化工学报 ›› 2025, Vol. 76 ›› Issue (7): 3226-3234.DOI: 10.11949/0438-1157.20241123
张晓钰( ), 兰金鑫, 黎昕, 曹石林, 高海丽, 马晓娟(
), 兰金鑫, 黎昕, 曹石林, 高海丽, 马晓娟( )
)
收稿日期:2024-10-11
修回日期:2025-02-19
出版日期:2025-07-25
发布日期:2025-08-13
通讯作者:
马晓娟
作者简介:张晓钰(1999—),女,硕士研究生,1696408617@qq.com
基金资助:
Xiaoyu ZHANG( ), Jinxin LAN, Xin LI, Shilin CAO, Haili GAO, Xiaojuan MA(
), Jinxin LAN, Xin LI, Shilin CAO, Haili GAO, Xiaojuan MA( )
)
Received:2024-10-11
Revised:2025-02-19
Online:2025-07-25
Published:2025-08-13
Contact:
Xiaojuan MA
摘要:
离子液体1-乙基-3-甲基氢氧化咪唑([Emim]OH)是一种极具潜力的纤维素溶剂,但是因其结构不稳定限制了进一步的应用。为了提高 [Emim]OH的稳定性,通过密度泛函理论(DFT)计算以及实验选择合适的极性非质子溶剂二甲基亚砜(DMSO),构建1-乙基-3-甲基醋酸咪唑([Emim]Ac)-[Emim]OH-DMSO三元体系稳定氢氧盐离子液体且提高其对纤维素的溶解能力。研究结果表明,离子液体与DMSO摩尔比为1.25∶0.75且[Emim]Ac与 [Emim]OH摩尔比为2∶1时,竹浆在三元溶剂体系中的溶解度达9.9%,比在纯 [Emim]Ac中的溶解度提高了23.8%。经三元溶剂体系溶解再生后,纤维素聚合度从523下降至466,纤维素的晶型由Ⅰ型变为Ⅱ型。DMSO的加入不仅降低了混合离子液体体系黏度,黏度从21.2 Pa∙s下降至19.7 Pa∙s,还加快了纤维素的溶解。
中图分类号:
张晓钰, 兰金鑫, 黎昕, 曹石林, 高海丽, 马晓娟. [Emim]Ac-[Emim]OH-DMSO三元体系溶解纤维素的研究[J]. 化工学报, 2025, 76(7): 3226-3234.
Xiaoyu ZHANG, Jinxin LAN, Xin LI, Shilin CAO, Haili GAO, Xiaojuan MA. Study on dissolution of cellulose by [Emim]Ac-[Emim]OH-DMSO ternary system[J]. CIESC Journal, 2025, 76(7): 3226-3234.
| 比 例 | C2—H键长/Å | 氢键键长/Å | 结合能/ (kJ/mol) |
|---|---|---|---|
| DMSO/OH(1∶2)-[Emim]+ | 1.085 | 4.610 | -902.83 |
| DMSO/OH(2∶1)-[Emim]+ | 1.080 | 3.909 | -675.31 |
| DMF/OH(2∶1)-[Emim]+ | 1.081 | 3.957 | -856.96 |
表1 DMSO、DMF与[Emim]OH结合能及优化后阳离子咪唑环C2—H、阴阳离子氢键键长
Table 1 Binding energy of DMSO, DMF and [Emim]OH, C2—H and canionic hydrogen bond length of cationic imidazole ring
| 比 例 | C2—H键长/Å | 氢键键长/Å | 结合能/ (kJ/mol) |
|---|---|---|---|
| DMSO/OH(1∶2)-[Emim]+ | 1.085 | 4.610 | -902.83 |
| DMSO/OH(2∶1)-[Emim]+ | 1.080 | 3.909 | -675.31 |
