化工学报 ›› 2025, Vol. 76 ›› Issue (5): 2026-2041.DOI: 10.11949/0438-1157.20241221
张冰1( ), 李建惠1, 马欣蓉1, 陈杨1,2, 李晋平1,2, 李立博1,2(
), 李建惠1, 马欣蓉1, 陈杨1,2, 李晋平1,2, 李立博1,2( )
)
收稿日期:2024-10-31
修回日期:2024-12-16
出版日期:2025-05-25
发布日期:2025-06-13
通讯作者:
李立博
作者简介:张冰(1999—),女,硕士研究生,tyutzhangbing@163.com
基金资助:
Bing ZHANG1( ), Jianhui LI1, Xinrong MA1, Yang CHEN1,2, Jinping LI1,2, Libo LI1,2(
), Jianhui LI1, Xinrong MA1, Yang CHEN1,2, Jinping LI1,2, Libo LI1,2( )
)
Received:2024-10-31
Revised:2024-12-16
Online:2025-05-25
Published:2025-06-13
Contact:
Libo LI
摘要:
金属有机骨架(MOFs)材料因其具有高的比表面积和孔隙率、可调的孔径、丰富多样的结构等优势在气体吸附分离、催化、传感等领域展现出巨大的应用潜力。然而,传统MOF材料的合成以溶剂热法为主,不仅要消耗大量高值有机溶剂,且生产过程能耗高、产量低、废液难处理,不符合化工绿色发展的要求。蒸气相辅助合成法具有溶剂用量小、反应流程少、周期短等优势,近年来在MOF材料合成与改性方面受到广泛关注,有望为MOF材料的合成提供一条绿色高效的新途径。综述了蒸气相辅助法制备MOF基材料的研究进展,阐述了该方法在MOF材料合成及改性领域的研究进展,展望了该方法的发展前景。
中图分类号:
张冰, 李建惠, 马欣蓉, 陈杨, 李晋平, 李立博. 蒸气相辅助法制备MOF基材料的研究进展[J]. 化工学报, 2025, 76(5): 2026-2041.
Bing ZHANG, Jianhui LI, Xinrong MA, Yang CHEN, Jinping LI, Libo LI. Research progress of MOF preparation by steam-assisted method[J]. CIESC Journal, 2025, 76(5): 2026-2041.
| 合成方法 | 优点 | 缺点 | MOF | 比表面积/ (m2/g) | 时空产率/ (kg/(m3·d)) | 文献 |
|---|---|---|---|---|---|---|
| 溶剂热合成法 | 适用范围广 | 高温高压、废液难以处理 | MOF-5 | 2900 | — | [ |
| 电化学合成法 | 合成效率高 | 能耗高、普适性差 | Cu-MOF | 1820 | — | [ |
| 微波加热法 | 效率高,反应时间短 | 微波设备成本高 | Ni-MOF-74 | 1252 | — | [ |
| Mg-MOF-74 | 1416 | — | [ | |||
| UiO-66 | 1052 | 7163 | [ | |||
| 超声波化学法 | 快速结晶 | 产品质量低 | MOF-1 | — | — | [ |
| 微通道合成技术法 | 反应速率快 | 设备加工成本高、狭窄的通道易堵塞 | MOF-808 | 1600 | 95000 | [ |
| 室温溶液合成法 | 简单、廉价、绿色 | 单体溶解度差、普适性差 | UiO-66-(COOH)2 | 890 | — | [ |
| HKUST-1 | 1749 | 1842 | [ | |||
| 机械研磨法 | 环境友好、经济高效 | 产品质量低、稳定性差 | ZIF-8 | 37 | — | [ |
表1 MOF不同合成方法的总结
Table 1 Summary of different synthesis methods of MOF
| 合成方法 | 优点 | 缺点 | MOF | 比表面积/ (m2/g) | 时空产率/ (kg/(m3·d)) | 文献 |
|---|---|---|---|---|---|---|
| 溶剂热合成法 | 适用范围广 | 高温高压、废液难以处理 | MOF-5 | 2900 | — | [ |
| 电化学合成法 | 合成效率高 | 能耗高、普适性差 | Cu-MOF | 1820 | — | [ |
| 微波加热法 | 效率高,反应时间短 | 微波设备成本高 | Ni-MOF-74 | 1252 | — | [ |
| Mg-MOF-74 | 1416 | — | [ | |||
| UiO-66 | 1052 | 7163 | [ | |||
| 超声波化学法 | 快速结晶 | 产品质量低 | MOF-1 | — | — | [ |
| 微通道合成技术法 | 反应速率快 | 设备加工成本高、狭窄的通道易堵塞 | MOF-808 | 1600 | 95000 | [ |
| 室温溶液合成法 | 简单、廉价、绿色 | 单体溶解度差、普适性差 | UiO-66-(COOH)2 | 890 | — | [ |
| HKUST-1 | 1749 | 1842 | [ | |||
| 机械研磨法 | 环境友好、经济高效 | 产品质量低、稳定性差 | ZIF-8 | 37 | — | [ |
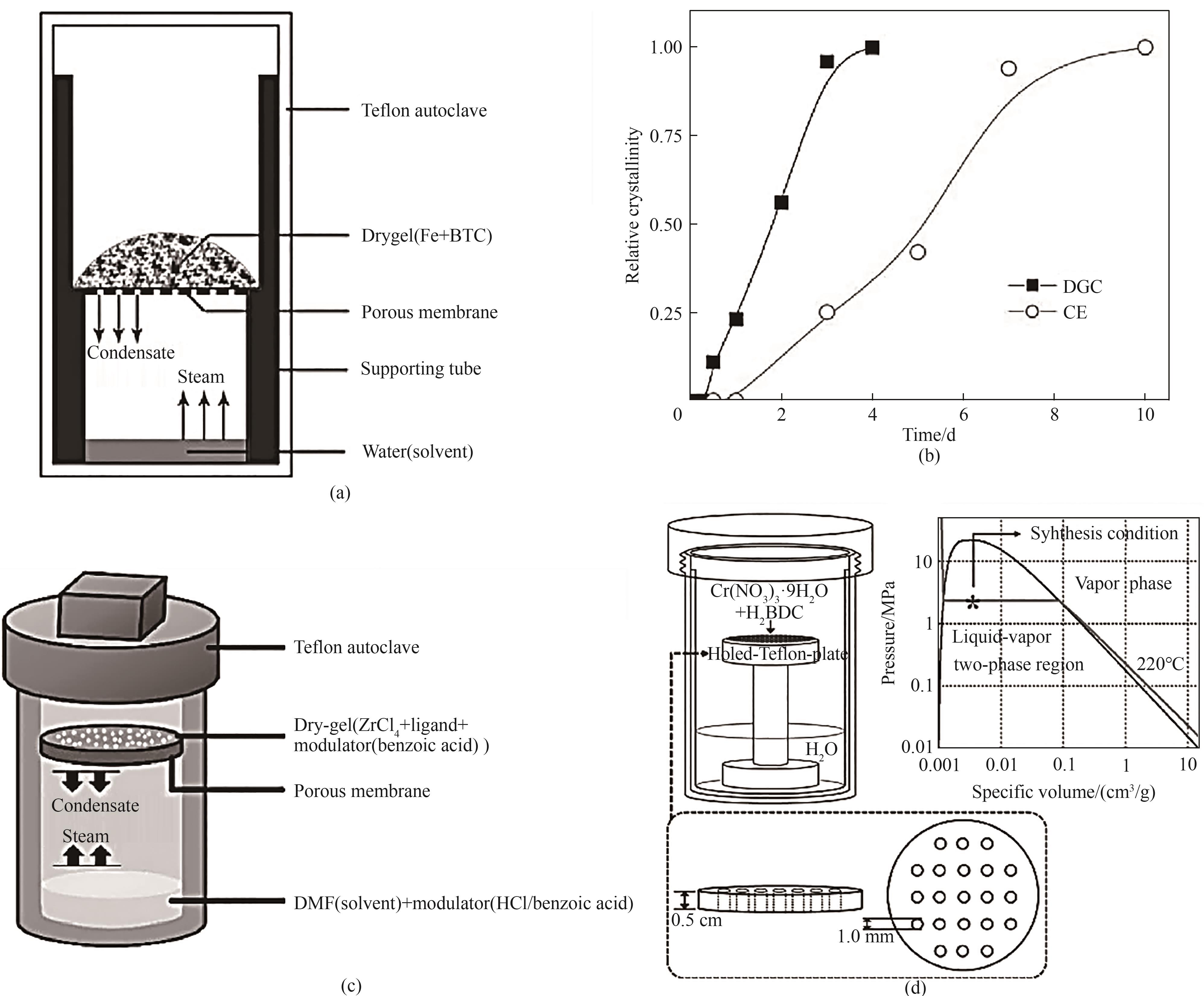
图4 (a)蒸气相辅助法合成MIL-100(Fe)的装置示意图;(b)蒸气相辅助法与电合成的MIL-100(Fe)相对结晶度曲线对比[36];(c)蒸气相辅助法合成UiO型MOF的装置示意图[37];(d)蒸气相辅助法合成MIL-101的反应器图(左)和孔洞聚四氟乙烯板(左下),水的P-V相图(右)[40]
Fig.4 (a) Schematic diagram of the steam-assisted synthesis of MIL-100(Fe); (b) Relative crystallinity curves of MIL-100(Fe) by steam-assisted method and electrosynthesis[36]; (c) Schematic diagram of the device for the synthesis of UiO-type MOF by steam-assisted method[37]; (d) Reactor diagram of steam-assisted synthesis of MIL-101 (left) and holed-Teflon plate (left-bottom), P-V phase diagram of water (right)[40]
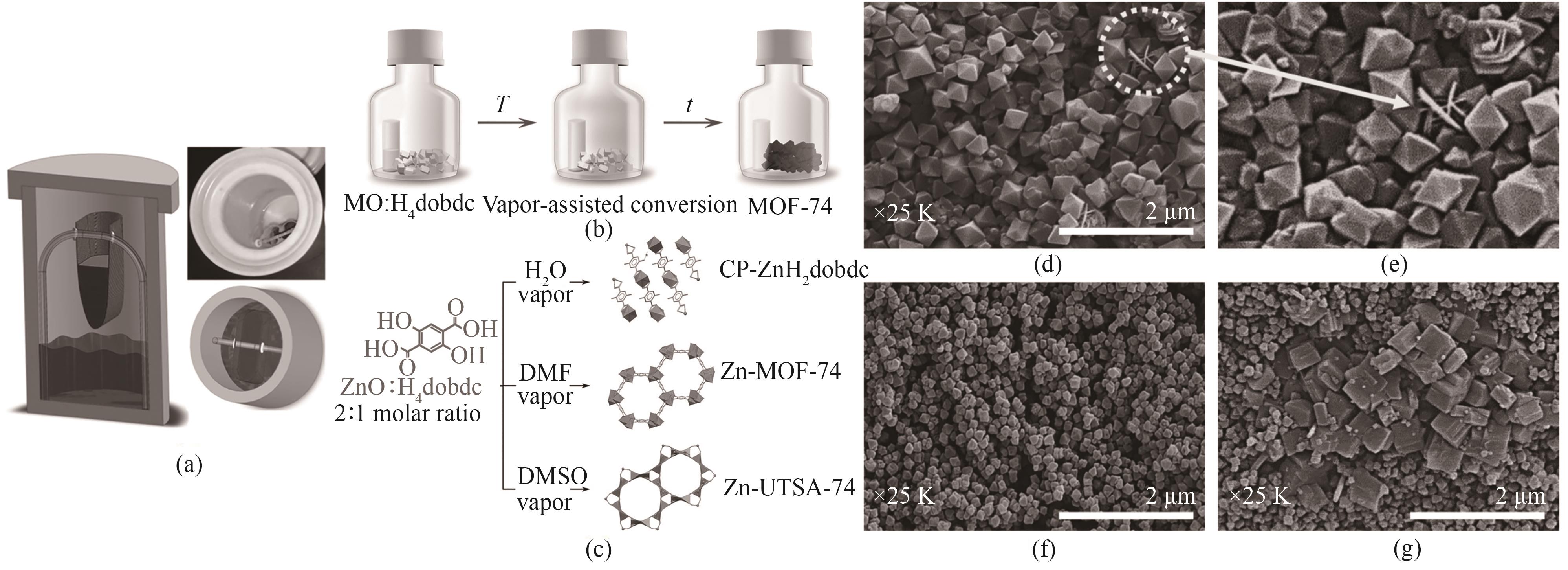
