 |
| 19 | 72.15 | 72.11 | -0.04 |
| 20 | 67.75 | 67.31 | -0.44 |
| 21 | 78.92 | 79.13 | 0.21 |
| 22 | 82.39 | 81.43 | -0.96 |
| 项目 | 优化条件Ⅰ | 优化条件Ⅱ |
|---|---|---|
| 产物分布/%(质量分数) | ||
| 干气(H2-C2) | 17.33 | 21.63 |
| 其中乙烯 | 10.94 | 12.77 |
| 液化气(C3~C4) | 52.00 | 47.56 |
| 其中丙烯 | 27.97 | 27.56 |
| 其中丁烯 | 16.71 | 15.29 |
| 汽油(C5~200℃) | 13.06 | 13.14 |
| 柴油(200~350℃) | 5.40 | 3.78 |
| 重油(>350℃) | 1.10 | 0.70 |
| 焦炭 | 11.12 | 13.20 |
| 产物产率合计/%(质量分数) | 100 | 100 |
| 馏分转化率/%(质量分数) | 80.45 | 82.39 |
| 氢平衡/%(质量分数) | ||
| 干气(H2-C2) | 23.68 | 30.17 |
| 其中乙烯 | 11.32 | 13.20 |
| 液化气(C3~C4) | 55.43 | 49.90 |
| 其中丙烯 | 28.94 | 28.49 |
| 其中丁烯 | 17.29 | 15.80 |
| 汽油(C5~200℃) | 12.01 | 11.63 |
| 柴油(200~350℃) | 3.52 | 2.24 |
| 重油(>350℃) | 0.53 | 0.33 |
| 焦炭 | 4.83 | 5.73 |
| 氢平衡合计/%(质量分数) | 100 | 100 |
表5 两个优化条件下的产物分布及氢平衡数据
Table 5 Product distribution and hydrogen balance data for two optimized conditions
| 项目 | 优化条件Ⅰ | 优化条件Ⅱ |
|---|---|---|
| 产物分布/%(质量分数) | ||
| 干气(H2-C2) | 17.33 | 21.63 |
| 其中乙烯 | 10.94 | 12.77 |
| 液化气(C3~C4) | 52.00 | 47.56 |
| 其中丙烯 | 27.97 | 27.56 |
| 其中丁烯 | 16.71 | 15.29 |
| 汽油(C5~200℃) | 13.06 | 13.14 |
| 柴油(200~350℃) | 5.40 | 3.78 |
| 重油(>350℃) | 1.10 | 0.70 |
| 焦炭 | 11.12 | 13.20 |
| 产物产率合计/%(质量分数) | 100 | 100 |
| 馏分转化率/%(质量分数) | 80.45 | 82.39 |
| 氢平衡/%(质量分数) | ||
| 干气(H2-C2) | 23.68 | 30.17 |
| 其中乙烯 | 11.32 | 13.20 |
| 液化气(C3~C4) | 55.43 | 49.90 |
| 其中丙烯 | 28.94 | 28.49 |
| 其中丁烯 | 17.29 | 15.80 |
| 汽油(C5~200℃) | 12.01 | 11.63 |
| 柴油(200~350℃) | 3.52 | 2.24 |
| 重油(>350℃) | 0.53 | 0.33 |
| 焦炭 | 4.83 | 5.73 |
| 氢平衡合计/%(质量分数) | 100 | 100 |
| 项目 | 实验值 | 计算值 | 绝对误差 |
|---|---|---|---|
| 密度(20℃)/(kg/m3) | 862.8 | 864.3 | 1.5 |
| 黏度(50℃)/(mm2/s) | 27.2 | 28.1 | 0.9 |
| 残炭/%(质量分数) | 3.07 | 3.10 | 0.03 |
| 元素组成/%(质量分数) | |||
| C | 86.11 | 86.16 | 0.05 |
| H | 13.55 | 13.50 | 0.05 |
| S | 0.12 | 0.12 | 0 |
| N | 0.11 | 0.11 | 0 |
| 蒸馏数据/℃ | |||
| 初馏点 | 73.6 | 74.3 | 0.7 |
| 10%(质量分数) | 209.2 | 211.5 | 2.3 |
| 30%(质量分数) | 383.8 | 384.7 | 0.9 |
| 50%(质量分数) | 448.2 | 451.6 | 3.4 |
| 70%(质量分数) | 602.2 | 601.5 | 0.7 |
| 90%(质量分数) | 707.2 | 706.4 | 0.8 |
| 95%(质量分数) | 741.2 | 742.5 | 1.3 |
| 馏分切割/%(质量分数) | |||
| 石脑油(C5~200℃) | 9.56 | 9.54 | 0.02 |
| 柴油(200~350℃) | 19.58 | 19.59 | 0.01 |
| VGO(350~500℃) | 26.56 | 26.52 | 0.04 |
| VR(>500℃) | 44.30 | 44.35 | 0.05 |
表6 大庆原油性质的实验值与模型计算值
Table 6 Experimental and model calculated value of Daqing crude oil properties
| 项目 | 实验值 | 计算值 | 绝对误差 |
|---|---|---|---|
| 密度(20℃)/(kg/m3) | 862.8 | 864.3 | 1.5 |
| 黏度(50℃)/(mm2/s) | 27.2 | 28.1 | 0.9 |
| 残炭/%(质量分数) | 3.07 | 3.10 | 0.03 |
| 元素组成/%(质量分数) | |||
| C | 86.11 | 86.16 | 0.05 |
| H | 13.55 | 13.50 | 0.05 |
| S | 0.12 | 0.12 | 0 |
| N | 0.11 | 0.11 | 0 |
