化工学报 ›› 2025, Vol. 76 ›› Issue (2): 686-694.DOI: 10.11949/0438-1157.20241074
• 分离工程 • 上一篇
崔家馨( ), 殷梦凡, 郑涛, 刘晗, 张睿, 刘植昌, 刘海燕, 徐春明, 孟祥海(
), 殷梦凡, 郑涛, 刘晗, 张睿, 刘植昌, 刘海燕, 徐春明, 孟祥海( )
)
收稿日期:2024-09-25
修回日期:2024-11-04
出版日期:2025-03-25
发布日期:2025-03-10
通讯作者:
孟祥海
作者简介:崔家馨(1997—),女,博士研究生,2796867498@qq.com
基金资助:
Jiaxin CUI( ), Mengfan YIN, Tao ZHENG, Han LIU, Rui ZHANG, Zhichang LIU, Haiyan LIU, Chunming XU, Xianghai MENG(
), Mengfan YIN, Tao ZHENG, Han LIU, Rui ZHANG, Zhichang LIU, Haiyan LIU, Chunming XU, Xianghai MENG( )
)
Received:2024-09-25
Revised:2024-11-04
Online:2025-03-25
Published:2025-03-10
Contact:
Xianghai MENG
摘要:
石脑油中烯烃/烷烃的分离是石油化工过程原料优化和产品精制的需求,液液萃取是烯烃/烷烃分离的重要途径。以1-己烯/正己烷为模型化合物,探讨了铝铜双金属离子液体选择性萃取烯烃的可行性。结果表明,铝铜双金属离子液体对1-己烯/正己烷具有较好的分离性能,在剂油质量比为2、萃取温度10℃的条件下,1-己烯萃取选择性为6.33,优于常规咪唑型离子液体和传统有机溶剂。此外,铝铜双金属离子液体具有良好的重复使用性能。通过同步辐射X射线吸收精细结构光谱(XAFS)和密度泛函理论(DFT)揭示了双金属离子液体阴离子结构。利用量子化学计算手段对离子液体与1-己烯、正己烷相互作用进行计算分析,解释了双金属离子液体选择性分离1-己烯的原理。
中图分类号:
崔家馨, 殷梦凡, 郑涛, 刘晗, 张睿, 刘植昌, 刘海燕, 徐春明, 孟祥海. 铝铜双金属离子液体在1-己烯/正己烷分离中的应用[J]. 化工学报, 2025, 76(2): 686-694.
Jiaxin CUI, Mengfan YIN, Tao ZHENG, Han LIU, Rui ZHANG, Zhichang LIU, Haiyan LIU, Chunming XU, Xianghai MENG. Application of aluminum-copper bimetallic ionic liquids in 1-hexene/n-hexane separation[J]. CIESC Journal, 2025, 76(2): 686-694.
| 萃取剂 | S | D | PI |
|---|---|---|---|
| N-甲酰基吗啉 | 2.12 | 0.046 | 0.10 |
| N-甲基吡咯烷酮 | 1.31 | 0.301 | 0.39 |
| 乙二醇 | 2.25 | 0.028 | 0.06 |
| [Bmim]Cl-1.0FeCl3 | 2.40 | 0.041 | 0.10 |
| [Bmim]Cl-1.0AlCl3 | 2.39 | 0.036 | 0.09 |
[Bmim]Cl-1.0CuCl [Bmim]Cl-1.5CuCl [Bmim]Cl-2.0CuCl | 2.96 4.39 6.41 | 0.034 0.034 0.039 | 0.10 0.15 0.25 |
| [Bmim]NTf2 | 1.82 | 0.142 | 0.26 |
| [Bmim]BF4 | 2.45 | 0.032 | 0.08 |
| [Bmim]PF6 | 2.53 | 0.042 | 0.11 |
| [Bmim]Cl-1.0AlCl3+[Bmim]Cl-2.0CuCl | 3.24 | 0.040 | 0.13 |
| [Bmim]Cl-0.6AlCl3-1.0CuCl | 6.33 | 0.073 | 0.46 |
表1 萃取剂的烯烃分离性能
Table 1 Olefin separation performance of extractants
| 萃取剂 | S | D | PI |
|---|---|---|---|
| N-甲酰基吗啉 | 2.12 | 0.046 | 0.10 |
| N-甲基吡咯烷酮 | 1.31 | 0.301 | 0.39 |
| 乙二醇 | 2.25 | 0.028 | 0.06 |
| [Bmim]Cl-1.0FeCl3 | 2.40 | 0.041 | 0.10 |
| [Bmim]Cl-1.0AlCl3 | 2.39 | 0.036 | 0.09 |