图5 (a)蒸气相辅助法合成MOF-74装置的内部结构[38];(b)蒸气相辅助合成MOF-74的装置:金属氧化物(MO)和H4dobdc配体以2∶1的比例混合[39];(c)ZnO与H4dobdc在不同蒸气条件下反应得到的产物[39];(d)采用溶剂热合成法合成MIL-101的SEM图;(e)图(d)的局部放大;蒸气相辅助法合成MIL-101的SEM图:(f) Cr/H2BDC=1/1,(g) Cr/H2BDC=1/1.2[40]
Fig.5 (a) Diagram of the internal installation of the steam-assisted synthesis MOF-74[38]; (b) Steam-assisted synthesis of MOF-74∶metal oxide (MO) and H4dobdc ligand are mixed in a ratio of 2∶1[39]; (c) Products obtained by the reaction ZnO with H4 dobdc under different steam conditions[39]; (d) SEM image of MIL-101 synthesized by solvent-thermal synthesis method; (e) The expanded images of figure (d); SEM diagram of steam-assisted synthesis MIL-101: (f) Cr/H2BDC=1/1, (g) Cr/H2BDC=1/1.2[40]
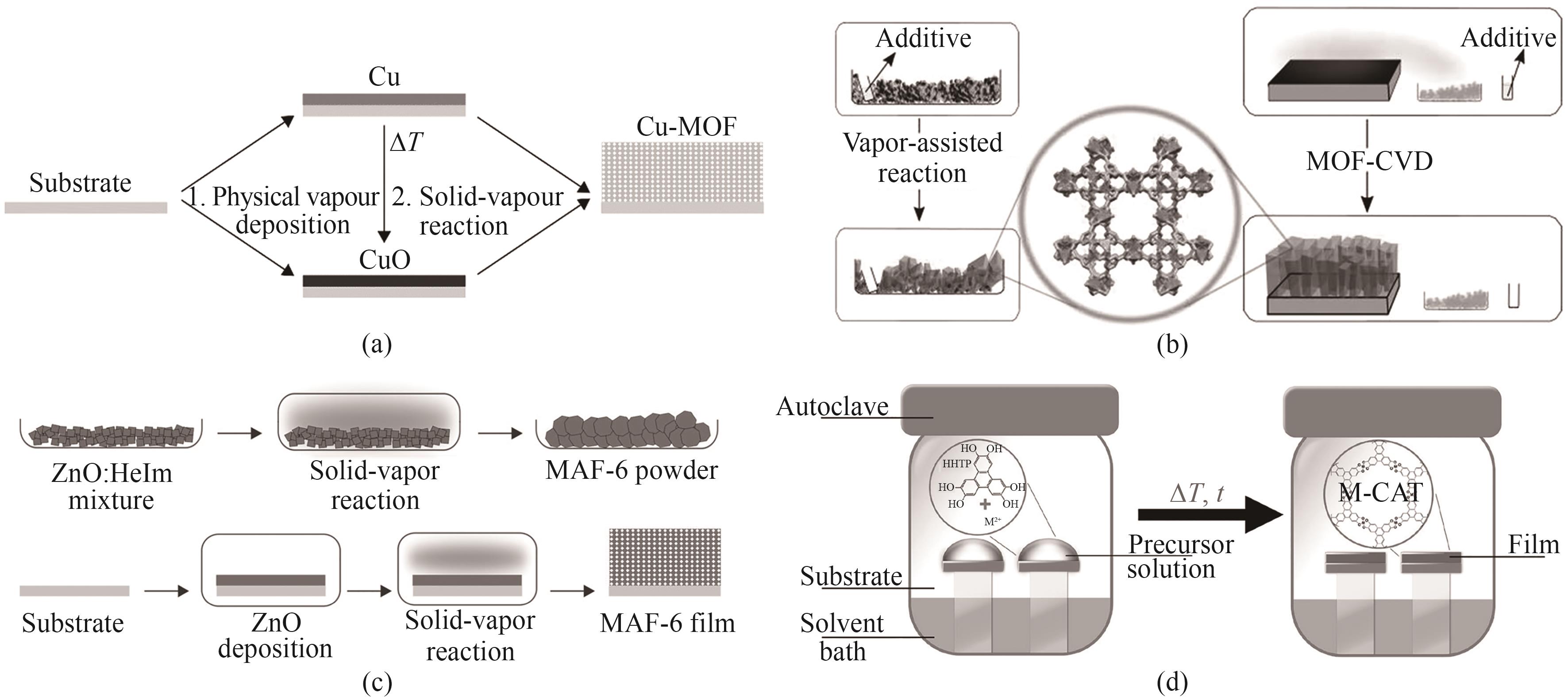
图8 (a)化学气相沉积Cu基MOF薄膜流程示意图[55];(b)气相辅助合成HKUST-1粉末(左)和HKUST-1膜的示意图(右)[56];(c)蒸气相辅助反应制备大孔MAF-6粉末(上)和薄膜的合成示意图(下)[57];(d)化学气相沉积法制备CAT-1膜装置流程图[58]
Fig.8 (a) Chemical vapor deposition Cu-based MOF film flow diagram[55]; (b) Steam-assisted synthesis of HKUST-1 powder (left) and HKUST-1 film (right)[56]; (c) Schematic diagram of preparation of large-pore MAF-6 powder (top) and thin film by steam-assisted method (bottom)[57]; (d) Flow chart of CAT-1 film device prepared by chemical vapor deposition[58]
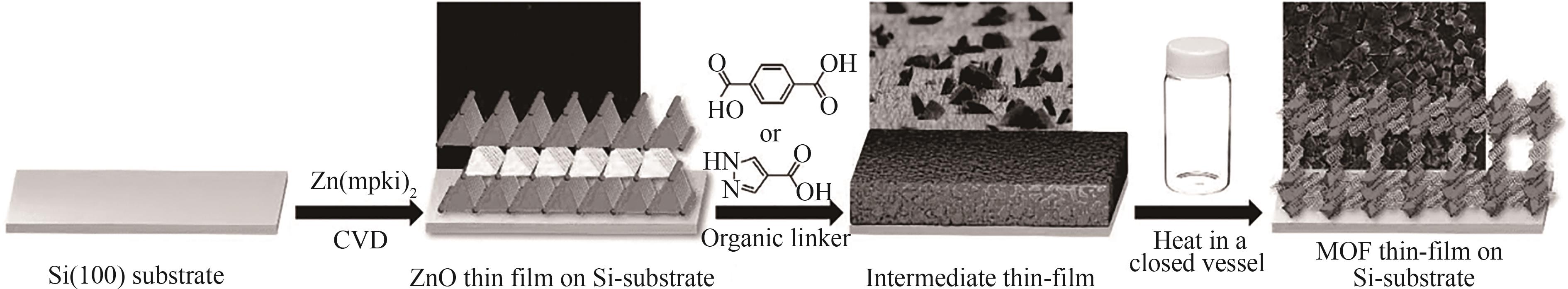
图9 ZnO薄膜的制备并将其转化为MOF薄膜的示意图[SEM显微图像展示了Si(100)衬底上MOF形成的各个阶段][59]
Fig.9 Schematic diagram of the preparation of ZnO thin film and conversion into MOF thin film[SEM microscopic images show the various stages of MOF formation on Si (100) substrate][59]
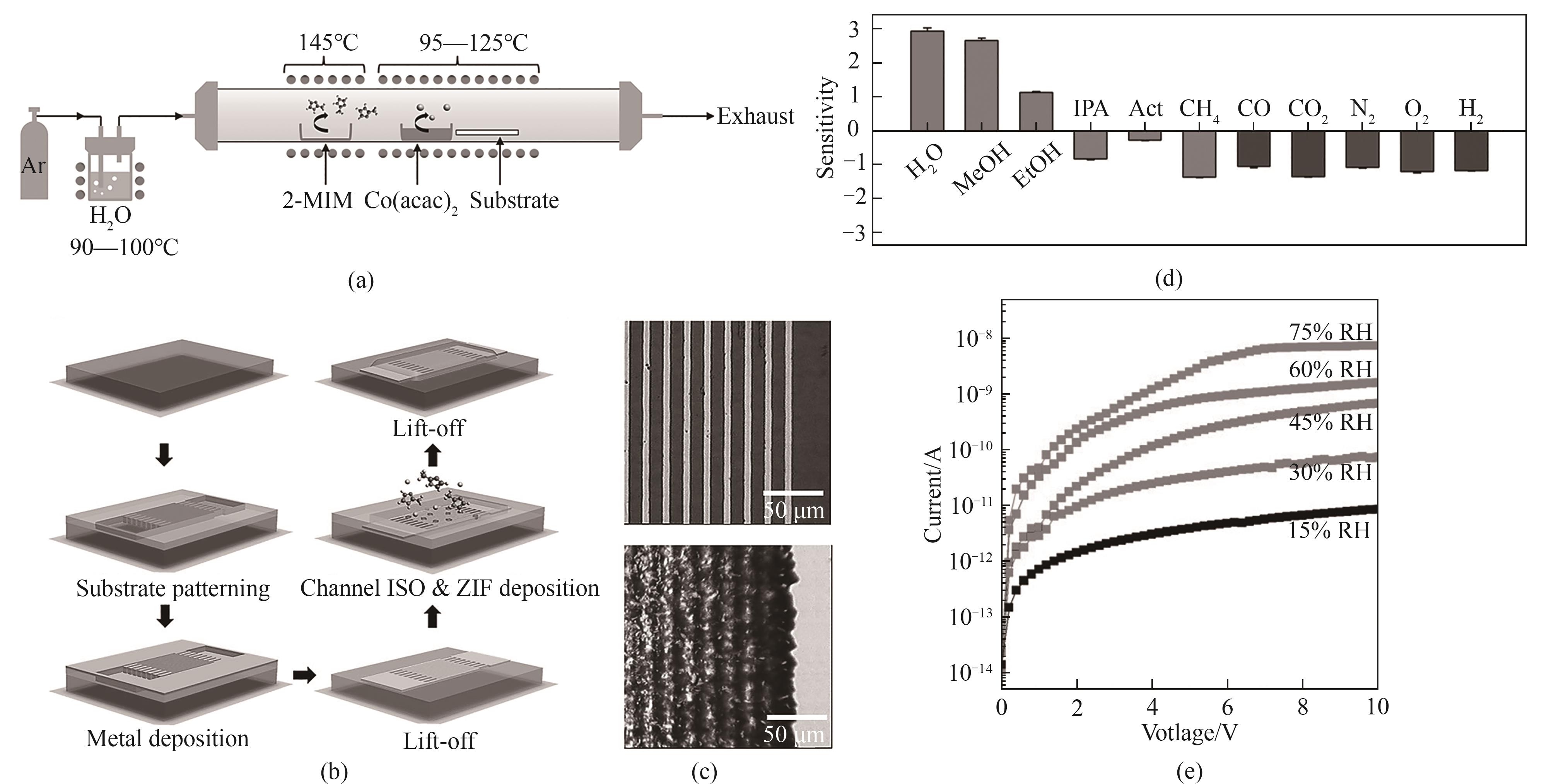
图10 (a)CVD炉中Co(acac)2和2-MIM的蒸气流辅助反应在基质上生长ZIF-67的示意图;(b)ZIF-67化学电阻器的制备示意图;(c)ZIF-67沉积前和沉积后的电极光学图像;(d)CVD ZIF-67化学电阻器在不同环境条件下的气敏性;(e)CVD ZIF-67化学电阻器在不同湿度条件下的输出特性[60]
Fig.10 (a) Schematic diagram of the growth of ZIF-67 on substrate by the steam-flow assisted reaction of Co(acac)2 and 2-MIM in a CVD furnace; (b) Schematic diagram of preparation of ZIF-67 chemical resistor; (c) Optical images of ZIF-67 electrodes before and after deposition; (d) Gas sensitivity of CVD ZIF-67 chemical resistors under different environmental conditions; (e) Output characteristics of CVD ZIF-67 chemical resistor under different humidity conditions[60]
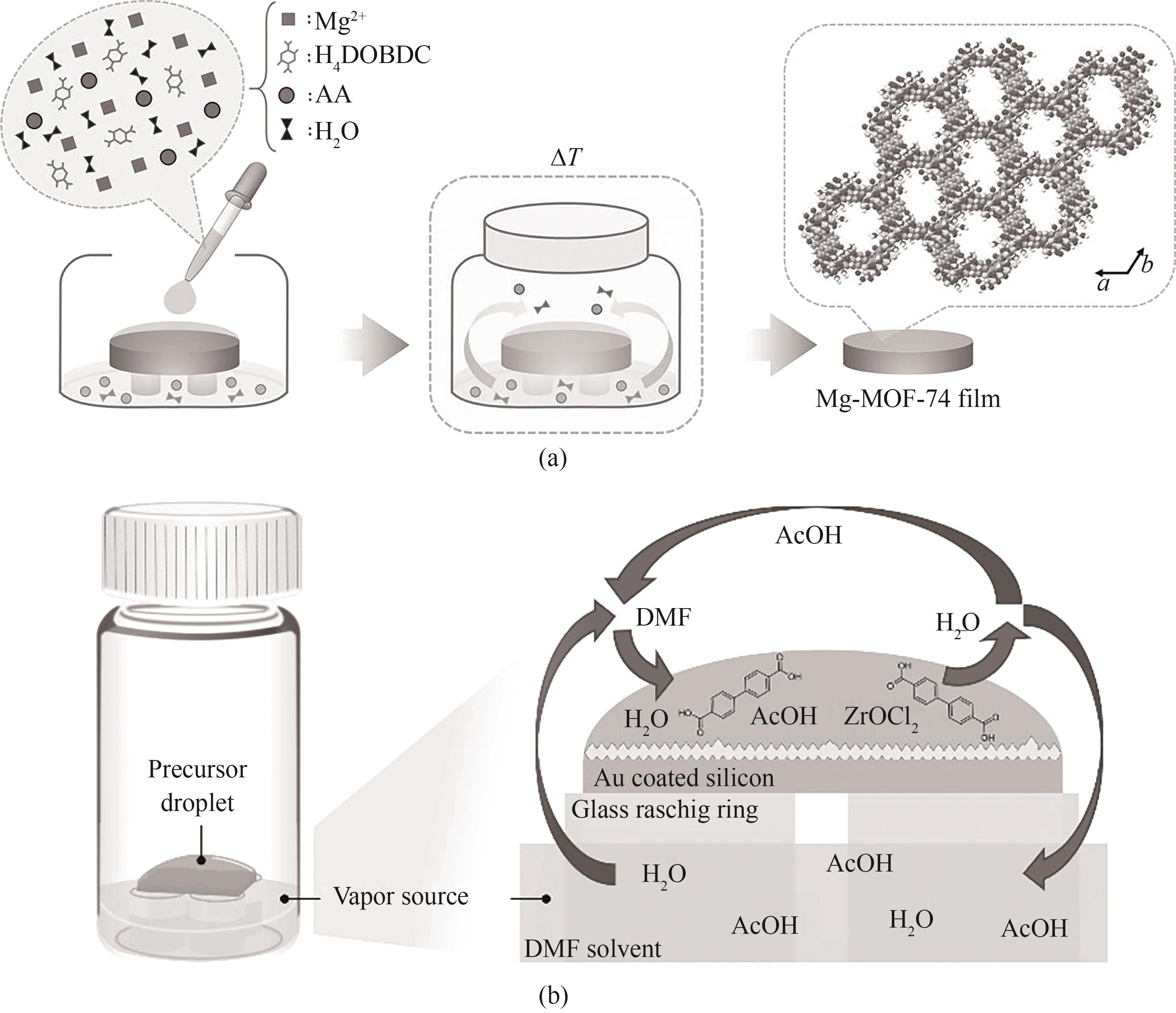
图11 (a)合成高质量Mg-MOF-74薄膜的CVD工艺示意图[62];(b)真空反应装置中平衡过程[63]
Fig.11 (a) CVD process diagram for synthesizing high quality Mg-MOF-74 film[62]; (b) Overview of equilibrium processes in vacuum reaction units[63]
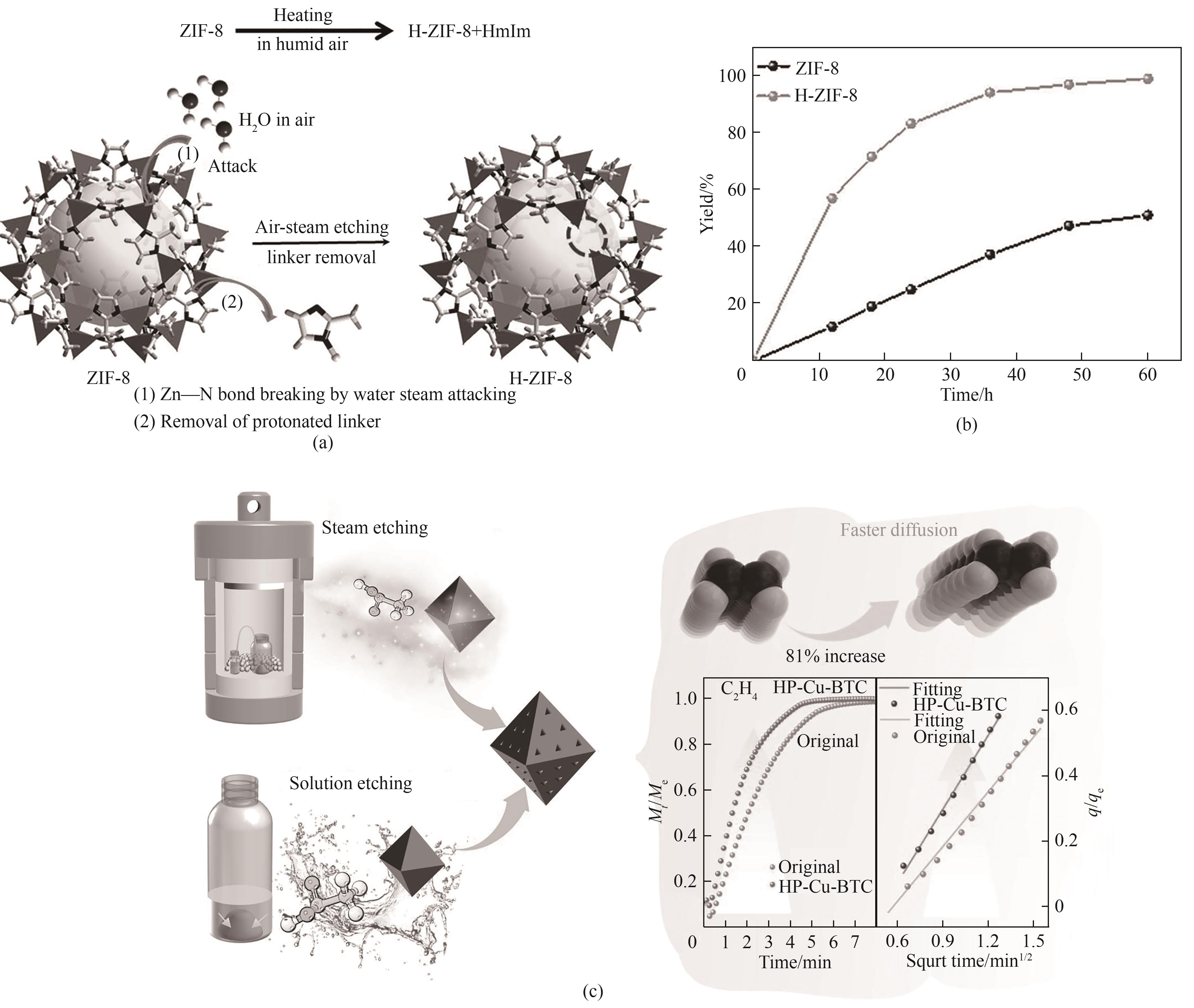
图12 (a)H-ZIF-8中产生分级多孔结构的空气-蒸气刻蚀工艺基本原理示意图;(b)ZIF-8和H-ZIF-8样品在环氧氯丙烷催化CO2环加成中的催化性能[69];(c)蒸气刻蚀、溶液刻蚀制备HP-Cu-BTC的方法示意图及气体分离性能[70]
Fig.12 (a) Schematic diagram of the basic principle of the air-steam etching process for producing layered porous structures in H-ZIF-8; (b) Catalytic performance of ZIF-8 and H-ZIF-8 samples in the cycloaddition of CO2 catalyzed by epichlorohydrin[69]; (c) Method diagram of preparation of HP-Cu-BTC by steam etching and solution etching and gas separation performance diagram[70]
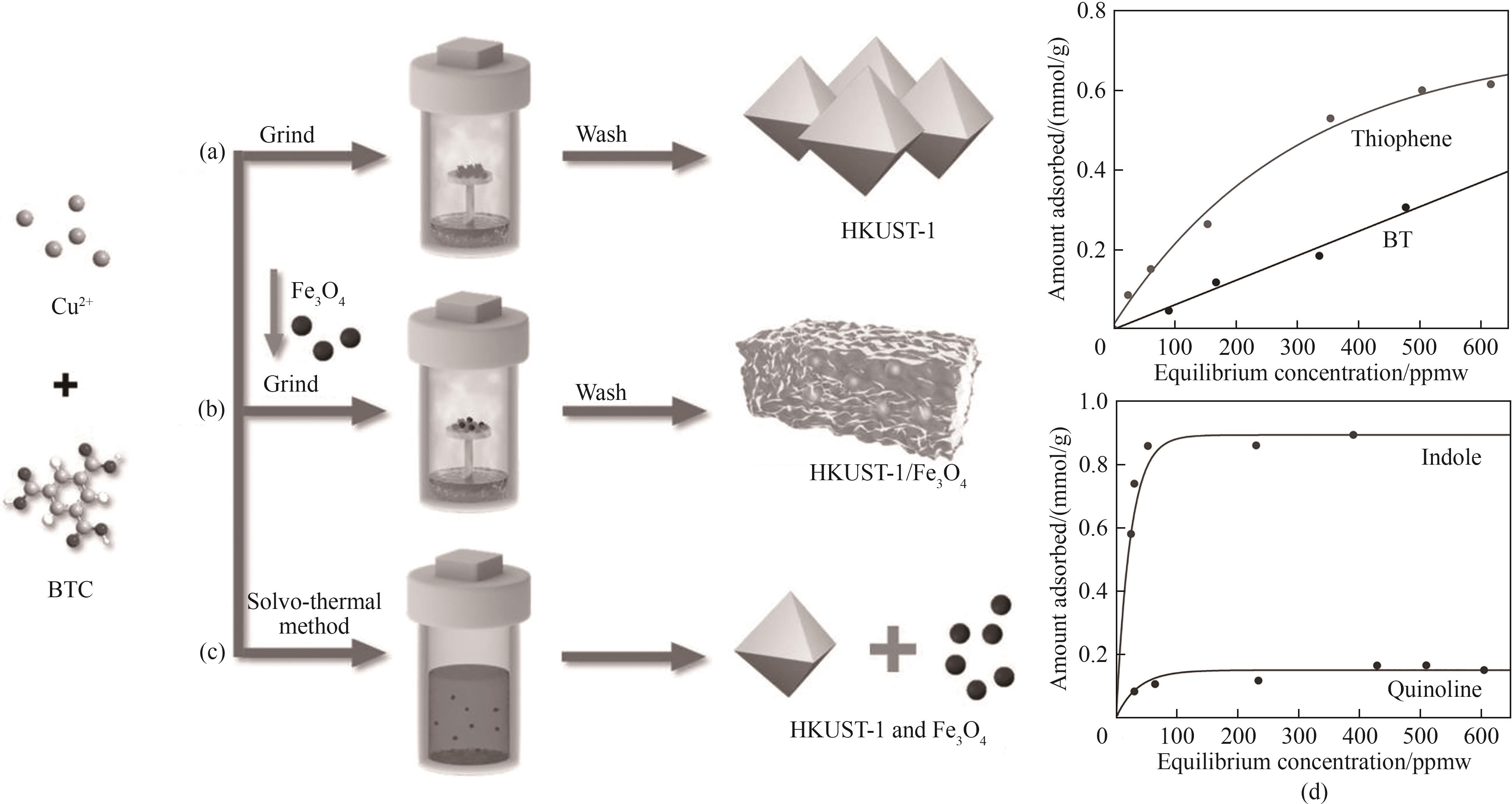