| DMF/OH(2∶1)-[Emim]+ | 1.081 | 3.957 | -856.96 |
| DMSO/DMF-纤维二糖 | 编号 | 氢键 | 氢键键长/Å | 平均键长/Å | 结合能/(kJ/mol) |
|---|---|---|---|---|---|
| DMSO-纤维二糖 | 1 | O1-H…O7 | 1.829 | 1.829 | -63.11 |
| 2 | O4′-H…O7 | 1.835 | 2.280 | -76.54 | |
| C6′-H…O7 | 2.724 | 2.280 | -76.54 | ||
| 3 | O3′-H…O7 | 1.787 | 1.787 | -96.45 | |
| DMF-纤维二糖 | 1 | O1-H…O8 | 1.726 | 1.726 | -53.71 |
| 2 | O4′-H…O8 | 1.788 | 2.211 | -64.46 | |
| C6′-H…O8 | 2.633 | 2.211 | -64.46 | ||
| 3 | O3′-H…O8 | 1.804 | 1.804 | -61.10 |
表2 DMSO、DMF与纤维二糖间氢键及结合能
Table 2 Hydrogen bond and binding energy between DMSO, DMF and cellobiose
| DMSO/DMF-纤维二糖 | 编号 | 氢键 | 氢键键长/Å | 平均键长/Å | 结合能/(kJ/mol) |
|---|---|---|---|---|---|
| DMSO-纤维二糖 | 1 | O1-H…O7 | 1.829 | 1.829 | -63.11 |
| 2 | O4′-H…O7 | 1.835 | 2.280 | -76.54 | |
| C6′-H…O7 | 2.724 | 2.280 | -76.54 | ||
| 3 | O3′-H…O7 | 1.787 | 1.787 | -96.45 | |
| DMF-纤维二糖 | 1 | O1-H…O8 | 1.726 | 1.726 | -53.71 |
| 2 | O4′-H…O8 | 1.788 | 2.211 | -64.46 | |
| C6′-H…O8 | 2.633 | 2.211 | -64.46 | ||
| 3 | O3′-H…O8 | 1.804 | 1.804 | -61.10 |
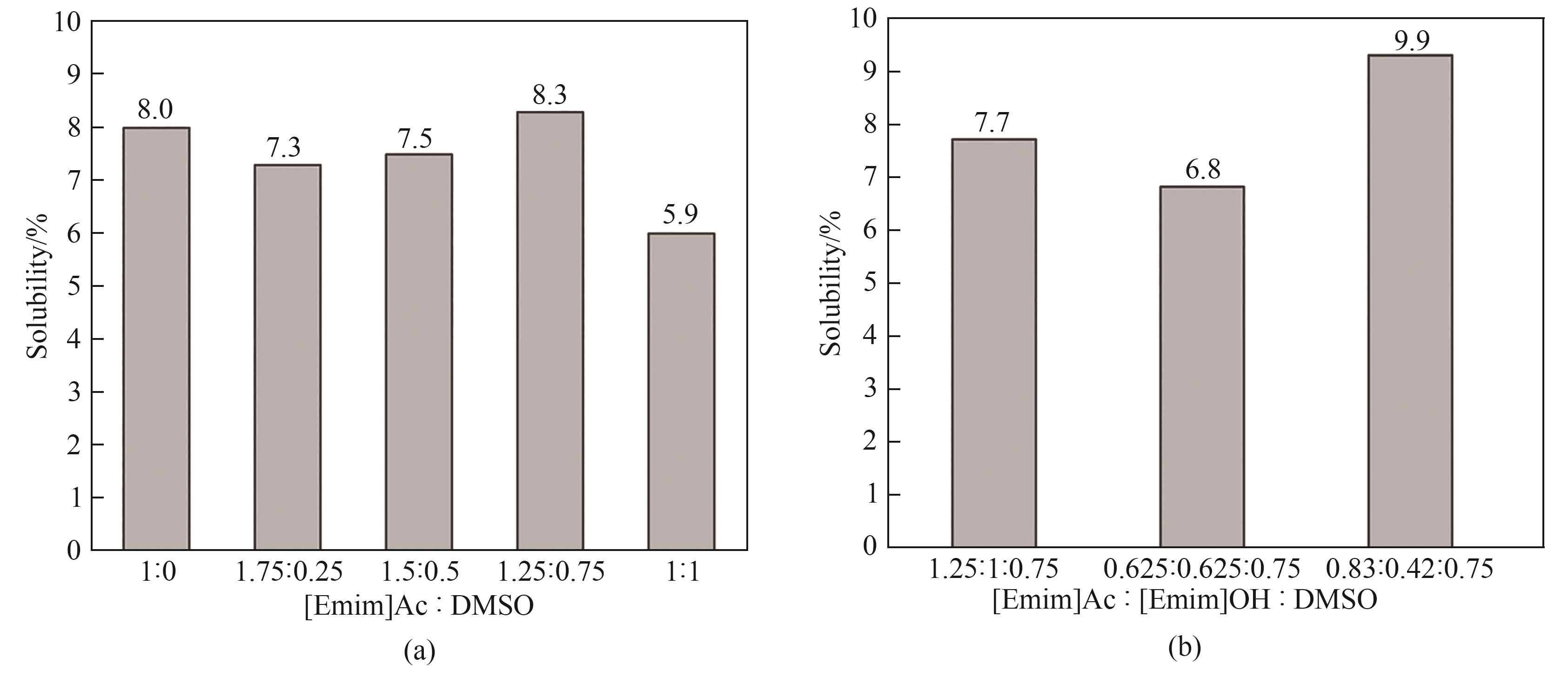
图4 [Emim]Ac-DMSO 及 [Emim]Ac-[Emim]OH-DMSO混合离子液体中纤维素的溶解度
Fig. 4 Solubility of cellulose in [Emim]Ac-DMSO and [Emim]Ac-[Emim]OH-DMSO mixed ionic liquids
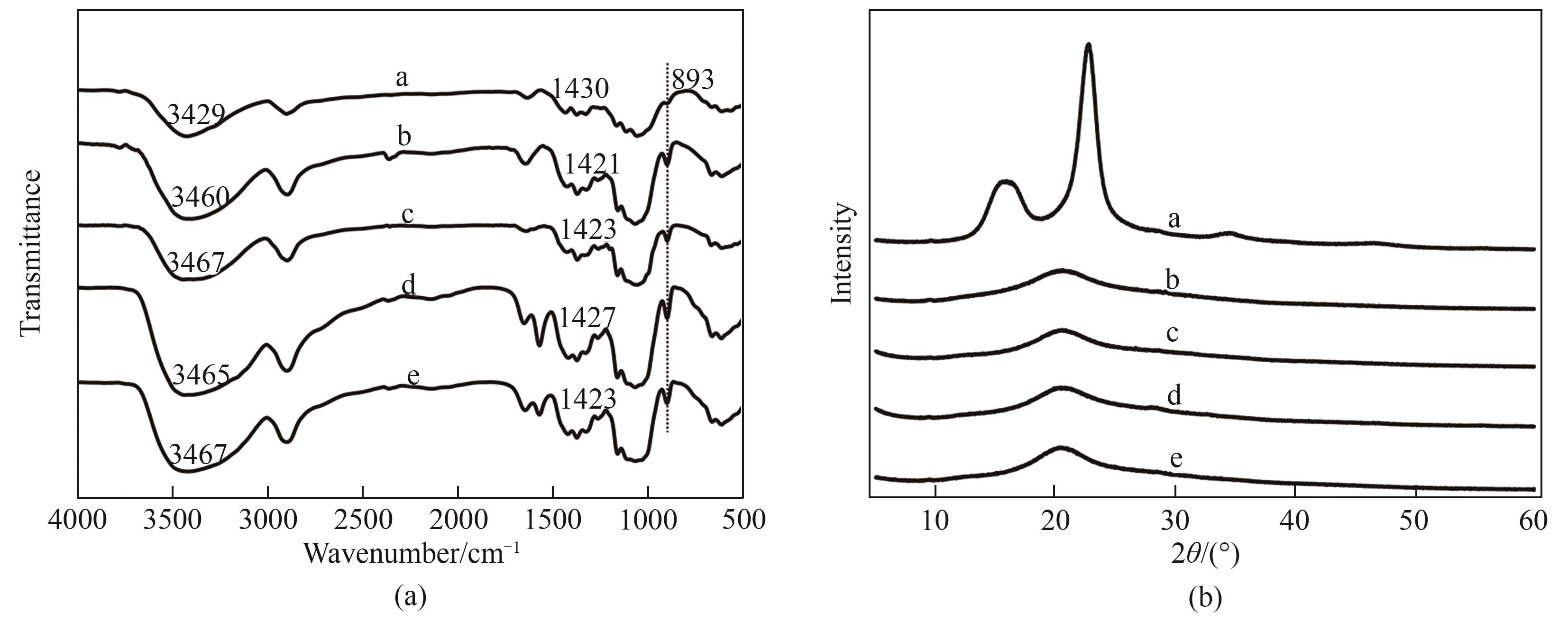
图6 纤维素及再生纤维素的 FTIR 谱图(a)和XRD谱图(b)[a为原纤维素,b~e分别为经 [Emim]Ac、[Emim]Ac/DMSO(1.25∶0.75)、[Emim]Ac/[Emim]OH/DMSO(0.625∶0.625∶0.75)、[Emim]Ac/[Emim]OH/DMSO(0.83∶0.42∶0.75)溶解再生的纤维素]
Fig.6 FTIR spectra (a) and XRD patterns (b) of cellulose and regenerated cellulose [a was protocellulose; b—e were dissolved and regenerated cellulose obtained by [Emim]Ac, [Emim]Ac/DMSO (1.25∶0.75), [Emim]Ac/[Emim]OH/DMSO (0.625∶0.625∶0.75), [Emim]Ac/[Emim]OH/DMSO (0.83∶0.42∶0.75), respectively)