[Bmim]Cl-1.0CuCl [Bmim]Cl-1.5CuCl [Bmim]Cl-2.0CuCl | 2.96 4.39 6.41 | 0.034 0.034 0.039 | 0.10 0.15 0.25 |
| [Bmim]NTf2 | 1.82 | 0.142 | 0.26 |
| [Bmim]BF4 | 2.45 | 0.032 | 0.08 |
| [Bmim]PF6 | 2.53 | 0.042 | 0.11 |
| [Bmim]Cl-1.0AlCl3+[Bmim]Cl-2.0CuCl | 3.24 | 0.040 | 0.13 |
| [Bmim]Cl-0.6AlCl3-1.0CuCl | 6.33 | 0.073 | 0.46 |
| Path | N | R/Å | σ2/Å2 |
|---|---|---|---|
| Cu—Cl | 2.00 | 2.21 | 0.013 |
| Cu—Al | 1.45 | 2.93 | 0.024 |
表2 Cu K-edge XAFS的曲线拟合参数
Table 2 Curve-fit parameters for Cu K-edge XAFS
| Path | N | R/Å | σ2/Å2 |
|---|---|---|---|
| Cu—Cl | 2.00 | 2.21 | 0.013 |
| Cu—Al | 1.45 | 2.93 | 0.024 |
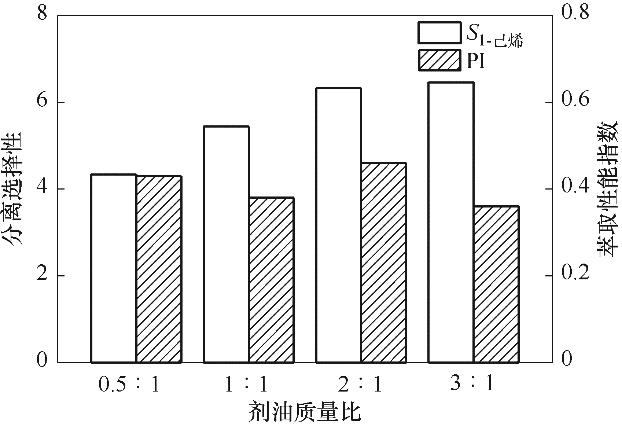
图6 剂油质量比对分离选择性和萃取性能指数的影响
Fig.6 Influence of the mass ratio of solvent to feed on the separation selectivity and extraction performance index of the extraction
| 离子液体+1-己烯/正己烷 | ΔE/(kJ/mol) |
|---|---|
| [Bmim][CuCl2]+1-己烯 | -89.79 |
| [Bmim][CuAlCl5]+1-己烯 | -138.08 |
| [Bmim][CuCl2]+正己烷 | -24.89 |
| [Bmim][CuAlCl5]+正己烷 | -19.99 |
表3 离子液体与1-己烯/正己烷之间的结合能比较
Table 3 Comparison of binding energies of ionic liquids with 1-hexene and n-hexane
| 离子液体+1-己烯/正己烷 | ΔE/(kJ/mol) |
|---|---|
| [Bmim][CuCl2]+1-己烯 | -89.79 |
| [Bmim][CuAlCl5]+1-己烯 | -138.08 |
| [Bmim][CuCl2]+正己烷 | -24.89 |
| [Bmim][CuAlCl5]+正己烷 | -19.99 |
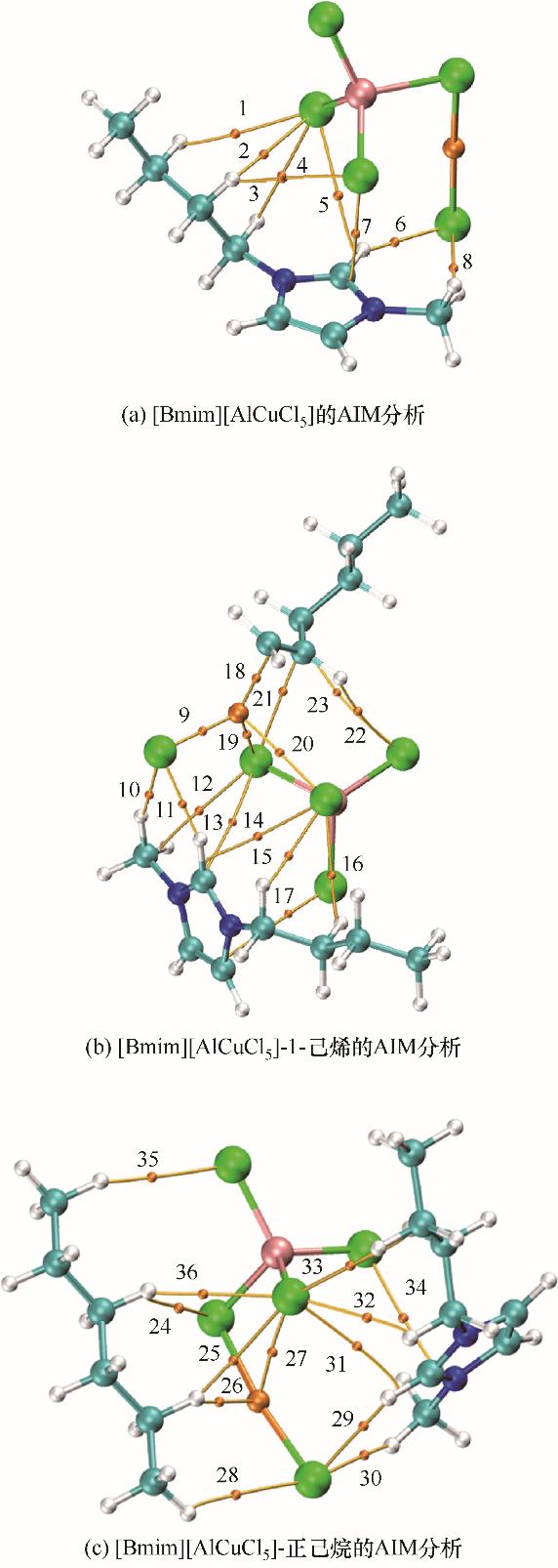
图10 [Bmim][AlCuCl5]、[Bmim][AlCuCl5]-1-己烯及[Bmim][AlCuCl5]-正己烷的AIM分析(球体代表原子;N:蓝色;H:白色;C:青色;Cl:绿色;Cu:橙色;Al:粉色)
Fig.10 The AIM analysis for [Bmim][AlCuCl5], [Bmim][AlCuCl5]-1-hexene and [Bmim][AlCuCl5]-n-hexane (spheres represent the atoms; N: blue; H: white; C: cyan; Cl: green; Cu: orange; Al: pink)
| 体系 | BCP | 原子标签 | ρ(BCP) | ∇2ρ(BCP) | H(BCP) | |
|---|---|---|---|---|---|---|
| [Bmim][AlCuCl5] | 1 | C—H…Cl | 0.0046 | 0.0159 | 0.0009 | |
| 2 | C—H…Cl | 0.0072 | 0.0241 | 0.0012 | ||
| 3 | C—H…Cl | 0.0077 | 0.0257 | 0.0013 | ||
| 4 | C—H…Cl | 0.0045 | 0.0135 | 0.0007 | ||
| 5 | C—H…Cl | 0.0041 | 0.0142 | 0.0008 | ||
| 6 | C—H…Cl | 0.0174 | 0.0532 | 0.0019 | ||
| 7 | C…Cl | 0.0082 | 0.0279 | 0.0013 | ||
| 8 | C—H…Cl | 0.0131 | 0.0397 | 0.0017 | ||
| [Bmim][AlCuCl5]-1-己烯 | 9 | Cu…Cl | 0.0783 | 0.2614 | 0.0238 | |
| 10 | C—H…Cl | 0.0110 | 0.0328 | 0.0015 | ||