| 蒸馏数据/℃ | |||
| 初馏点 | 73.6 | 74.3 | 0.7 |
| 10%(质量分数) | 209.2 | 211.5 | 2.3 |
| 30%(质量分数) | 383.8 | 384.7 | 0.9 |
| 50%(质量分数) | 448.2 | 451.6 | 3.4 |
| 70%(质量分数) | 602.2 | 601.5 | 0.7 |
| 90%(质量分数) | 707.2 | 706.4 | 0.8 |
| 95%(质量分数) | 741.2 | 742.5 | 1.3 |
| 馏分切割/%(质量分数) | |||
| 石脑油(C5~200℃) | 9.56 | 9.54 | 0.02 |
| 柴油(200~350℃) | 19.58 | 19.59 | 0.01 |
| VGO(350~500℃) | 26.56 | 26.52 | 0.04 |
| VR(>500℃) | 44.30 | 44.35 | 0.05 |
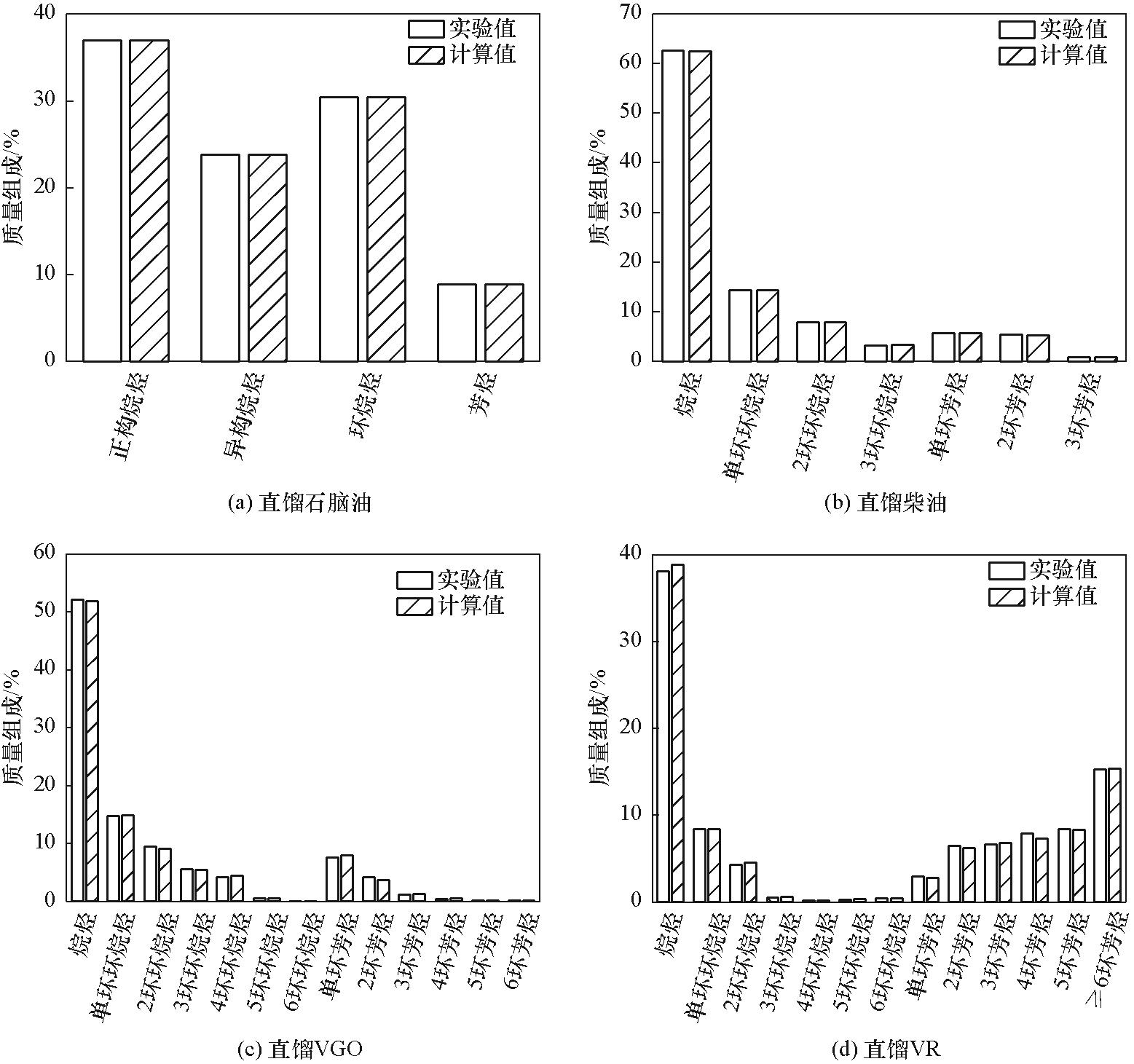
图7 原油组成模型中不同馏分油烃族组成计算值与实验值对比
Fig.7 Comparison of calculated and experimental values of hydrocarbon compositions of different fractions in crude oil composition model
| 反应类型 | 代表性反应 |
|---|---|
| 烷烃裂解反应 | |
| 正构烷烃中间裂解 |  |
| 异构烷烃中间裂解 |  |
| 烷烃裂解生成氢气 |  |
| 烷烃裂解生成甲烷 |  |
| 烷烃裂解生成乙烷 |  |
| 烷烃裂解生成丙烷或丁烷 |  |
 | |
| 烷烃脱硫 |  |
| 烯烃裂解反应 | |
| 烯烃中间裂解 |  |
| 烯烃裂解生成乙烯 |  |
| 烯烃裂解生成丙烯或丁烯 |  |
 | |
| 环裂解反应 | |
| 环侧链裂解 | 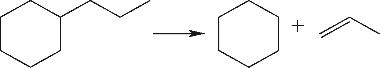 |
| 开环 |  |
| 含硫环开环 | 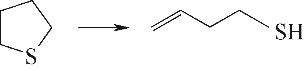 |
| 氢转移反应 | |
| 烯烃与活性氢 | 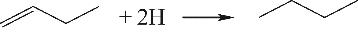 |
| 烯烃与环烯烃Ⅰ | 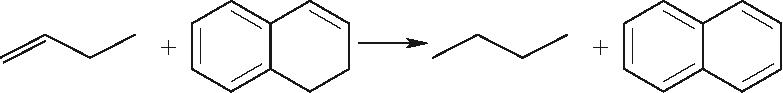 |
| 烯烃与环烯烃Ⅱ | 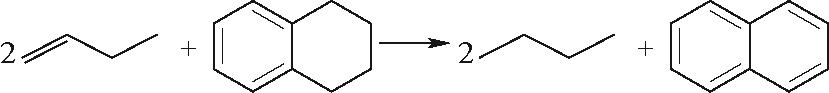 |
| 烯烃与环烷烃 |  |
| 异构化反应 | |
| 烷烃异构化 |  |
| 烯烃异构化 | 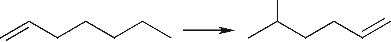 |
| 缩合反应 | |
| 环缩合Ⅰ | 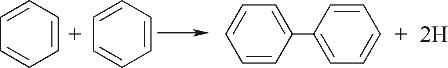 |
| 环缩合Ⅱ | 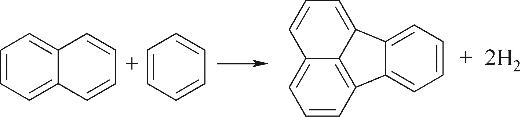 |
| 环缩合Ⅲ | 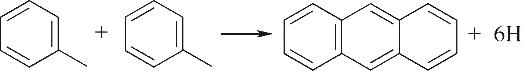 |
| 环脱氢反应 | |
| 环脱氢Ⅰ | 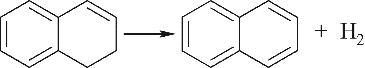 |
| 环脱氢Ⅱ | 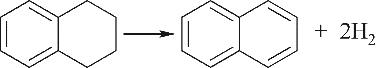 |
| 环脱氢Ⅲ | 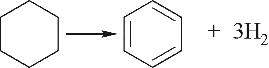 |
| 含硫环脱氢 | 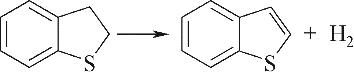 |
| 环化反应 | |
| 烯烃环化 | 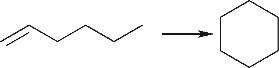 |
| 烯烃叠合 | 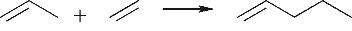 |
| 环侧链闭合 | 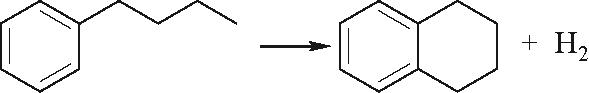 |
表7 原油催化裂解的代表性反应规则
Table 7 Representative reaction rules for catalytic cracking of crude oil
| 反应类型 | 代表性反应 |
|---|---|
| 烷烃裂解反应 | |
| 正构烷烃中间裂解 |  |
| 异构烷烃中间裂解 |  |
| 烷烃裂解生成氢气 |  |
| 烷烃裂解生成甲烷 |  |
| 烷烃裂解生成乙烷 |  |
| 烷烃裂解生成丙烷或丁烷 |  |
 | |
| 烷烃脱硫 |  |
| 烯烃裂解反应 | |
| 烯烃中间裂解 |  |
| 烯烃裂解生成乙烯 |  |
| 烯烃裂解生成丙烯或丁烯 |  |
 | |
| 环裂解反应 | |
| 环侧链裂解 |  |
| 开环 |  |
| 含硫环开环 |  |
| 氢转移反应 | |
| 烯烃与活性氢 | 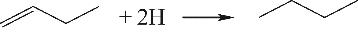 |
| 烯烃与环烯烃Ⅰ |  |
| 烯烃与环烯烃Ⅱ |  |
| 烯烃与环烷烃 |  |
| 异构化反应 | |
| 烷烃异构化 |  |
| 烯烃异构化 | 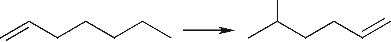 |
| 缩合反应 | |
| 环缩合Ⅰ |  |
| 环缩合Ⅱ |  |
| 环缩合Ⅲ |  |
| 环脱氢反应 | |
| 环脱氢Ⅰ |  |
| 环脱氢Ⅱ |  |
| 环脱氢Ⅲ |  |
| 含硫环脱氢 |  |
| 环化反应 | |
| 烯烃环化 |  |
| 烯烃叠合 | 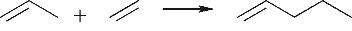 |
| 环侧链闭合 |  |
| 项目 | A | a | b | ||
|---|---|---|---|---|---|
| 烷烃裂解反应 | |||||
| 正构烷烃中间裂解 | 3.08×104 | 673 | 79534 | ||
| 异构烷烃中间裂解 | 1.38×105 | 656 | 68713 | ||