| 样品 | 结晶度/ % | 聚合度(DP) |
|---|---|---|
| Raw | 64.0 | 523 |
| [Emim]Ac | 19.3 | 423 |
| [Emim]Ac/[Emim]OH/DMSO(0.625∶0.625∶0.75) | 20.2 | 359 |
| [Emim]Ac/[Emim]OH/DMSO(0.83∶0.42∶0.75) | 28.8 | 466 |
表3 纤维素及再生纤维素的结晶度和聚合度
Table 3 Crystallinity and polymerization of cellulose and regenerated cellulose
| 样品 | 结晶度/ % | 聚合度(DP) |
|---|---|---|
| Raw | 64.0 | 523 |
| [Emim]Ac | 19.3 | 423 |
| [Emim]Ac/[Emim]OH/DMSO(0.625∶0.625∶0.75) | 20.2 | 359 |
| [Emim]Ac/[Emim]OH/DMSO(0.83∶0.42∶0.75) | 28.8 | 466 |
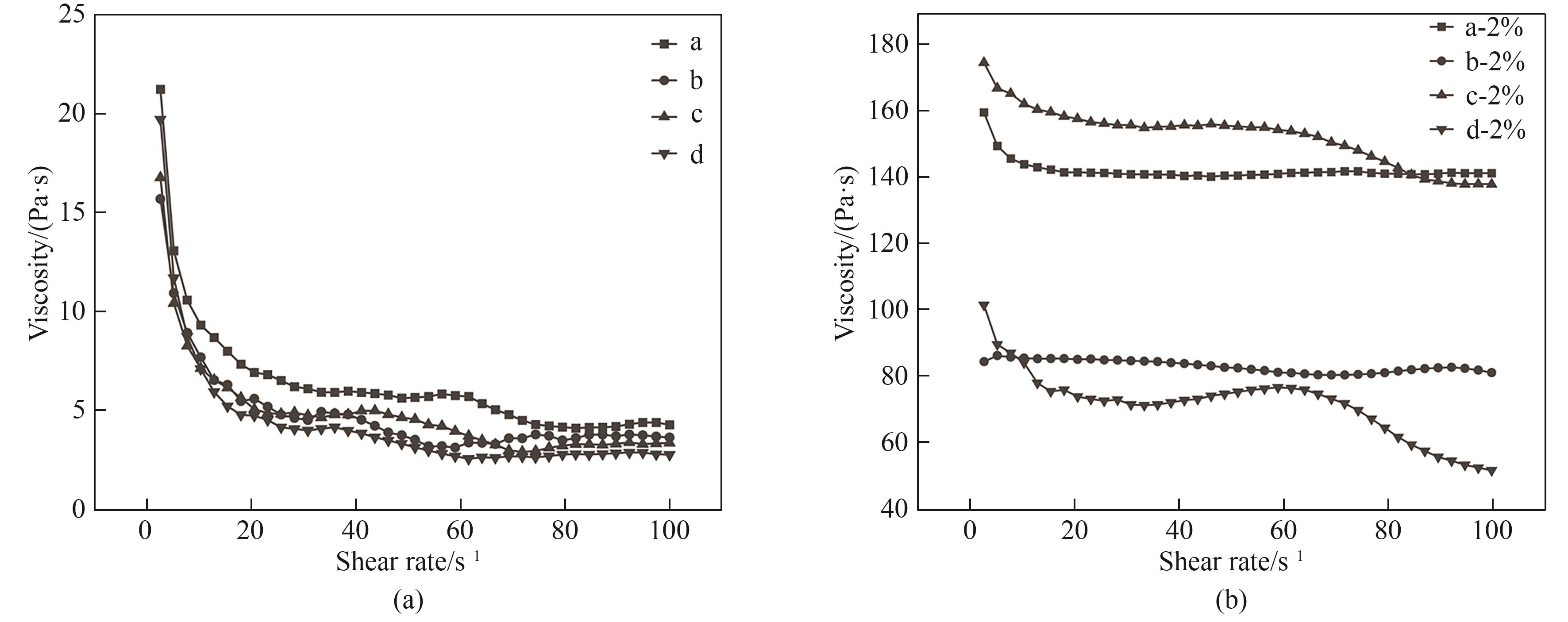
图7 离子液体和溶解2%纤维素的离子液体的黏度随剪切速率的变化
Fig.7 Viscosity changes of ionic liquid and ionic liquid dissolved 2% cellulose with shear rate a—[Emim]Ac; b—[Emim]Ac/DMSO (1.25∶0.75); c—[Emim]Ac/[Emim]OH/DMSO (0.625∶0.625∶0.75); d—[Emim]Ac/[Emim]OH/DMSO (0.83∶0.42∶0.75)
| [1] | French A D. Glucose, not cellobiose, is the repeating unit of cellulose and why that is important[J]. Cellulose, 2017, 24(11): 4605-4609. |
| [2] | Arend M. Immunolocalization of (1, 4)-galactan in tension wood fibers of poplar[J]. Tree Physiology, 2008, 28(8): 1263-1267. |
| [3] | Klemm D, Heablein B, Fink H, et al. Cellulose: fascinating biopolymer and sustainable raw material[J]. Angewandte Chemie International Edition, 2005, 44(22): 3358-3393. |
| [4] | Jedvert K, Heinze T. Cellulose modification and shaping—a review[J]. Journal of Polymer Engineering, 2017, 37(9): 845-860. |
| [5] | Zhang J M, Zhang H, Wu J, et al. NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids[J]. Physical Chemistry Chemical Physics, 2010, 12(8): 1941-1947. |
| [6] | Kraemer T R, Reyes G, Cartes M, et al. Ionic liquid interactions with cellulose and the effect of water[J]. Cellulose, 2024, 31(11): 6597-6610. |