| 11 | C—H…Cl | 0.0217 | 0.0631 | 0.0015 | ||
| 12 | C…Cl | 0.0065 | 0.0239 | 0.0013 | ||
| 13 | C…Cl | 0.0036 | 0.0122 | 0.0008 | ||
| 14 | C…Cl | 0.0050 | 0.0168 | 0.0009 | ||
| 15 | C—H…Cl | 0.0068 | 0.0209 | 0.0010 | ||
| 16 | C—H…Cl | 0.0068 | 0.0232 | 0.0012 | ||
| 17 | C—H…Cl | 0.0077 | 0.0259 | 0.0013 | ||
| 18 | Cu…C | 0.0831 | 0.2393 | -0.0257 | ||
| 19 | Cu…Cl | 0.0463 | 0.1723 | -0.0061 | ||
| 20 | Cu…Cl | 0.0155 | 0.0433 | -0.0001 | ||
| 21 | C—H…Cl | 0.0075 | 0.0268 | 0.0014 | ||
| 22 | C—H…Cl | 0.0047 | 0.0152 | 0.0008 | ||
| 23 | C—H…Cl | 0.0027 | 0.0097 | 0.0006 | ||
| [Bmim][AlCuCl5]-正己烷 | 24 | C—H…Cl | 0.0052 | 0.0156 | 0.0008 | |
| 25 | C—H…Cl | 0.0064 | 0.0190 | 0.0009 | ||
| 26 | Cu…H | 0.0134 | 0.0337 | -0.0004 | ||
| 27 | Cu…Cl | 0.0145 | 0.0354 | -0.0003 | ||
| 28 | C—H…Cl | 0.0034 | 0.0110 | 0.0006 | ||
| 29 | C—H…Cl | 0.0176 | 0.0538 | 0.0019 | ||
| 30 | C—H…Cl | 0.0124 | 0.0376 | 0.0016 | ||
| 31 | C—H…Cl | 0.0049 | 0.0168 | 0.0010 | ||
| 32 | C—H…Cl | 0.0076 | 0.0257 | 0.0013 | ||
| 33 | C—H…Cl | 0.0041 | 0.0148 | 0.0009 | ||
| 34 | C—N…Cl | 0.0077 | 0.0269 | 0.0013 | ||
| 35 | C—H…Cl | 0.0038 | 0.0116 | 0.0006 | ||
| 36 | C—H…Cl | 0.0041 | 0.0143 | 0.0009 | ||
表4 BCPs拓扑性质
Table 4 Topological properties of BCPs
| 体系 | BCP | 原子标签 | ρ(BCP) | ∇2ρ(BCP) | H(BCP) | |
|---|---|---|---|---|---|---|
| [Bmim][AlCuCl5] | 1 | C—H…Cl | 0.0046 | 0.0159 | 0.0009 | |
| 2 | C—H…Cl | 0.0072 | 0.0241 | 0.0012 | ||
| 3 | C—H…Cl | 0.0077 | 0.0257 | 0.0013 | ||
| 4 | C—H…Cl | 0.0045 | 0.0135 | 0.0007 | ||
| 5 | C—H…Cl | 0.0041 | 0.0142 | 0.0008 | ||
| 6 | C—H…Cl | 0.0174 | 0.0532 | 0.0019 | ||
| 7 | C…Cl | 0.0082 | 0.0279 | 0.0013 | ||
| 8 | C—H…Cl | 0.0131 | 0.0397 | 0.0017 | ||
| [Bmim][AlCuCl5]-1-己烯 | 9 | Cu…Cl | 0.0783 | 0.2614 | 0.0238 | |
| 10 | C—H…Cl | 0.0110 | 0.0328 | 0.0015 | ||
| 11 | C—H…Cl | 0.0217 | 0.0631 | 0.0015 | ||
| 12 | C…Cl | 0.0065 | 0.0239 | 0.0013 | ||
| 13 | C…Cl | 0.0036 | 0.0122 | 0.0008 | ||
| 14 | C…Cl | 0.0050 | 0.0168 | 0.0009 | ||
| 15 | C—H…Cl | 0.0068 | 0.0209 | 0.0010 | ||
| 16 | C—H…Cl | 0.0068 | 0.0232 | 0.0012 | ||
| 17 | C—H…Cl | 0.0077 | 0.0259 | 0.0013 | ||
| 18 | Cu…C | 0.0831 | 0.2393 | -0.0257 | ||