| 烷烃裂解生成氢气 | 3.51×108 | 576 | 146477 | ||
| 烷烃裂解生成甲烷 | 2.12×109 | 667 | 103426 | ||
| 烷烃裂解生成乙烷 | 3.74×108 | 658 | 87353 | ||
| 烷烃裂解生成丙烷或丁烷 | 3.18×106 | 620 | 80306 | ||
| 烷烃脱硫 | 4.55×103 | 592 | 62238 | ||
| 烯烃裂解反应 | |||||
| 烯烃中间裂解 | 6.04×106 | 631 | 55904 | ||
| 烯烃裂解生成乙烯 | 1.67×106 | 668 | 66151 | ||
| 烯烃裂解生成丙烯或丁烯 | 1.19×107 | 633 | 63288 | ||
| 环裂解反应 | |||||
| 环侧链裂解 | 1.27×108 | 715 | 74334 | ||
| 开环 | 6.19×109 | 722 | 66427 | ||
| 含硫环开环 | 7.94×105 | 727 | 71035 | ||
| 氢转移反应 | |||||
| 烯烃与活性氢 | 1.29×1010 | 9158 | 24810 | ||
| 烯烃与环烯烃Ⅰ | 1.70×1016 | 1516 | 139450 | ||
| 烯烃与环烯烃Ⅱ | 6.58×1017 | 1531 | 136634 | ||
| 烯烃与环烷烃 | 1.32×1018 | 357 | 42635 | ||
| 异构化反应 | |||||
| 烷烃异构化 | 1.20×101 | 96 | 38783 | ||
| 烯烃异构化 | 1.50×101 | 361 | 53861 | ||
| 缩合反应 | |||||
| 环缩合Ⅰ | 1.03×105 | 148 | 23696 | ||
| 环缩合Ⅱ | 1.12×104 | 252 | 24132 | ||
| 环缩合Ⅲ | 1.82×104 | 351 | 25785 | ||
| 环脱氢反应 | |||||
| 环脱氢Ⅰ | 2.62×103 | 1365 | 74647 | ||
| 环脱氢Ⅱ | 1.86×104 | 1042 | 58149 | ||
| 环脱氢Ⅲ | 2.21×105 | 200 | 288 | ||
| 含硫环脱氢 | 1.27×103 | 719 | 2605 | ||
| 环化反应 | |||||
| 烯烃环化 | 5.24×109 | 233 | 153619 | ||
| 烯烃叠合 | 2.50×102 | 153 | 23057 | ||
| 环侧链闭合 | 7.50×102 | 228 | 55373 | ||
| 模型失活参数 | α | β | kA | kN | |
| 0.35 | -1.17 | 2.83 | 0.002 | ||
表8 原油催化裂解动力学参数
Table 8 Kinetic parameters of crude oil catalytic cracking
| 项目 | A | a | b | ||
|---|---|---|---|---|---|
| 烷烃裂解反应 | |||||
| 正构烷烃中间裂解 | 3.08×104 | 673 | 79534 | ||
| 异构烷烃中间裂解 | 1.38×105 | 656 | 68713 | ||
| 烷烃裂解生成氢气 | 3.51×108 | 576 | 146477 | ||
| 烷烃裂解生成甲烷 | 2.12×109 | 667 | 103426 | ||
| 烷烃裂解生成乙烷 | 3.74×108 | 658 | 87353 | ||
| 烷烃裂解生成丙烷或丁烷 | 3.18×106 | 620 | 80306 | ||
| 烷烃脱硫 | 4.55×103 | 592 | 62238 | ||
| 烯烃裂解反应 | |||||
| 烯烃中间裂解 | 6.04×106 | 631 | 55904 | ||
| 烯烃裂解生成乙烯 | 1.67×106 | 668 | 66151 | ||
| 烯烃裂解生成丙烯或丁烯 | 1.19×107 | 633 | 63288 | ||
| 环裂解反应 | |||||
| 环侧链裂解 | 1.27×108 | 715 | 74334 | ||
| 开环 | 6.19×109 | 722 | 66427 | ||
| 含硫环开环 | 7.94×105 | 727 | 71035 | ||
| 氢转移反应 | |||||
| 烯烃与活性氢 | 1.29×1010 | 9158 | 24810 | ||
| 烯烃与环烯烃Ⅰ | 1.70×1016 | 1516 | 139450 | ||
| 烯烃与环烯烃Ⅱ | 6.58×1017 | 1531 | 136634 | ||
| 烯烃与环烷烃 | 1.32×1018 | 357 | 42635 | ||
| 异构化反应 | |||||
| 烷烃异构化 | 1.20×101 | 96 | 38783 | ||
| 烯烃异构化 | 1.50×101 | 361 | 53861 | ||
| 缩合反应 | |||||
| 环缩合Ⅰ | 1.03×105 | 148 | 23696 | ||
| 环缩合Ⅱ | 1.12×104 | 252 | 24132 | ||
| 环缩合Ⅲ | 1.82×104 | 351 | 25785 | ||
| 环脱氢反应 | |||||
| 环脱氢Ⅰ | 2.62×103 | 1365 | 74647 | ||
| 环脱氢Ⅱ | 1.86×104 | 1042 | 58149 | ||
| 环脱氢Ⅲ | 2.21×105 | 200 | 288 | ||
| 含硫环脱氢 | 1.27×103 | 719 | 2605 | ||
| 环化反应 | |||||
| 烯烃环化 | 5.24×109 | 233 | 153619 | ||
| 烯烃叠合 | 2.50×102 | 153 | 23057 | ||
| 环侧链闭合 | 7.50×102 | 228 | 55373 | ||
| 模型失活参数 | α | β | kA | kN | |
| 0.35 | -1.17 | 2.83 | 0.002 | ||