图13 (a)蒸气相辅助法合成HKUST-1的原理图;(b)、(c)分别为蒸气相辅助法和溶剂热法合成HKUST-1/Fe3O4复合材料的合成原理图;(d)HKUST-1/Fe3O4复合材料对噻吩和苯并噻吩(上)、吲哚和喹啉(下)的吸附性能[44]
Fig.13 (a) Schematic diagram of synthesis of HKUST-1 by steam-assisted method; (b),(c) Schematic diagram of synthesis of HKUST-1/Fe3O4 composites by steam-assisted and solvothermal methods; (d) Adsorption properties of HKUST-1/Fe3O4 composites for thiophene and benzothiophene (top), indole and quinoline (bottom)[44]
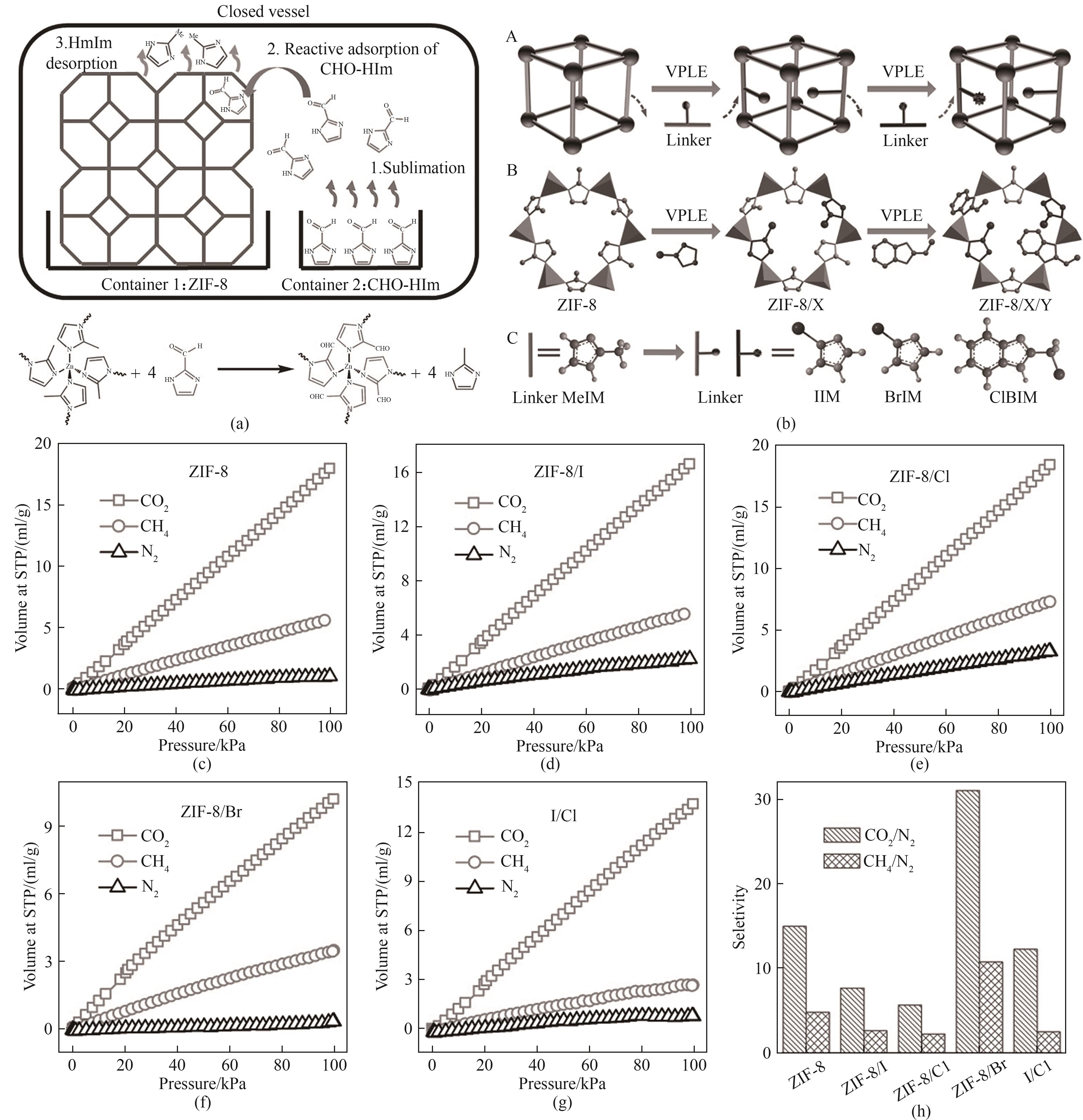
图15 (a)VPLE过程的示意图[76];(b)多连接体VPLE策略示意图;(c) ZIF-8对CO2、CH4和N2的吸附等温线;(d) ZIF-8/I的气体吸附等温线;(e) ZIF-8/Cl的气体吸附等温线;(f) ZIF-8/Br的气体吸附等温线;(g) ZIF-8/I/Cl的气体吸附等温线;(h) ZIF-8和连接体交换的ZIF的CO2/N2和CH4/N2选择性[77]
Fig.15 (a) Schematic diagram of the VPLE process[76]; (b) Schematic diagram of multi-connector VPLE strategy; (c) The adsorption isotherm of CO2, CH4 and N2 by ZIF-8; (d) Gas adsorption isotherm of ZIF-8/I; (e) Gas adsorption isotherm of ZIF-8/Cl; (f) Gas adsorption isotherm of ZIF-8/Br; (g) Gas adsorption isotherm of ZIF-8/I/Cl; (h) CO2/N2 and CH4/N2 selectivity of ZIF exchanged between ZIF-8 and the linker[77]
| 1 | Yaghi O M, Li G M, Li H L. Selective binding and removal of guests in a microporous metal-organic framework[J]. Nature, 1995, 378: 703-706. |
| 2 | Yaghi O M, O'Keeffe M, Ockwig N W, et al. Reticular synthesis and the design of new materials[J]. Nature, 2003, 423(6941): 705-714. |
| 3 | Férey G. Hybrid porous solids: past, present, future[J]. Chemical Society Reviews, 2008, 37(1): 191-214. |
| 4 | Dutta S, Walden M, Sinelshchikova A, et al. Cradle-to-gate environmental impact assessment of commercially available metal-organic frameworks manufacturing[J]. Advanced Functional Materials, 2024, 34(52): 2410751. |
| 5 | 崔希利, 邢华斌. 金属有机框架材料分离低碳烃的研究进展[J]. 化工学报, 2018, 69(6): 2339-2352. |
| Cui X L, Xing H B. Separation of light hydrocarbons with metal-organic frameworks[J]. CIESC Journal, 2018, 69(6): 2339-2352. | |
| 6 | 李建惠, 兰天昊, 陈杨, 等. MOF复合材料在气体吸附分离中的研究进展[J]. 化工学报, 2021, 72(1): 167-179. |
| Li J H, Lan T H, Chen Y, et al. Research progress of MOF-based composites for gas adsorption and separation[J]. CIESC Journal, 2021, 72(1): 167-179. | |
| 7 | Zhang L, Yu B, Wang M, et al. Ethane triggered gate-opening in a flexible-robust metal-organic framework for ultra-high purity ethylene purification[J]. Angewandte Chemie International Edition, 2025, 64(7): e202418853. |
| 8 | Feng Y, Yan W, Kang Z X, et al. Thermal treatment optimization of porous MOF glass and polymer for improving gas permeability and selectivity of mixed matrix membranes[J]. Chemical Engineering Journal, 2023, 465: 142873. |
| 9 | Gao Z Z, Li B J, Li Z, et al. Free-standing metal-organic framework membranes made by solvent-free space-confined conversion for efficient H2/CO2 separation[J]. ACS Applied Materials & Interfaces, 2023, 15(15): 19241-19249. |
| 10 | Vikal A, Maurya R, Patel P, et al. Exploring metal-organic frameworks (MOFs) in drug delivery: a concise overview of synthesis approaches, versatile applications, and current challenges[J]. Applied Materials Today, 2024, 41: 102443. |