| 19 | Cu…Cl | 0.0463 | 0.1723 | -0.0061 | ||
| 20 | Cu…Cl | 0.0155 | 0.0433 | -0.0001 | ||
| 21 | C—H…Cl | 0.0075 | 0.0268 | 0.0014 | ||
| 22 | C—H…Cl | 0.0047 | 0.0152 | 0.0008 | ||
| 23 | C—H…Cl | 0.0027 | 0.0097 | 0.0006 | ||
| [Bmim][AlCuCl5]-正己烷 | 24 | C—H…Cl | 0.0052 | 0.0156 | 0.0008 | |
| 25 | C—H…Cl | 0.0064 | 0.0190 | 0.0009 | ||
| 26 | Cu…H | 0.0134 | 0.0337 | -0.0004 | ||
| 27 | Cu…Cl | 0.0145 | 0.0354 | -0.0003 | ||
| 28 | C—H…Cl | 0.0034 | 0.0110 | 0.0006 | ||
| 29 | C—H…Cl | 0.0176 | 0.0538 | 0.0019 | ||
| 30 | C—H…Cl | 0.0124 | 0.0376 | 0.0016 | ||
| 31 | C—H…Cl | 0.0049 | 0.0168 | 0.0010 | ||
| 32 | C—H…Cl | 0.0076 | 0.0257 | 0.0013 | ||
| 33 | C—H…Cl | 0.0041 | 0.0148 | 0.0009 | ||
| 34 | C—N…Cl | 0.0077 | 0.0269 | 0.0013 | ||
| 35 | C—H…Cl | 0.0038 | 0.0116 | 0.0006 | ||
| 36 | C—H…Cl | 0.0041 | 0.0143 | 0.0009 | ||
| 1 | 石博文, 朱楠, 海红莲, 等. 煤制油费托α-烯烃增值利用及发展展望[J]. 合成材料老化与应用, 2023, 52(5): 113-116. |
| Shi B W, Zhu N, Hai H L, et al. Value-added utilization and development prospect of Fischer-α-olefin from coal to oil[J]. Synthetic Materials Aging and Application, 2023, 52(5): 113-116. | |
| 2 | 白玫. ACO技术制备烯烃工艺研究及展望[J]. 化工与医药工程, 2017, 38(3): 18-23. |
| Bai M. Research of ACO technique used in preparation of olefins and expectation[J]. Chemical and Pharmaceutical Engineering, 2017, 38(3): 18-23. | |
| 3 | Lin Y H, Yu L, Ullah S, et al. Temperature-programmed separation of hexane isomers by a porous calcium chloranilate metal-organic framework[J]. Angewandte Chemie International Edition, 2022, 61(50): e202214060. |
| 4 | Sholl D S, Lively R P. Seven chemical separations to change the world[J]. Nature, 2016, 532(7600): 435-437. |
| 5 | Zhu L, Li F F, Zhu J Q, et al. Liquid-liquid equilibria of ternary systems of 1-hexene/hexane and extraction solvents[J]. Chemical Papers, 2016, 70(5): 585-593. |
| 6 | 容凡丁, 丁泽相, 曹义风, 等. 离子液体强化不饱和键差异化合物分离的研究进展[J]. 化工进展, 2024, 43(1): 198-214. |
| Rong F D, Ding Z X, Cao Y F, et al. Progress in enhanced separation of compounds differing in unsaturated bonds by ionic liquids[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 198-214. | |