| 1 | Alotaibi F M, González-Cortés S, Alotibi M F, et al. Enhancing the production of light olefins from heavy crude oils: turning challenges into opportunities[J]. Catalysis Today, 2018, 317: 86-98. |
| 2 | Zhou X, Sun Z Z, Yan H, et al. Produce petrochemicals directly from crude oil catalytic cracking, a techno-economic analysis and life cycle society-environment assessment[J]. Journal of Cleaner Production, 2021, 308: 127283. |
| 3 | Zhou X, Li S F, Wang Y, et al. Crude oil hierarchical catalytic cracking for maximizing chemicals production: pilot-scale test, process optimization strategy, techno-economic-society-environment assessment[J]. Energy Conversion and Management, 2022, 253: 115149. |
| 4 | Alabdullah M A, Gomez A R, Vittenet J, et al. A viewpoint on the refinery of the future: catalyst and process challenges[J]. ACS Catalysis, 2020, 10(15): 8131-8140. |
| 5 | Amghizar I, Vandewalle L A, Van Geem K M, et al. New trends in olefin production[J]. Engineering, 2017, 3(2): 171-178. |
| 6 | Tullo A H. The future of oil is in chemicals, not fuels[J]. Chemical and Engineering News, 2019, 97(8): 26-29. |
| 7 | Al-Samhan M, Al-Fadhli J, Al-Otaibi A M, et al. Prospects of refinery switching from conventional to integrated: an opportunity for sustainable investment in the petrochemical industry[J]. Fuel, 2022, 310: 122161. |
| 8 | 马文明, 朱根权, 杨超, 等. 一种将原油转化成石油化工产品的集成方法和集成装置: 112745915A[P]. 2021-05-04. |
| Ma W M, Zhu G Q, Yang C, et al. An integrated method and device for converting crude oil into petrochemical products: 112745915A[P]. 2021-05-04. | |
| 9 | 李春义, 郭阳玲, 许乃文, 等. 一种烃类催化裂解制低碳烯烃催化剂及其制备方法: 111203225A[P]. 2020-05-29. |
| Li C Y, Guo Y L, Xu N W, et al. A catalyst for catalytic cracking of hydrocarbons to produce low-carbon olefins and its preparation method: 111203225A[P]. 2020-05-29. | |
| 10 | Corma A, Corresa E, Mathieu Y, et al. Crude oil to chemicals: light olefins from crude oil[J]. Catalysis Science & Technology, 2017, 7(1): 12-46. |
| 11 | Sayed E, Shafi R, Akhras A R Z, et al. Integrated slurry hydroprocessing and steam pyrolysis of crude oil to produce petrochemicals: US20170342336[P]. 2017-11-30. |
| 12 | Oprins M, Ward M, Velasco R, et al. An integrated hydrotreating and steam pyrolysis process for the direct processing of a crude oil to produce olefnic and aromatic petrochemicals: US2019352569[P]. 2018-02-02. |
| 13 | Chen J W, Xu Y H. Catalytic Cracking Process and Engineering(Version 3)[M]. Beijing: China Petrochemical Press, 2015. |
| 14 | Wang X Q, Shu X T. Heavy Oil Catalytic Cracking to Olefins[M]. Beijing: China Petrochemical Press, 2015. |
| 15 | 贺安新, 侯利国, 靳凤英, 等. 原油(重油)直接制化学品(DPC)技术在惠州石化的工业应用[J]. 炼油技术与工程, 2023, 53(4): 34-38. |