| 11 | Hou Q Q, Zhou S, Wei Y Y, et al. Balancing the grain boundary structure and the framework flexibility through bimetallic metal-organic framework (MOF) membranes for gas separation[J]. Journal of the American Chemical Society, 2020, 142(21): 9582-9586. |
| 12 | Lan T H, Li L B, Chen Y, et al. Opportunities and critical factors of porous metal-organic frameworks for industrial light olefins separation[J]. Materials Chemistry Frontiers, 2020, 4(7): 1954-1984. |
| 13 | Yang M X, Xiao L F, Chen W T, et al. Recent advances on metal-organic framework-based electrochemical sensors for determination of organic small molecules[J]. Talanta, 2024, 280: 126744. |
| 14 | Zhang Y, Yu X H, Hou Y W, et al. Current research status of MOF materials for catalysis applications[J]. Molecular Catalysis, 2024, 555: 113851. |
| 15 | Li H, Li L B, Lin R B, et al. Porous metal-organic frameworks for gas storage and separation: status and challenges[J]. EnergyChem, 2019, 1(1): 100006. |
| 16 | Felix Sahayaraj A, Joy Prabu H, Maniraj J, et al. Metal-organic frameworks (MOFs): the next generation of materials for catalysis, gas storage, and separation[J]. Journal of Inorganic and Organometallic Polymers and Materials, 2023, 33(7): 1757-1781. |
| 17 | Shi L, Zhong Y L, Cao H H, et al. A hetero-supermolecular-building-block strategy for the assembly of porous (3,12,24)-connected uru metal-organic frameworks[J]. Nature Synthesis, 2024, 3(12): 1560-1566. |
| 18 | Li H L, Eddaoudi M, O’Keeffe M, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J]. Nature, 1999, 402: 276-279. |
| 19 | Han J L, He X D, Liu J, et al. Determining factors in the growth of MOF single crystals unveiled by in situ interface imaging[J]. Chem, 2022, 8(6): 1637-1657. |
| 20 | Li H, Qin Z, Yang X F, et al. Growth pattern control and nanoarchitecture engineering of metal-organic framework single crystals by confined space synthesis[J]. ACS Central Science, 2022, 8(6): 718-728. |
| 21 | Zhang P, Kang X C, Tao L M, et al. A new route for the rapid synthesis of metal-organic frameworks at room temperature[J]. CCS Chemistry, 2023, 5(6): 1462-1469. |
| 22 | Chen Z J, Wang X J, Noh H, et al. Scalable, room temperature, and water-based synthesis of functionalized zirconium-based metal-organic frameworks for toxic chemical removal[J]. CrystEngComm, 2019, 21(14): 2409-2415. |
| 23 | Mueller U, Schubert M, Teich F, et al. Metal-organic frameworks—prospective industrial applications[J]. Journal of Materials Chemistry, 2006, 16(7): 626-636. |
| 24 | Wu X F, Bao Z B, Yuan B, et al. Microwave synthesis and characterization of MOF-74 (M=Ni, Mg) for gas separation[J]. Microporous and Mesoporous Materials, 2013, 180: 114-122. |
| 25 | Ogura Y, Taniya K, Horie T, et al. Process intensification of synthesis of metal organic framework particles assisted by ultrasound irradiation[J]. Ultrasonics Sonochemistry, 2023, 96: 106443. |
| 26 | Qiu L G, Li Z Q, Wu Y, et al. Facile synthesis of nanocrystals of a microporous metal-organic framework by an ultrasonic method and selective sensing of organoamines[J]. Chemical Communications, 2008(31): 3642-3644. |