| 7 | 吴沛文, 荀苏杭, 蒋伟, 等. 离子液体反应型萃取燃油脱硫研究进展[J]. 化工学报, 2021, 72(1): 276-291. |
| Wu P W, Xun S H, Jiang W, et al. Recent progress on extractive desulfurization of fuel oils through reactions based on ionic liquids as solvents and catalysts[J]. CIESC Journal, 2021, 72(1): 276-291. | |
| 8 | 吕玉苗, 陈伟, 王艳磊, 等. 离子液体二维结构制备及其特性研究进展[J]. 化学学报, 2021, 79(4): 443-458. |
| Lyu Y M, Chen W, Wang Y L, et al. Research progress on the preparation and properties of two dimensional structure of ionic liquids[J]. Acta Chimica Sinica, 2021, 79(4): 443-458. | |
| 9 | 李瑞, 崔现宝, 吴添, 等. 基于COSMO-SAC模型的离子液体萃取剂的选择[J]. 化工学报, 2013, 64(2): 452-469. |
| Li R, Cui X B, Wu T, et al. Selection of ionic liquid solvent for liquid-liquid extraction based on COSMO-SAC model[J]. CIESC Journal, 2013, 64(2): 452-469. | |
| 10 | Xing H B, Zhao X, Li R L, et al. Improved efficiency of ethylene/ethane separation using a symmetrical dual nitrile-functionalized ionic liquid[J]. ACS Sustainable Chemistry & Engineering, 2013, 1(11): 1357-1363. |
| 11 | Wang Y, Hao W Y, Jacquemin J, et al. Enhancing liquid-phase olefin-paraffin separations using novel silver-based ionic liquids[J]. Journal of Chemical & Engineering Data, 2015, 60(1): 28-36. |
| 12 | Li H, Zhang Z S, Sun G L, et al. Performance and mechanism of the separation of C8 α-olefin from F-T synthesis products using novel Ag-DES[J]. AIChE Journal, 2021, 67(8): e17252. |
| 13 | Yu G R, Deng L Y, Abdeltawab A A, et al. Functional solution composed of Cu(Ⅰ) salt and ionic liquids to separate propylene from propane[J]. Industrial & Engineering Chemistry Research, 2014, 53(34): 13430-13435. |
| 14 | Yu G R, Zhang L, Alhumaydhi I A, et al. Separation of propylene and propane by alkylimidazolium thiocyanate ionic liquids with Cu+ salt[J]. Separation and Purification Technology, 2015, 156: 356-362. |
| 15 | 张睿, 董淑媛, 伍洛, 等. 小分子烷烃与烯烃在离子液体中的溶解性能[J]. 化工学报, 2020, 71(10): 4674-4687. |
| Zhang R, Dong S Y, Wu L, et al. Solubility of light alkanes and alkenes in ionic liquids[J]. CIESC Journal, 2020, 71(10): 4674-4687. | |
| 16 | Chen X C, Ming S M, Wu X Y, et al. Cu(Ⅰ)-based ionic liquids as potential absorbents to separate propylene and propane[J]. Separation Science and Technology, 2013, 48(15): 2317-2323. |