| He A X, Hou L G, Jin F Y, et al. Industrial application of direct petroleum to chemicals (DPC) technology in Huizhou petrochemical company[J]. Petroleum Refinery Engineering, 2023, 53(4): 34-38. | |
| 16 | 沙特阿美韩国原油直接制化学品项目开工[J]. 石油化工技术与经济, 2023, 39(2): 48. |
| Saudi Aramco South Korea crude oil direct chemicals project started[J]. Technology & Economics in Petrochemicals, 2023, 39(2): 48. | |
| 17 | 吴青. 原油(重油)制化学品的技术及其进展(Ⅱ): 重油催化裂解与DPC碱催化技术[J]. 炼油技术与工程, 2022, 52(8): 1-7, 15. |
| Wu Q. Technology and progress in crude oil to chemicals(Ⅱ): Catalytic cracking technology and DPC basic catalytic cutting technology[J]. Petroleum Refinery Engineering, 2022, 52(8): 1-7, 15. | |
| 18 | 原油催化裂解技术实现全球首次工业化应用[J]. 石油化工设计, 2021, 38(2): 12. |
| Catalytic cracking technology of crude oil realizes the first industrial application in the world[J]. Petrochemical Design, 2021, 38(2): 12. | |
| 19 | 张伟清. S-Oil公司的Shaheen原油制化学品综合体取得突破性进展[J]. 石油炼制与化工, 2023, 54(9): 50. |
| Zhang W Q. Breakthrough progress has been made in S-Oil's Shaheen crude oil chemical complex[J]. Petroleum Processing and Petrochemicals, 2023, 54(9): 50. | |
| 20 | 许友好, 汪燮卿, 舒兴田. 原油最大化生产化工原料的技术思考及相关技术开发[J]. 石油炼制与化工, 2019, 50(11): 1-10. |
| Xu Y H, Wang X Q, Shu X T. Technical consideration and relevant technological development on maximizing chemicals from crude oil[J]. Petroleum Processing and Petrochemicals, 2019, 50(11): 1-10. | |
| 21 | Zhang D, Zong P J, Wang J Y, et al. Catalytic dehydrogenation cracking of crude oil to light olefins by structure and basicity/acidity adjustment of bifunctional metal/acid catalysts[J]. Fuel, 2023, 334: 126808. |
| 22 | Zhang D, Zong P J, Li J, et al. Fundamental studies and pilot verification of an olefins/aromatics-rich chemical production from crude oil dehydrogenation catalytic pyrolysis process[J]. Fuel, 2022, 310: 122435. |
| 23 | Al-Absi A A, Al-Khattaf S S. Conversion of Arabian light crude oil to light olefins via catalytic and thermal cracking[J]. Energy & Fuels, 2018, 32(8): 8705-8714. |
| 24 | Al-Absi A A, Aitani A M, Al-Khattaf S S. Thermal and catalytic cracking of whole crude oils at high severity[J]. Journal of Analytical and Applied Pyrolysis, 2020, 145: 104705. |
| 25 | Al-Khattaf S, Saeed M R, Aitani A, et al. Catalytic cracking of light crude oil to light olefins and naphtha over E-cat and MFI: microactivity test versus advanced cracking evaluation and the effect of high reaction temperature[J]. Energy & Fuels, 2018, 32(5): 6189-6199. |
| 26 | Alabdullah M, Rodriguez-Gomez A, Shoinkhorova T, et al. One-step conversion of crude oil to light olefins using a multi-zone reactor[J]. Nature Catalysis, 2021, 4: 233-241. |