| [7] | Deetlefs M, Seddon K R, Shara M. Predicting physical properties of ionic liquids[J]. Physical Chemistry Chemical Physics, 2006, 8(5): 642-649. |
| [8] | Abe M, Kuroda K, Sato D, et al. Effects of polarity, hydrophobicity, and density of ionic liquids on cellulose solubility[J]. Physical Chemistry Chemical Physics, 2015, 17(48): 32276-32282. |
| [9] | Zhang J M, Xu L L, Yu J, et al. Understanding cellulose dissolution: effect of the cation and anion structure of ionic liquids on the solubility of cellulose[J]. Science China Chemistry, 2016, 59(11): 1421-1429. |
| [10] | Lu X M, Xu S J, Chen J Z, et al. Cellulose dissolution in ionic liquid from hydrogen bonding perspective: first-principles calculations[J]. Cellulose, 2023, 30(7): 4181-4195. |
| [11] | 鲁兴美. DFT研究纤维素在离子液体中的溶解机理及EmimOH溶剂体系的开发[D]. 福州: 福建农林大学, 2023. |
| Lu X M. DFT study on dissolution mechanism of cellulose in ionic liquids and development of EmimOH solvent system[D]. Fuzhou: Fujian Agriculture and Forestry University, 2023. | |
| [12] | Zhao Y L, Liu X M, Wang J J, et al. Insight into the cosolvent effect of cellulose dissolution in imidazolium-based ionic liquid systems[J]. The Journal of Physical Chemistry B, 2013, 117(30): 9042-9049. |
| [13] | Ni L F, Lin C M, Zhang H, et al. Synergistic action of EmimAc and aqueous NaOH for selective dissolution of hemicellulose for cellulose purification[J]. Cellulose, 2021, 28(3): 1331-1338. |
| [14] | Delley B. A scattering theoretic approach to scalar relativistic corrections on bonding[J]. International Journal of Quantum Chemistry, 1998, 69(3): 423-433. |