| 17 | Wentink A E, Kockmann D, Kuipers N J M, et al. Effect of C6-olefin isomers on π-complexation for purification of 1-hexene by reactive extractive distillation[J]. Separation and Purification Technology, 2005, 43(2): 149-162. |
| 18 | Capracotta M D, Sullivan R M, Martin J D. Sorptive reconstruction of CuMCl4 (M = Al and Ga) upon small-molecule binding and the competitive binding of CO and ethylene[J]. Journal of the American Chemical Society, 2006, 128(41): 13463-13473. |
| 19 | Anantharaj R, Banerjee T. COSMO-RS-based screening of ionic liquids as green solvents in denitrification studies[J]. Industrial & Engineering Chemistry Research, 2010, 49(18): 8705-8725. |
| 20 | Adamo C, Barone V. Toward reliable density functional methods without adjustable parameters: the PBE0 model[J]. Journal of Chemical Physics, 1999, 110(13): 6158-6170. |
| 21 | Neese F. Software update: the ORCA program system, version 4.0[J]. WIREs Computational Molecular Science, 2018, 8(1): e1327. |
| 22 | Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory[J]. Journal of Computational Chemistry, 2011, 32(7): 1456-1465. |
| 23 | Grimme S, Antony J, Ehrlich S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. The Journal of Chemical Physics, 2010, 132(15): 154104. |
| 24 | 倪清, 来锦波, 彭东岳, 等. 离子液体萃取分离烃类化合物的研究进展[J]. 化工进展, 2022, 41(2): 619-627. |
| Ni Q, Lai J B, Peng D Y, et al. Progress in extraction separation of hydrocarbons by ionic liquids[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 619-627. | |
| 25 | Sasaki T, Tada M, Zhong C M, et al. Immobilized metal ion-containing ionic liquids: preparation, structure and catalytic performances in kharasch addition reaction and suzuki cross-coupling reactions[J]. Journal of Molecular Catalysis A: Chemical, 2008, 279(2): 200-209. |
| 26 | Fulton J L, Hoffmann M M, Darab J G. An X-ray absorption fine structure study of copper(Ⅰ) chloride coordination structure in water up to 325℃[J]. Chemical Physics Letters, 2000, 330(3/4): 300-308. |
| 27 | Schäafer H, Binnewies M, Laumanns R, et al. CuAl2Cl8. Darstellung und kristallstruktur[J]. Zeitschrift Für Anorganische und Allgemeine Chemie, 1980, 461(1): 31-34. |