| 27 | Bagi S, Yuan S, Rojas-Buzo S, et al. A continuous flow chemistry approach for the ultrafast and low-cost synthesis of MOF-808[J]. Green Chemistry, 2021, 23(24): 9982-9991. |
| 28 | Katsenis A D, Puškarić A, Štrukil V, et al. In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework[J]. Nature Communications, 2015, 6: 6662. |
| 29 | Wang C, Zhang F F, Yang J F, et al. Rapid and HF-free synthesis of MIL-100(Cr) via steam-assisted method[J]. Materials Letters, 2019, 252: 286-288. |
| 30 | Taddei M, Casati N, Steitz D A, et al. In situ high-resolution powder X-ray diffraction study of UiO-66 under synthesis conditions in a continuous-flow microwave reactor[J]. CrystEngComm, 2017, 19(23): 3206-3214. |
| 31 | Majano G, Pérez-Ramírez J. Scalable room-temperature conversion of copper(Ⅱ) hydroxide into HKUST-1 (Cu3(btc)2)[J]. Advanced Materials, 2013, 25(7): 1052-1057. |
| 32 | Xu W Y, Dong J X, Li J P, et al. A novel method for the preparation of zeolite ZSM-5[J]. Journal of the Chemical Society, Chemical Communications, 1990(10): 755-756. |
| 33 | Shi Q, Chen Z F, Song Z W, et al. Synthesis of ZIF-8 and ZIF-67 by steam-assisted conversion and an investigation of their tribological behaviors[J]. Angewandte Chemie International Edition, 2011, 50(3): 672-675. |
| 34 | Horcajada P, Surblé S, Serre C, et al. Synthesis and catalytic properties of MIL-100(Fe), an iron(Ⅲ) carboxylate with large pores[J]. Chemical Communications, 2007(27): 2820-2822. |
| 35 | Seo Y K, Yoon J W, Lee J S, et al. Large scale fluorine-free synthesis of hierarchically porous iron(Ⅲ) trimesate MIL-100(Fe) with a zeolite MTN topology[J]. Microporous and Mesoporous Materials, 2012, 157: 137-145. |
| 36 | Ahmed I, Jeon J, Khan N A, et al. Synthesis of a metal-organic framework, iron-benezenetricarboxylate, from dry gels in the absence of acid and salt[J]. Crystal Growth & Design, 2012, 12(12): 5878-5881. |
| 37 | Gökpinar S, Diment T, Janiak C. Environmentally benign dry-gel conversions of Zr-based UiO metal-organic frameworks with high yield and the possibility of solvent re-use[J]. Dalton Transactions, 2017, 46(30): 9895-9900. |
| 38 | Das A K, Vemuri R S, Kutnyakov I, et al. An efficient synthesis strategy for metal-organic frameworks: dry-gel synthesis of MOF-74 framework with high yield and improved performance[J]. Scientific Reports, 2016, 6: 28050. |
| 39 | Wauteraerts N, Tu M, Chanut N, et al. Vapor-assisted synthesis of the MOF-74 metal-organic framework family from zinc, cobalt, and magnesium oxides[J]. Dalton Transactions, 2023, 52(47): 17873-17880. |
| 40 | Kim J, Lee Y R, Ahn W S. Dry-gel conversion synthesis of Cr-MIL-101 aided by grinding: high surface area and high yield synthesis with minimum purification[J]. Chemical Communications, 2013, 49(69): 7647-7649. |
| 41 | Férey G, Mellot-Draznieks C, Serre C, et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area[J]. Science, 2005, 309(5743): 2040-2042. |