| [15] | Delley B. From molecules to solids with the DMol3 approach[J]. Journal of Chemical Physics, 2000, 113(18): 7756-7764. |
| [16] | Ndruru S T C L, Wahyuningrum D, Bundjali B, et al. Green synthesis of [EMIm]Ac ionic liquid for plasticizing MC-based biopolymer electrolyte membranes[J]. Bulletin of Chemical Reaction Engineering & Catalysis, 2019, 14(2): 345-357. |
| [17] | Mansikkamäki P, Lahtinen M, Rissanen K. The conversion from cellulose Ⅰ to cellulose Ⅱ in NaOH mercerization performed in alcohol-water systems: an X-ray powder diffraction study[J]. Carbohydrate Polymers, 2007, 68(1): 35-43. |
| [18] | Shakourian-Fard M, Ghenaatian H R, Kamath G, et al. Unraveling the effect of nitrogen doping on graphene nanoflakes and the adsorption properties of ionic liquids: a DFT study[J]. Journal of Molecular Liquids, 2020, 312: 113400. |
| [19] | 何宏艳. 离子液体中离子对结构及氢键相互作用研究[D]. 北京: 中国科学院大学, 2013. |
| He H Y. Study on ion pair structure and hydrogen bond interaction in ionic liquids[D]. Beijing: University of Chinese Academy of Sciences, 2013. | |
| [20] | Hunt P A, Ashworth C R, Matthews R P. Hydrogen bonding in ionic liquids[J]. Chemical Society Reviews, 2015, 44(5): 1257-1288. |
| [21] | 兰嫒, 李欣达, 张玥, 等. DMSO对纤维素在咪唑型离子液体中溶解性能的影响[J]. 材料科学与工程学报, 2015, 33(1): 93-97. |
| Lan Y, Li X D, Zhang Y, et al. Effect of DMSO addition on dissolution of cellulose in ionic liquid[J]. Journal of Materials Science and Engineering, 2015, 33(1): 93-97. | |
| [22] | Li T, Li S X, Kong W Q, et al. A nanofluidic ion regulation membrane with aligned cellulose nanofibers[J]. Science Advances, 2019, 5(2): eaau4238. |
| [23] | 李承杰. 纤维素在离子液体中溶解及再生过程的分子动力学模拟[D]. 青岛: 青岛大学, 2022. |
| Li C J. Molecular dynamics simulation of cellulose dissolution and regeneration in ionic liquids[D]. Qingdao: Qingdao University, 2022. | |