| 28 | Safarik D J, Eldridge R B. Olefin/paraffin separations by reactive absorption: a review[J]. Industrial & Engineering Chemistry Research, 1998, 37(7): 2571-2581. |
| 29 | Bader R F W. Atoms in molecules[J]. Accounts of Chemical Research, 1985, 18(1): 9-15. |
| 30 | Lipkowski P, Grabowski S J, Robinson T L, et al. Properties of the C—H…H dihydrogen bond: an ab initio and topological analysis[J]. The Journal of Physical Chemistry A, 2004, 108(49): 10865-10872. |
| 31 | Cremer D, Kraka E. Chemical bonds without bonding electron density—Does the difference electron-density analysis suffice for a description of the chemical bond?[J]. Angewandte Chemie International Edition in English, 1984, 23(8): 627-628. |
| [1] | 冯海军, 章冰璇, 周健. 图神经网络模型预测和解释离子液体毒性的研究[J]. 化工学报, 2025, 76(1): 93-106. |
| [2] | 李匡奚, 于佩潜, 王江云, 魏浩然, 郑志刚, 冯留海. 微气泡旋流气浮装置内流动分析与结构优化[J]. 化工学报, 2024, 75(S1): 223-234. |
| [3] | 谢慧慧, 姜佳鑫, 王鑫, 李正, 郭鑫, 吕欣然, 王凌云, 刘杨. 深共晶溶剂聚合物包覆膜传输分离铂、钯的研究[J]. 化工学报, 2024, 75(S1): 235-243. |
| [4] | 邱知, 谭明. 聚离子液体膜的制备及其在低钠高钾健康酱油中的应用[J]. 化工学报, 2024, 75(S1): 244-250. |
| [5] | 刘律, 刘洁茹, 范亮亮, 赵亮. 基于层流效应的被动式颗粒分离微流控方法研究[J]. 化工学报, 2024, 75(S1): 67-75. |
| [6] | 唐昊, 胡定华, 李强, 张轩畅, 韩俊杰. 抗加速度双切线弧流道内气泡动力学行为数值与可视化研究[J]. 化工学报, 2024, 75(9): 3074-3082. |
| [7] | 李彦熹, 王晔春, 谢向东, 王进芝, 王江, 周煜, 潘盈秀, 丁文涛, 郭烈锦. 蜗壳式多通道气液旋流分离器结构优化及分离特性研究[J]. 化工学报, 2024, 75(8): 2875-2885. |
| [8] | 罗小平, 侯云天, 范一杰. 逆流相分离结构微细通道流动沸腾传热与均温性[J]. 化工学报, 2024, 75(7): 2474-2485. |
| [9] | 张颂红, 赵欣怡, 楼小玲, 沈绍传, 贠军贤. 阳离子交换纳晶胶分离乳过氧化物酶的研究[J]. 化工学报, 2024, 75(7): 2574-2582. |
| [10] | 秦晓巧, 谭宏博, 温娜. 储能式低温空分系统热力学与经济性分析[J]. 化工学报, 2024, 75(7): 2409-2421. |
| [11] | 杜海燕, 朱凯, 游峰, 王金凤, 赵一帆, 张楠, 李英. 用于应变传感器的自愈合抗冻离子水凝胶[J]. 化工学报, 2024, 75(7): 2709-2722. |
| [12] | 周文轩, 刘珍, 张福建, 张忠强. 高通量-高截留率时间维度膜法水处理机理研究[J]. 化工学报, 2024, 75(7): 2583-2593. |
| [13] | 张香港, 常玉龙, 汪华林, 江霞. 废弃秸秆等生物质低能耗非相变秒级干燥[J]. 化工学报, 2024, 75(7): 2433-2445. |
| [14] | 张广宇, 付然飞, 孙冰, 袁俊聪, 冯翔, 杨朝合, 徐伟. CO2-环氧丙烷合成碳酸丙烯酯:氢键供体效应研究[J]. 化工学报, 2024, 75(6): 2243-2251. |
| [15] | 霍宗伟, 牛亚宾, 潘艳秋. 油水膜分离中高黏度油滴行为研究和影响因素分析[J]. 化工学报, 2024, 75(6): 2262-2273. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号