| 42 | Latroche M, Surblé S, Serre C, et al. Hydrogen storage in the giant-pore metal-organic frameworks MIL-100 and MIL-101[J]. Angewandte Chemie International Edition, 2006, 45(48): 8227-8231. |
| 43 | Horcajada P, Serre C, Vallet-Regí M, et al. Metal-organic frameworks as efficient materials for drug delivery[J]. Angewandte Chemie International Edition, 2006, 45(36): 5974-5978. |
| 44 | Tan P, Xie X Y, Liu X Q, et al. Fabrication of magnetically responsive HKUST-1/Fe3O4 composites by dry gel conversion for deep desulfurization and denitrogenation[J]. Journal of Hazardous Materials, 2017, 321: 344-352. |
| 45 | Chen Y, Yang C Y, Wang X Q, et al. Vapor phase solvents loaded in zeolite as the sustainable medium for the preparation of Cu-BTC and ZIF-8[J]. Chemical Engineering Journal, 2017, 313: 179-186. |
| 46 | Liu Y T, Chen H, Li T, et al. Balancing the crystallinity and film formation of metal-organic framework membranes through in situ modulation for efficient gas separation[J]. Angewandte Chemie International Edition, 2023, 62(37): e202309095. |
| 47 | Wu M M, Sun Y W, Ji T T, et al. Fabrication of water-stable MOF-808 membrane for efficient salt/dye separation[J]. Journal of Membrane Science, 2023, 686: 122023. |
| 48 | Shah M, McCarthy M C, Sachdeva S, et al. Current status of metal-organic framework membranes for gas separations: promises and challenges[J]. Industrial & Engineering Chemistry Research, 2012, 51(5): 2179-2199. |
| 49 | Luo L X, Hou L X, Cui X P, et al. Self-condensation-assisted chemical vapour deposition growth of atomically two-dimensional MOF single-crystals[J]. Nature Communications, 2024, 15(1): 3618. |
| 50 | Fu M, Liu Y L, Lyu Q, et al. Sustainable vapor-phase deposition and applications of MOF films and membranes: a critical review[J]. Separation and Purification Technology, 2025, 356: 129883. |
| 51 | Choe M, Koo J Y, Park I, et al. Chemical vapor deposition of edge-on oriented 2D conductive metal-organic framework thin films[J]. Journal of the American Chemical Society, 2022, 144(37): 16726-16731. |
| 52 | Hachem K, Ansari M J, Saleh R O, et al. Methods of chemical synthesis in the synthesis of nanomaterial and nanoparticles by the chemical deposition method: a review[J]. BioNanoScience, 2022, 12(3): 1032-1057. |
| 53 | Salmi L D, Heikkilä M J, Puukilainen E, et al. Studies on atomic layer deposition of MOF-5 thin films[J]. Microporous and Mesoporous Materials, 2013, 182: 147-154. |
| 54 | Stassen I, Styles M, Grenci G, et al. Chemical vapour deposition of zeolitic imidazolate framework thin films[J]. Nature Materials, 2016, 15(3): 304-310. |
| 55 | Stassin T, Rodríguez-Hermida S, Schrode B, et al. Vapour-phase deposition of oriented copper dicarboxylate metal-organic framework thin films[J]. Chemical Communications, 2019, 55(68): 10056-10059. |
| 56 | Rodríguez-Hermida S, Kravchenko D E, Wauteraerts N, et al. Vapor-assisted powder synthesis and oriented MOF-CVD thin films of the metal-organic framework HKUST-1[J]. Inorganic Chemistry, 2022, 61(45): 17927-17931. |