| 27 | Al-Khattaf S S, Ali S A. Catalytic cracking of Arab super light crude oil to light olefins: an experimental and kinetic study[J]. Energy & Fuels, 2018, 32(2): 2234-2244. |
| 28 | Usman A, Siddiqui M A B, Hussain A, et al. Catalytic cracking of crude oil to light olefins and naphtha: experimental and kinetic modeling[J]. Chemical Engineering Research and Design, 2017, 120: 121-137. |
| 29 | 周鑫, 闫昊, 赵辉, 等. 原油直接催化裂解过程模拟与工艺参数优化[J]. 石油学报(石油加工), 2021, 37(6): 1216-1224. |
| Zhou X, Yan H, Zhao H, et al. Simulation of the crude oil direct catalytic cracking process and optimization of process parameters[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2021, 37(6): 1216-1224. | |
| 30 | Zhou X, Yang Q C, Yang S Q, et al. One-step leap in achieving oil-to-chemicals by using a two-stage riser reactor: molecular-level process model and multi-objective optimization strategy[J]. Chemical Engineering Journal, 2022, 444: 136684. |
| 31 | Wang G, Lan X Y, Xu C M, et al. Study of optimal reaction conditions and a modified residue catalytic cracking process for maximizing liquid products[J]. Industrial & Engineering Chemistry Research, 2009, 48(7): 3308-3316. |
| 32 | Sheng Q, Wang G, Liu Y J, et al. Pilot-scale evaluation of hydrotreating inferior coker gas oil prior to its fluid catalytic cracking[J]. Fuel, 2018, 226: 27-34. |
| 33 | Jin N, Wang G, Han S, et al. Hydroconversion behavior of asphaltenes under liquid-phase hydrogenation conditions[J]. Energy & Fuels, 2016, 30(4): 2594-2603. |
| 34 | 王刚. 高温短接触时间重油催化裂化反应规律研究[D]. 北京: 中国石油大学, 2006. |
| Wang G. Study on reaction law of heavy oil catalytic cracking at high temperature and short contact time[D]. Beijing: China University of Petroleum, 2006. | |
| 35 | 马文明, 李小斐, 朱根权, 等. 重油催化裂解多产轻质芳烃工艺的研究[J]. 石油炼制与化工, 2015, 46(8): 1-6. |
| Ma W M, Li X F, Zhu G Q, et al. Study on increasing light aromatics production in deep catalytic cracking of heavy oil[J]. Petroleum Processing and Petrochemicals, 2015, 46(8): 1-6. | |
| 36 | Meng X H, Xu C M, Gao J S, et al. Studies on catalytic pyrolysis of heavy oils: reaction behaviors and mechanistic pathways[J]. Applied Catalysis A: General, 2005, 294(2): 168-176. |
| 37 | Feng S, Cui C, Li K Y, et al. Molecular composition modelling of petroleum fractions based on a hybrid structural unit and bond-electron matrix (SU-BEM) framework[J]. Chemical Engineering Science, 2019, 201: 145-156. |
| 38 | Chen Z Y, Feng S, Zhang L Z, et al. Molecular-level kinetic modeling of heavy oil fluid catalytic cracking process based on hybrid structural unit and bond-electron matrix[J]. AIChE Journal, 2021, 67(1): e17027. |
| 39 | 张霖宙, 陈政宇, 吕文进, 等. 石油加工分子管理平台构建[J]. 中国科学: 化学, 2018, 48(4): 411-426. |