| [24] | 潘心悦, 杨祥建, 龚润竹, 等. 非衍生化溶剂体系及再生工艺对纤维素结构与性能的影响[J]. 中国造纸学报, 2023, 38(4): 107-115. |
| Pan X Y, Yang X J, Gong R Z, et al. Effect of non-derived solvent system and regeneration process on cellulose structure and properties[J]. Transactions of China Pulp and Paper, 2023, 38(4): 107-115. | |
| [25] | Li X, Li H C, Ling Z, et al. Room-temperature superbase-derived ionic liquids with facile synthesis and low viscosity: powerful solvents for cellulose dissolution by destroying the cellulose aggregate structure[J]. Macromolecules, 2020, 53(9): 3284-3295. |
| [26] | 马继玮, 姜泽明, 高鑫, 等. 离子液体中再生纤维素纤维的制备及表征[J]. 高分子材料科学与工程, 2019, 35(10): 176-182, 190. |
| Ma J W, Jiang Z M, Gao X, et al. Preparation and characterization of regenerated cellulose fibers in ionic liquid[J]. Polymer Materials Science & Engineering, 2019, 35(10): 176-182, 190. | |
| [27] | 马浩, 廖春燕, 樊梅林, 等. 羧酸根阴离子型功能化离子液体对纤维素的溶解性能[J]. 应用化学, 2018, 35(4): 449-456. |
| Ma H, Liao C Y, Fan M L, et al. Dissolution of cellulose in carboxylate-based task-specific ionic liquids[J]. Chinese Journal of Applied Chemistry, 2018, 35(4): 449-456. | |
| [28] | Knill C J, Kennedy J F. Degradation of cellulose under alkaline conditions[J]. Carbohydrate Polymers, 2003, 51(3): 281-300. |
| [29] | Le K A, Sescousse R, Budtova T. Influence of water on cellulose-EMIMAc solution properties: a viscometric study[J]. Cellulose, 2012, 19(1): 45-54. |
| [30] | 郑勇, 郑永军, 田大勇, 等. 3种氯铝酸离子液体的密度、黏度和电导率的研究[J]. 河南师范大学学报(自然科学版), 2019, 47(6): 65-70. |
| Zheng Y, Zheng Y J, Tian D Y, et al. The density, viscosity and electrical conductivity of three chloroaluminate-based ionic liquids[J]. Journal of Henan Normal University (Natural Science Edition), 2019, 47(6): 65-70. | |