| 57 | Stassin T, Stassen I, Marreiros J, et al. Solvent-free powder synthesis and MOF-CVD thin films of the large-pore metal-organic framework MAF-6[J]. Chemistry of Materials, 2020, 32(5): 1784-1793. |
| 58 | Mähringer A, Jakowetz A C, Rotter J M, et al. Oriented thin films of electroactive triphenylene catecholate-based two-dimensional metal-organic frameworks[J]. ACS Nano, 2019, 13(6): 6711-6719. |
| 59 | Medishetty R, Zhang Z J, Sadlo A, et al. Fabrication of zinc-dicarboxylate- and zinc-pyrazolate-carboxylate-framework thin films through vapour-solid deposition[J]. Dalton Transactions, 2018, 47(40): 14179-14183. |
| 60 | Huang J K, Saito N, Cai Y C, et al. Steam-assisted chemical vapor deposition of zeolitic imidazolate framework[J]. ACS Materials Letters, 2020, 2(5): 485-491. |
| 61 | Tu M, Kravchenko D E, Xia B Z, et al. Template-mediated control over polymorphism in the vapor-assisted formation of zeolitic imidazolate framework powders and films[J]. Angewandte Chemie International Edition, 2021, 60(14): 7553-7558. |
| 62 | Kim K J, Culp J T, Ohodnicki P R, et al. Synthesis of high-quality Mg-MOF-74 thin films via vapor-assisted crystallization[J]. ACS Applied Materials & Interfaces, 2021, 13(29): 35223-35231. |
| 63 | Gschwind W, McCarthy B D, Suremann N F, et al. The influence of water in the vapor-assisted conversion synthesis of UiO-67 MOF thin films[J]. European Journal of Inorganic Chemistry, 2023, 26(27): e202300216. |
| 64 | 杨东晓, 熊启钊, 王毅, 等. 多级孔MOF的制备及其吸附分离应用研究进展[J]. 化工进展, 2024, 43(4): 1882-1896. |
| Yang D X, Xiong Q Z, Wang Y, et al. Progress in the preparation of hierarchically porous MOF and applications in adsorption and separation[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1882-1896. | |
| 65 | Koo J, Hwang I C, Yu X J, et al. Hollowing out MOFs: hierarchical micro-and mesoporous MOFs with tailorable porosity via selective acid etching[J]. Chemical Science, 2017, 8(10): 6799-6803. |
| 66 | Xi D Y, Sun Q M, Xu J, et al. In situ growth-etching approach to the preparation of hierarchically macroporous zeolites with high MTO catalytic activity and selectivity[J]. Journal of Materials Chemistry A, 2014, 2(42): 17994-18004. |
| 67 | McNamara N D, Hicks J C. Chelating agent-free, vapor-assisted crystallization method to synthesize hierarchical microporous/mesoporous MIL-125 (Ti)[J]. ACS Applied Materials & Interfaces, 2015, 7(9): 5338-5346. |
| 68 | Hou C C, Wang Y, Zou L L, et al. A gas-steamed MOF route to P-doped open carbon cages with enhanced Zn-ion energy storage capability and ultrastability[J]. Advanced Materials, 2021, 33(31): e2101698. |
| 69 | Huang H L, Sun Y X, Jia X M, et al. Air-steam etched construction of hierarchically porous metal-organic frameworks[J]. Chinese Journal of Chemistry, 2021, 39(6): 1538-1544. |
| 70 | Chen Y, Dai Y H, Xiong Q Z, et al. Synthesis of hierarchically porous Cu-BTC through phase-controlled etching[J]. Chemical Engineering Science, 2024, 297: 120293. |
| 71 | 代艳辉, 熊启钊, 房强, 等. 原位蒸汽辅助法用于一步制备多级孔Cu-BTC[J]. 化工学报, 2024, 75(9): 3329-3337. |