| Zhang L Z, Chen Z Y, Lyu W J, et al. Development of petroleum refining molecular management modeling platform[J]. Scientia Sinica Chimica, 2018, 48(4): 411-426. | |
| 40 | Chen Z Y, Wang G, Zhao S Q, et al. A molecular kinetic model for heavy gas oil catalytic pyrolysis to light olefins[J]. AIChE Journal, 2023, 69(8): e18116. |
| 41 | Chen Z Y, Sun N, Zhang L Z, et al. Molecular-level modeling for naphtha olefin reduction in FCC subsidiary riser: from laboratory reactor to pilot plant[J]. Chemical Engineering Journal, 2022, 437: 135429. |
| 42 | Cui C, Billa T, Zhang L Z, et al. Molecular representation of the petroleum gasoline fraction[J]. Energy & Fuels, 2018, 32(2): 1525-1533. |
| 43 | Cui C, Zhang L Z, Ma Y J, et al. Computer-aided gasoline compositional model development based on GC-FID analysis[J]. Energy & Fuels, 2018, 32(8): 8366-8373. |
| 44 | Corma A, Orchillés A V. Current views on the mechanism of catalytic cracking[J]. Microporous and Mesoporous Materials, 2000, 35: 21-30. |
| 45 | Ralph B. Molecular Modeling in Heavy Hydrocarbon Conversions[M]. Boca Raton, Florida, USA: CRC Press, 2005. |
| 46 | Kotrel S, Knözinger H, Gates B C. The Haag-Dessau mechanism of protolytic cracking of alkanes[J]. Microporous and Mesoporous Materials, 2000, 35: 11-20. |
| 47 | Shertukde P V, Marcelin G, Sill G A, et al. Study of the mechanism of the cracking of small alkane molecules on HY zeolites[J]. Journal of Catalysis, 1992, 136(2): 446-462. |
| 48 | 许友好, 崔守业, 汪燮卿. FCC汽油烯烃双分子裂化反应及其与双分子氢转移反应之比的研究[J]. 石油炼制与化工, 2007, 38(9): 1-5. |
| Xu Y H, Cui S Y, Wang X Q. Study on the bimolecular catalytic cracking reaction and its ratio to the bimolecular hydrogen transfer reaction[J]. Petroleum Processing and Petrochemicals, 2007, 38(9): 1-5. | |
| 49 | Miyaji A, Iwase Y, Nishitoba T, et al. Influence of zeolite pore structure on product selectivities for protolysis and hydride transfer reactions in the cracking of n-pentane[J]. Physical Chemistry Chemical Physics, 2015, 17(7): 5014-5032. |
| 50 | Krannila H, Haag W O, Gates B C. Monomolecular and bimolecular mechanisms of paraffin cracking: n-butane cracking catalyzed by HZSM-5[J]. Journal of Catalysis, 1992, 135(1): 115-124. |
| 51 | Yang M, Zhang L Z, Wang G, et al. Fischer-Tropsch wax catalytic cracking for the production of low olefin and high octane number gasoline: experiment and molecular level kinetic modeling study[J]. Fuel, 2021, 303: 121226. |
| 52 | He S, Lucio-Vega J, Zhang L Z, et al. Integrating visualization methods with chemical kinetics model solution and editing tools[J]. Energy & Fuels, 2017, 31(9): 9881-9889. |