| [31] | Xu A R, Zhang Y J, Zhao Y, et al. Cellulose dissolution at ambient temperature: role of preferential solvation of cations of ionic liquids by a cosolvent[J]. Carbohydrate Polymers, 2013, 92(1): 540-544. |
| [1] | 徐鹏国, 孟子衡, 朱干宇, 李会泉, 王晨晔, 孙振华, 田国才. 粗碳酸锂CO2微气泡深度碳化工艺与动力学研究[J]. 化工学报, 2025, 76(7): 3325-3338. |
| [2] | 孙文浩, 田君, 张锟, 刘娜, 曹宝森, 梁晓嫱. 锂离子电池用高热稳定性新型隔膜的研究新进展[J]. 化工学报, 2025, 76(6): 2524-2543. |
| [3] | 刘鑫, 郑皓仁, 陈强, 丁静怡, 黄康, 徐至. 全钒液流电池用纤维素纳米晶掺杂混合基质膜[J]. 化工学报, 2025, 76(5): 2294-2303. |
| [4] | 李家顺, 李旺, 秦祖赠, 苏通明, 谢新玲, 纪红兵. 聚酰亚胺增强木质纤维素纳米纤丝气凝胶制备及其油水分离性能研究[J]. 化工学报, 2025, 76(5): 2169-2185. |
| [5] | 张奇, 张睿, 郑涛, 曹欣, 刘植昌, 刘海燕, 徐春明, 张荣, 孟祥海. 基于分子模拟的新型双阳离子质子型离子液体捕集CO2研究[J]. 化工学报, 2025, 76(2): 797-811. |
| [6] | 崔家馨, 殷梦凡, 郑涛, 刘晗, 张睿, 刘植昌, 刘海燕, 徐春明, 孟祥海. 铝铜双金属离子液体在1-己烯/正己烷分离中的应用[J]. 化工学报, 2025, 76(2): 686-694. |
| [7] | 张闯德, 陈黎. 优势通道对多孔介质中多相反应输运过程影响的孔隙尺度研究[J]. 化工学报, 2025, 76(1): 161-172. |
| [8] | 邹吉军, 刘宝宏, 史成香, 潘伦, 张香文. 综纤维素衍生物转化合成生物航空燃料的非均相催化剂研究进展[J]. 化工学报, 2025, 76(1): 1-17. |
| [9] | 张思文, 顾海明, 赵善辉. 纳米氧化铁对纤维素化学链气化的分子反应机理[J]. 化工学报, 2025, 76(1): 363-373. |
| [10] | 冯海军, 章冰璇, 周健. 图神经网络模型预测和解释离子液体毒性的研究[J]. 化工学报, 2025, 76(1): 93-106. |
| [11] | 李玲玉, 胡鑫, 成怀刚, 赵云, 安东, 马玉军, 金家豪, 于旭东, 张卫东. K+(Mg2+), Ca2+//Cl--H2O三元水盐体系的等温蒸发成盐相区[J]. 化工学报, 2025, 76(1): 120-130. |
| [12] | 邱知, 谭明. 聚离子液体膜的制备及其在低钠高钾健康酱油中的应用[J]. 化工学报, 2024, 75(S1): 244-250. |
| [13] | 王新月, 徐小虎, 张海洋, 尹春华. 维生素A醋酸酯/环糊精包合及性质研究[J]. 化工学报, 2024, 75(S1): 321-328. |
| [14] | 赵武灵, 满奕. 基于变分编码器的纳米纤维素分子结构预测模型框架研究[J]. 化工学报, 2024, 75(9): 3221-3230. |
| [15] | 杜海燕, 朱凯, 游峰, 王金凤, 赵一帆, 张楠, 李英. 用于应变传感器的自愈合抗冻离子水凝胶[J]. 化工学报, 2024, 75(7): 2709-2722. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号