| Dai Y H, Xiong Q Z, Fang Q, et al. In situ steam-assisted method for one-step synthesis of hierarchically porous Cu-BTC[J]. CIESC Journal, 2024, 75(9): 3329-3337. | |
| 72 | Cohen S M. Postsynthetic methods for the functionalization of metal-organic frameworks[J]. Chemical Reviews, 2012, 112(2): 970-1000. |
| 73 | Liu L J, Li L, Ziebel M E, et al. Metal-diamidobenzoquinone frameworks via post-synthetic linker exchange[J]. Journal of the American Chemical Society, 2020, 142(10): 4705-4713. |
| 74 | Su P C, Tu M, Ameloot R, et al. Vapor-phase processing of metal-organic frameworks[J]. Accounts of Chemical Research, 2022, 55(2): 186-196. |
| 75 | Park K S, Ni Z, Côté A P, et al. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(27): 10186-10191. |
| 76 | Marreiros J, Van Dommelen L, Fleury G, et al. Vapor-phase linker exchange of the metal-organic framework ZIF-8: a solvent-free approach to post-synthetic modification[J]. Angewandte Chemie International Edition, 2019, 58(51): 18471-18475. |
| 77 | Wu W F, Su J Y, Jia M M, et al. Vapor-phase linker exchange of metal-organic frameworks[J]. Science Advances, 2020, 6(18): eaax7270. |
| 78 | Kim I S, Ahn S, Vermeulen N A, et al. The synthesis science of targeted vapor-phase metal-organic framework postmodification[J]. Journal of the American Chemical Society, 2020, 142(1): 242-250. |
| 79 | De S, Quan G C, Gikonyo B, et al. Vapor-phase infiltration inside a microporous porphyrinic metal-organic framework for postsynthesis modification[J]. Inorganic Chemistry, 2020, 59(14): 10129-10137. |
| [1] | 张耀辉, 班宇杰, 杨维慎. 以蒸气加工法制备和修饰金属-有机框架膜[J]. 化工学报, 2025, 76(5): 2070-2086. |
| [2] | 赵浩帆, 任豪杰, 刘宗凯, 董冠英, 张亚涛. MOFs玻璃膜在气体分离领域的研究进展[J]. 化工学报, 2025, 76(5): 2042-2054. |
| [3] | 宁丹东, 李建惠, 陈杨, 李晋平, 李立博. MIL-101(Cr)批量化生产中的絮凝工艺研究[J]. 化工学报, 2025, 76(5): 2327-2336. |
| [4] | 赵海钎, 陈方, 陈涛, 郭建维, 林文静, 杨楚芬. 叶酸修饰的pH响应共聚物混合胶束用于抗癌药物递送[J]. 化工学报, 2025, 76(4): 1702-1710. |
| [5] | 产文, 余万, 王岗, 苏华山, 黄芬霞, 胡涛. 改进回热布局的Allam循环热力、经济性能分析和双目标优化[J]. 化工学报, 2025, 76(4): 1680-1692. |
| [6] | 朱峰, 赵跃, 马凤翔, 刘伟. 改性UIO-66对SF6/N2混合气体及其分解产物的吸附特性[J]. 化工学报, 2025, 76(4): 1604-1616. |
| [7] | 晋伊浩, 罗俊欣, 胡章茂, 王唯, 殷谦. 亲水改性硫酸镁/膨胀蛭石复合材料的吸附储热性能[J]. 化工学报, 2025, 76(4): 1852-1862. |
| [8] | 张静, 元跃, 刘艳梅, 王智文, 陈涛. 生物法制备衣康酸研究进展[J]. 化工学报, 2025, 76(3): 909-921. |
| [9] | 李远华, 凌思棋, 封科军, 冯颖, 郭于菁, 谢世桓. 基于cMOFs的固定化脂肪酶微反应器的构筑及其扁桃酸催化应用[J]. 化工学报, 2025, 76(3): 1170-1179. |
| [10] | 李京润, 杨思宇, 刘庆辉, 潘安, 王嘉岳, 符小贵, 余皓. 大规模风电耦合火电制氢多情景下不同运行策略分析[J]. 化工学报, 2025, 76(3): 1191-1206. |
| [11] | 徐艳焦, 楼琳瑾, 樊茁钦, 张浩淼, 王靖岱, 阳永荣. 甲基铝氧烷的改性技术研究进展[J]. 化工学报, 2025, 76(2): 454-465. |
| [12] | 应昕, 杜淼, 潘鹏举, 单国荣. 高折射率聚硫氨酯的合成、结构与性能[J]. 化工学报, 2025, 76(2): 858-867. |
| [13] | 贾晶宇, 孔德齐, 沈圆辉, 张东辉, 李文彬, 唐忠利. 合成氨反应器尾气变压吸附氨分离工艺的模拟与分析[J]. 化工学报, 2025, 76(2): 718-730. |
| [14] | 殷梦凡, 王倩, 郑涛, 姬奎, 王绍贵, 郭辉, 林志强, 张睿, 孙晖, 刘海燕, 刘植昌, 徐春明, 孟祥海, 王月平. 可再生能源电解水制氢-低温低压合成氨万吨级工业示范流程设计[J]. 化工学报, 2025, 76(2): 825-834. |
| [15] | 纪之骄, 张晓方, 甘汶, 薛云鹏. 载体对单原子电催化剂合成氨性能的影响与调控策略[J]. 化工学报, 2025, 76(1): 18-39. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号