| 53 | Chen Z Y, Guan D, Zhang X J, et al. A mass-temperature decoupled discretization strategy for large-scale molecular-level kinetic model[J]. Chemical Engineering Science, 2022, 249: 117348. |
| 54 | Matsumoto H. Linear free energy relationships in heterogeneous catalysis(Ⅷ): Isomerization of alkylbenzenes over solid acid catalysts[J]. Journal of Catalysis, 1968, 11(3): 211-219. |
| 55 | Evans M G, Polanyi M. Some applications of the transition state method to the calculation of reaction velocities, especially in solution[J]. Transactions of the Faraday Society, 1935, 31: 875. |
| 56 | Bell R, The theory of reactions involving proton transfers[C]//Proceedings of the Royal Society of London. Series A-Mathematical and Physical Sciences, 1997, 154 (882): 414-429. |
| 57 | Jacob S M, Gross B, Voltz S E, et al. A lumping and reaction scheme for catalytic cracking[J]. AIChE Journal, 1976, 22(4): 701-713. |
| 58 | 陈政宇. 分子尺度反应动力学模型构建及在重油催化转化过程中的应用[D]. 北京: 中国石油大学, 2019. |
| Chen Z Y. Construction of molecular scale reaction kinetics model and its application in catalytic conversion of heavy oil[D]. Beijing: China University of Petroleum, 2019. | |
| 59 | Xiong K, Lu C X, Wang Z F, et al. Kinetic study of catalytic cracking of heavy oil over an in situ crystallized FCC catalyst[J]. Fuel, 2015, 142: 65-72. |
| [1] | 彭子林, 周蕾, 邓庆航, 叶光华, 周兴贵. 包含偏硅酸影响的3D NAND磷酸湿法刻蚀动力学[J]. 化工学报, 2025, 76(2): 645-653. |
| [2] | 李舒月, 王欢, 周少强, 毛志宏, 张永民, 王军武, 吴秀花. 重质颗粒流态化研究现状与展望[J]. 化工学报, 2025, 76(2): 466-483. |
| [3] | 何传超, 周静红, 曹约强, 施尧, 周兴贵. Ag/SiO2催化草酸酯加氢制乙醇酸甲酯的床层-颗粒双尺度耦合模拟研究[J]. 化工学报, 2025, 76(2): 654-666. |
| [4] | 张珂, 任维杰, 王梦娜, 范凯锋, 常丽萍, 李佳斌, 马涛, 田晋平. Bunsen反应产物在微通道中的液-液两相混合特性[J]. 化工学报, 2025, 76(2): 623-636. |
| [5] | 张鑫源, 何呈祥, 李亚婷, 朱春英, 马友光, 付涛涛. 微通道内液液非均相传质的模拟和实验研究方法进展[J]. 化工学报, 2025, 76(2): 484-503. |
| [6] | 王瀚彬, 胡帅, 毕丰雷, 李隽森, 贺来宾. 新型波纹翅片金属氢化物反应器的放氢性能有限元分析[J]. 化工学报, 2025, 76(1): 221-230. |
| [7] | 高羡明, 杨汶轩, 卢少辉, 任晓松, 卢方财. 双槽道结构对超疏水表面液滴合并弹跳的影响[J]. 化工学报, 2025, 76(1): 208-220. |
| [8] | 韩启沃, 刘永峰, 裴普成, 张璐, 姚圣卓. 工作温度对PEMFC水分布、质子传输及性能影响分析[J]. 化工学报, 2025, 76(1): 374-384. |
| [9] | 邓志诚, 杨欢, 王斯民, 王家瑞. 微混燃烧器中微管结构对氢燃料掺混效果与燃烧性能影响[J]. 化工学报, 2025, 76(1): 335-347. |
| [10] | 张思文, 顾海明, 赵善辉. 纳米氧化铁对纤维素化学链气化的分子反应机理[J]. 化工学报, 2025, 76(1): 363-373. |
| [11] | 黄娜, 蒋云龙, 王东涵, 吴明婷, 蒋雪莉, 钟豫. 通道振动频率对超临界正癸烷裂解流动换热影响的数值研究[J]. 化工学报, 2025, 76(1): 173-183. |
| [12] | 刘萍, 邱雨生, 李世婧, 孙瑞奇, 申晨. 微通道内纳米流体传热流动特性[J]. 化工学报, 2025, 76(1): 184-197. |
| [13] | 韩志敏, 周相宇, 张宏宇, 徐志明. 不同粗糙元结构下CaCO3污垢局部沉积特性[J]. 化工学报, 2025, 76(1): 151-160. |
| [14] | 任冠宇, 张义飞, 李新泽, 杜文静. 翼型印刷电路板式换热器流动传热特性数值研究[J]. 化工学报, 2024, 75(S1): 108-117. |
| [15] | 杨勇, 祖子轩, 李煜坤, 王东亮, 范宗良, 周怀荣. T型圆柱形微通道内CO2碱液吸收数值模拟[J]. 化工学报, 2024, 75(S1): 135-142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号